Introduction
Crocin is an effective water-soluble active monomer
extracted from Crocus sativus, a plant that is used in
traditional Chinese medicine (1). It
is reported that crocin has a number of beneficial properties, such
as for the treatment of myocardial ischemia and hypoxia; improving
behavior and cognition; and anti-lipid peroxidation,
anti-atherosclerosis and antitumor effects (1–4).
Proteoglycans extracted from C. sativus can promote the
activity of macrophages and promotes immune regulation and invasion
resistance (5). Crocin can
effectively inhibit the activity of free radicals and xanthine
oxidase, thereby acting as an antioxidant (6). In addition, crocin has
anti-inflammatory effects and has been used as an adjuvant for
various inflammatory diseases (7,8).
The incidence of colorectal cancer has increased in
China, with only lung cancer and gastric cancer showing higher
incidences (9). Early colorectal
cancer lacks clear and typical symptoms and most patients with
colorectal cancer are already at an advanced stage and at risk of
metastasis upon diagnosis. As such, the optimal point for beginning
treatment has already passed and the prognosis is not as favorable
(10). Inflammation is involved in
the occurrence and development of colon cancer (11). For example, interleukin (IL)-1β, IL-6
and tumor necrosis factor (TNF)-α are involved in all aspects of
colon cancer (12,13). However, the anticancer and
anti-inflammatory functions of crocin in colon cancer cells have
not been investigated.
In the present study, proliferation and apoptosis of
colon cancer cells, inflammatory responses in colon cancer cells,
as well as chemokine release from colon cancer cells was
investigated following treatment with crocin. The signaling
pathways that are regulated by crocin were also examined.
Materials and methods
Cells
HCT116 cells (The Cell Bank of Type Culture
Collection of the Chinese Academy of Sciences) were cultured in
McCoy's 5A (Modified) Medium (16600082; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Thermo Fisher Scientific, Inc.) at
37°C and 5% CO2. Prior to western blot analysis, HCT116
cells were treated with Stattic (HY-13818; MedChemExpress),
according to the manufacturer's instructions, to inhibit the
activity of the STAT3 signaling pathway.
MTT assay
HCT116 cells in the logarithmic growth phase were
digested, resuspended at a density of 2×104 cells/ml and
seeded into 96-well plates at 37°C and 5% CO2. To test
the effect of crocin on the proliferation of HCT116 cells, cells
were treated with either a high or low dose of crocin. The cells in
the high-dose group were treated with 271.18 µM crocin and those in
the low-dose group were treated with 135.6 µM crocin at 37°C for
24, 48 or 72 h. To these the inhibitory rate of crocin, after
adhesion of the cells to the surface of the plate at 37°C for 8 h,
the medium was replaced with McCoy's 5A (Modified) Medium
containing 10% FBS and varying concentrations of crocin (50, 100,
200, 400, 800 and 1,600 µM; ES-0329; Extrasynthese) following
previous studies (14,15). The control group was cultured in
medium without drugs. Each concentration was examined in
triplicate. After culture at 37°C and 5% CO2 for 24, 48,
72, 96, 120 or 144 h, the medium was replaced with serum-free
medium. In the dark, 5 mg/ml MTT solution (20 µl) was added onto
the cells which were then incubated at 37°C for 4 h. Subsequently,
the medium was discarded, and DMSO (150 µl) was added into each
well before shaking at 37°C in the dark for 5 min. Then, the
absorbance was read at 570 nm using a microplate reader (DG5033A;
Nanjing Huadong Electronics Co., Ltd.), and this reflected cell
viability or number. The following formula was used: Inhibition
rate of drugs on cell proliferation (%)=(1-absorbance of drug
group/absorbance of control group) ×100%. Growth curves were
plotted using time (h) as the x-axis, and absorbance as the y-axis.
The IC50 was calculated from inhibitory rates at 48
h.
Transwell assay
To test invasion ability, Matrigel® (BD
Biosciences) was thawed at 4°C overnight and diluted with
serum-free medium (dilution, 1:2). The mixture (50 µl) was evenly
applied to the upper chambers of Transwell plates (Merck KGaA) on
ice and incubated at 37°C for 1 h for solidification. HCT116 cells
(2×105 cells/well) from the control group or the crocin
treatment groups were seeded into the upper chamber containing
200-µl serum free medium at 37°C. A total of 500 µl medium
supplemented with 10% FBS was added into the lower chamber. After
24 h of incubation at 37°C, the chamber was removed and the cells
in the upper chamber were wiped off. After being fixed with 4%
formaldehyde at room temperature for 10 min, the membrane was
stained with Giemsa at room temperature for 15 min and observed
using a light microscope (in 5 random fields (magnification, ×200).
The number of invading cells was counted to evaluate the cell
invasion ability.
Hoechst 33342/propidium iodide (PI)
double staining
After being treated with crocin for 24 h, HCT116
cells were subjected with Hoechst 33342/PI double staining (cat.
no. C1056; Beyotime Institute of Biotechnology). The cells were
first washed with PBS twice, and 5 µl Hoechst stain and 5 µl PI
stain were added onto the cells before incubating at 4°C for 20–30
min. After staining, the cells were washed with PBS twice before
observing red (Hoechst) and blue (PI) fluorescence under a
fluorescence microscope (Axio Scope A1; Carl Zeiss AG) at a
magnification of ×100.
Flow cytometry
Cells (1×106) in each group were washed
with pre-cooled phosphate-buffered saline twice and subjected to
flow cytometry using Annexin V-FITC/PI Apoptosis Detection kit
(A211-01/02; Vazyme) following the manufacturer's protocol to
detect cell apoptosis. Cells with Annexin V-positive values were
considered early apoptotic cells, those with PI-positive values
were considered necrotic, and those with double positive values
were considered late apoptotic.
ELISA
Cell supernatant was centrifuged at 3,000 × g and
4°C for 10 min to eliminate cell debris. IL-6 (cat. no. ab46027),
TNF-α (cat. no. ab181421), IL-1β (cat. no. ab46052), macrophage
inflammatory protein (MIP)-2 (cat. no. ab184862), monocyte
chemoattractant protein (MCP)-1 (cat. no. ab100586), and IL-8 (cat.
no. ab46032) ELISA kits (Abcam) were used to determine the
concentrations of respective proteins in the cell supernatant. In
96-well microplates, standards (50 µl) and samples (10 µl serum and
40 µl diluent) were added into predefined wells, while blank wells
were left empty. In the wells for standards and samples,
horseradish peroxidase-labelled conjugates (100 µl) were added
before sealing the plates for incubation at 37°C for 1 h. After
washing the plates five times, substrates A (50 µl) and B (50 µl)
were added into each well. After incubation at 37°C for 15 min,
stop solution (50 µl) was added into each well, and absorbance of
each well was measured at 450 nm using a microplate reader within
15 min.
Reverse transcription-quantitative PCR
(RT-qPCR)
Cells (3×106) were directly lysed with 1
ml TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Total RNA was extracted using phenol chloroform method. The
concentration and quality of RNA was measured using ultraviolet
spectrophotometry (NanoDrop™ ND2000; Thermo Fisher Scientific,
Inc.). Subsequently, cDNA was obtained by RT from 1 µg RNA and
stored at −20°C. RT of mRNA was performed using TIANScript II cDNA
First Strand Synthesis kit (Tiangen Biotech Co., Ltd.) according to
the manufacturer's protocol. SuperReal PreMix (SYBR-Green) kit
(Tiangen Biotech Co., Ltd.) was used to detect mRNA expression,
using GAPDH as an internal reference. The reaction system (20 µl)
was composed of 10 µl SYBR Premix EXTaq, 0.5 µl forward primer
(STAT3, 5′-GGAGGAGGCATTCGGAAAG-3′; β-actin,
5′-AACGGCTCCGGCATGTGCAA-3′), 0.5 µl reverse primer (STAT3,
5′-TCGTTGGTGTCACACAGAT-3′; β-actin, 5′-CTTCTGACCCATGCCCACCA-3′), 2
µl cDNA and 7 µl ddH2O. The following thermocycling
conditions were used: Initial denaturation at 95°C for 5 min; 46
cycles of denaturation at 95°C for 20 sec and annealing at 55°C for
20 sec; and a final extension at 72°C for 30 sec (iQ5 system;
Bio-Rad Laboratories, Inc.). The 2−ΔΔCq method was used
to calculate the relative expression of target mRNA against GAPDH
(16). Each sample was tested in
triplicate.
Western blotting
Before lysis, cells (1×106) were
trypsinized and collected. Then, the cells were lysed with
precooled RIPA lysis buffer (600 µl; 50 mM Tris-base, 1 mM EDTA,
150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% Triton X-100, 1%
sodium deoxycholate; Beyotime Institute of Biotechnology) for 30
min on ice. The mixture was centrifuged at 11,000 × g and 4°C for
10 min. The concentration of protein within the supernatant was
determined by BCA protein concentration determination kit [RTP7102,
Real-Times (Beijing) Biotechnology Co., Ltd.]. The samples were
then mixed with 5X SDS loading buffer before denaturation in a
boiling water bath for 10 min. Afterwards, the samples (20 µg) were
subjected to 10% SDS-PAGE at 100 V. The resolved proteins were
transferred to PVDF membranes on ice (100 V, 2 h) and blocked with
5% skimmed milk at room temperature for 1 h. Then, the membranes
were incubated with rabbit anti-human phosphorylated (P)-STAT3
(1:1,500; ab30647; Abcam), STAT3 (1:1,000; ab68153; Abcam) or
β-actin (1:5,000; ab129348; Abcam) monoclonal primary antibodies at
4°C overnight. After extensive washing with PBS with Tween-20,
three times for 15 min, the membranes were incubated with goat
anti-rabbit horseradish peroxidase-conjugated secondary antibody
(1:3,000; ab6721; Abcam) for 1 h at room temperature before washing
with PBS with Tween-20, three times for 15 min. Then, the membrane
was developed with an ECL kit (Sigma-Aldrich; Merck KGaA) for
imaging. Image Lab v3.0 software (Bio-Rad Laboratories, Inc.) was
used to acquire and analyze imaging signals. The relative amounts
of target proteins were normalized against β-actin.
Statistical analysis
The results were analyzed using SPSS 20.0
statistical software (IBM Corp.). The data are shown as the mean ±
SD. Multigroup measurement data were analyzed using one-way ANOVAs,
followed by Student-Newman-Keuls post-hoc tests. Comparisons
between two groups were carried out using the Student's t-test.
Three repeats were performed for each experiment. P<0.05 was
considered to indicate a statistically significant difference.
Results
Crocin inhibits the proliferation of
HCT116 cells in a dose-dependent manner and a high dose of crocin
results in a lower level of proliferation
To calculate the IC50 of crocin on the
proliferation of HCT116 cells, the cells were treated with 50, 100,
200, 400, 800 or 1,600 µM crocin for 48 h and an MTT assay was
performed. These data showed that crocin inhibited the
proliferation of HCT116 cells in a dose-dependent manner and the
IC50 was 271.18±21.83 µM (Fig. 1A). The absorbance of HCT116 cells in
both the low- and high-dose groups was significantly lower than
that in the control group (P<0.05 for both), and that in
low-dose group was significantly higher than that in high-dose
group after 72 h (P<0.05; Fig.
1B). The results suggested that crocin inhibited the
proliferation of HCT116 cells in a dose-dependent manner, with
higher doses of crocin resulting in lower levels of
proliferation.
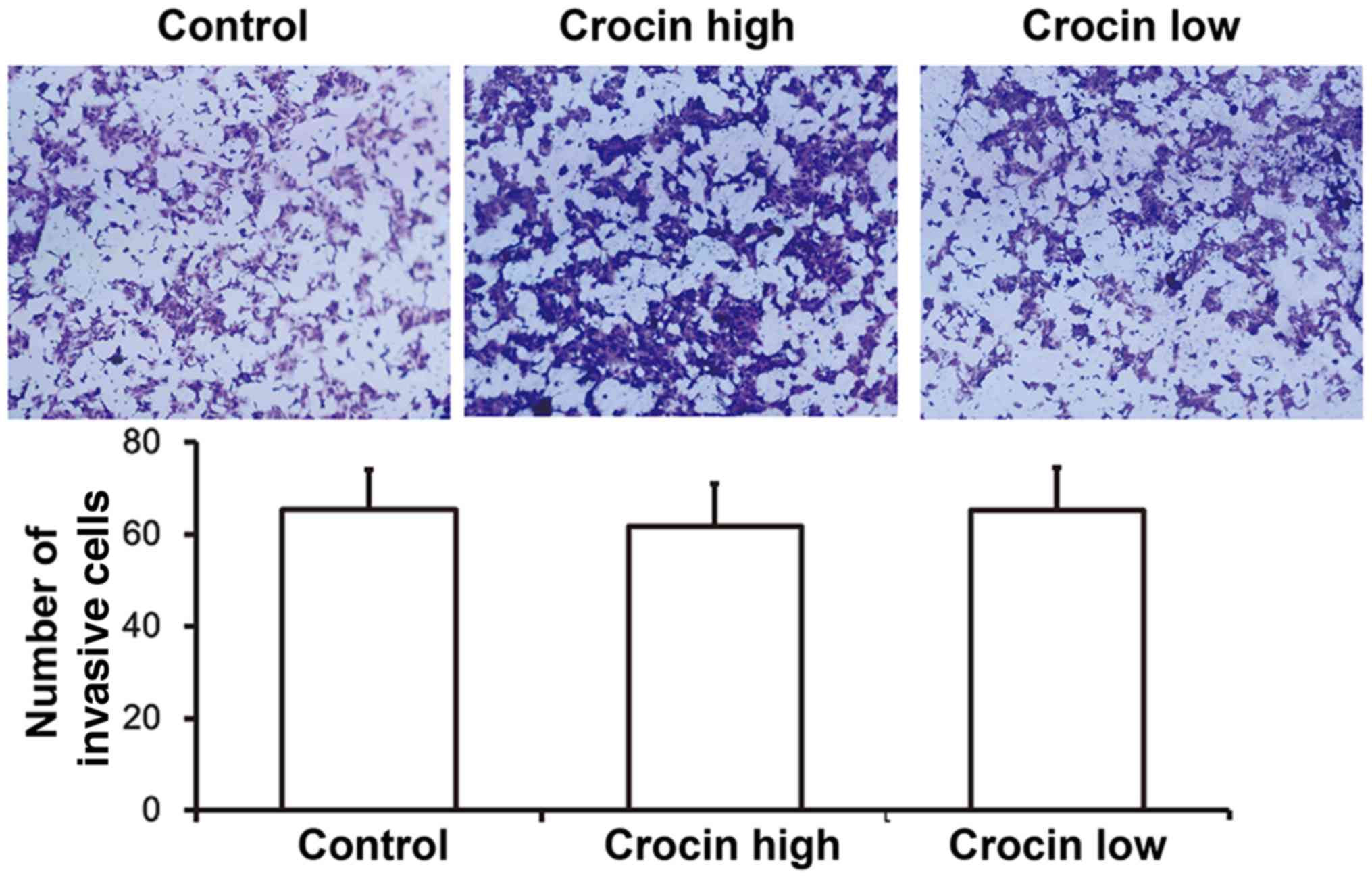
Crocin does not affect the invasion
ability of HCT116 cells
To evaluate the invasion of HCT116 cells, Transwell
assays were carried out. These data showed that number of invasive
cells in either the high-dose crocin group or the low-dose crocin
group was not different from that of the control group (P>0.05;
Fig. 2). These results indicated
that crocin did not affect the invasive capabilities of HCT116
cells.
Crocin increases the apoptosis of
HCT116 cells and a high dose of crocin leads to a higher level of
apoptosis
To examine the effect of crocin on the apoptosis of
HCT116 cells, flow cytometry and Hoechst/PI staining were carried
out after treatment with high-dose and low-dose crocin for 72 h.
These data showed that both high- and low-dose crocin treatment
induced significant apoptosis of HCT116 cells, and the apoptotic
rate in the low-dose group was significantly lower than that in the
high-dose group (P<0.05; Fig.
3A). Hoechst/PI staining showed a similar trend to the flow
cytometry data (Fig. 3B). The
results indicate that crocin increased the apoptosis of HCT116
cells and a higher dose of crocin led to a higher level of
apoptosis.
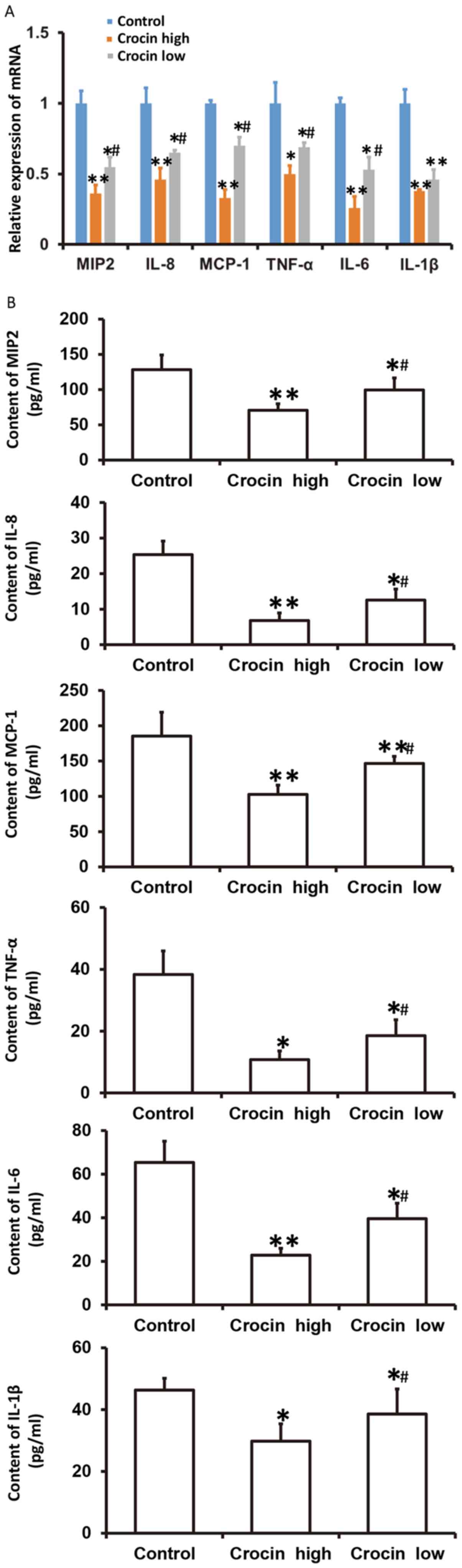
Crocin decreases the secretion of
chemokines and inflammatory factors from HCT116 cells and a high
dose of crocin causes reduced secretion of these factors
To examine how crocin influences the mRNA levels of
chemokines (MIP2, IL-8 and MCP-1) and inflammatory factors (TNF-α,
IL-6 and IL-1β), as well as the secretion in the culture
supernatant of these factors from HCT116 cells at 72 h following
treatment with crocin, RT-qPCR and ELISA were employed. These data
showed that the mRNA expression and secretion of MIP2, IL-8, MCP-1,
TNF-α, IL-6 and IL-1β in both the high- and low-dose crocin groups
were significantly lower than that in the control group
(P<0.05), and those in the low-dose group were significantly
higher than those in the high-dose group (P<0.05) (Fig. 4). The results suggested that crocin
decreased the secretion of chemokines and inflammatory factors from
HCT116 cells, and a high-dose of crocin had the most significant
effect.
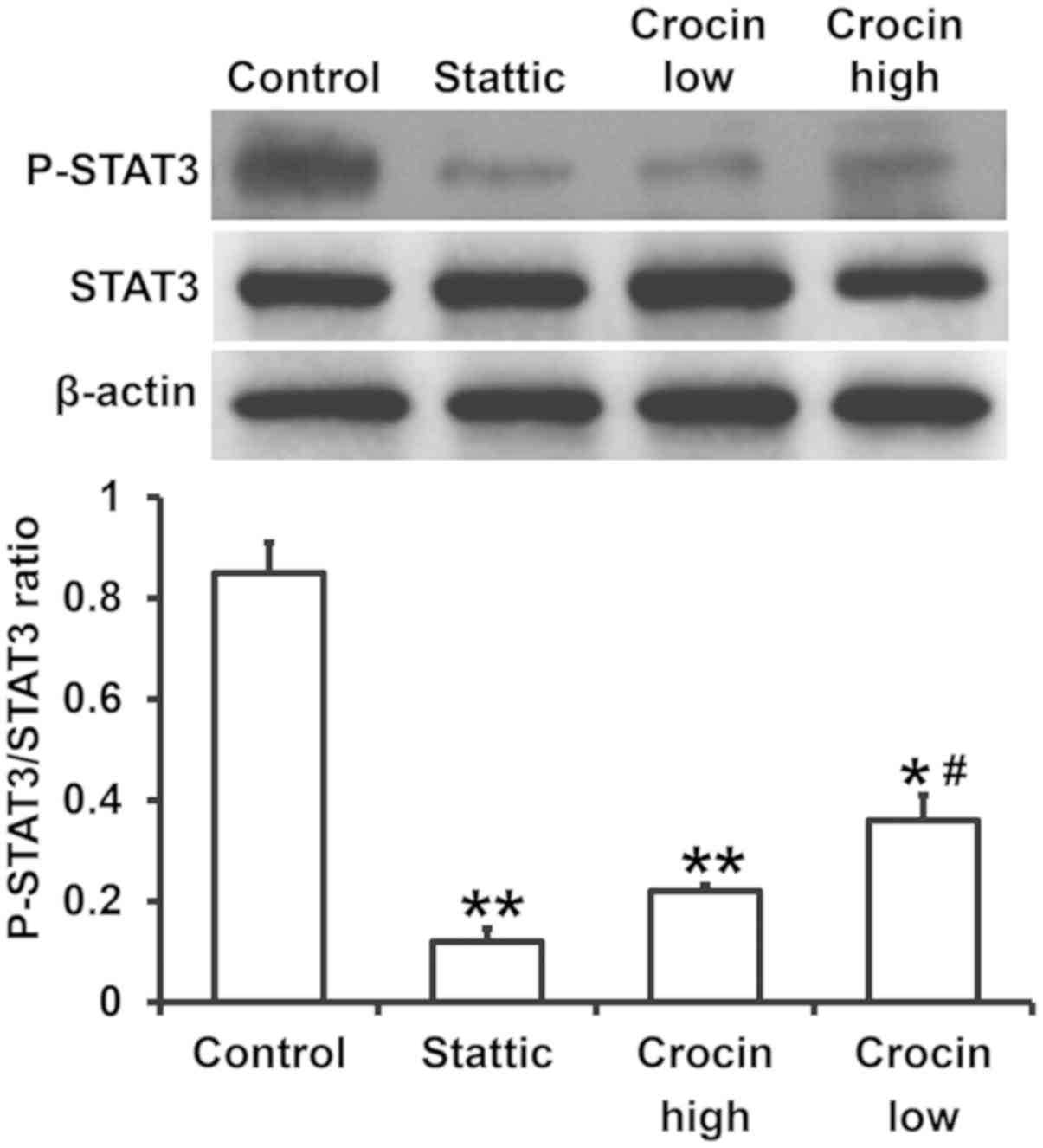
Crocin reduces the levels of P-STAT3,
and thereby reduces the release of cytokines
The secretion of chemokines and inflammatory factors
is regulated by the STAT3 signaling pathway (17). To examine the expression of proteins
related to the STAT3 signaling pathway, western blotting was used.
The data showed that the expression of P-STAT3 in the Stattic group
was significantly reduced compared to the control group
(P<0.05). Similarly, the expression of P-STAT3 in the high- and
low-dose crocin treatment groups was also significantly lower than
that in control group (P<0.05), and that in the low-dose group
was significantly higher than that in the high-dose group
(P<0.05) (Fig. 5). These results
indicated that crocin reduces the levels of P-STAT3, and thereby
reduced the release of cytokines.
Discussion
At present, surgical treatment combined with
radiotherapy, chemotherapy and molecular targeted therapy, is the
main treatment procedure for colorectal cancer, but the surgical
cure rate and postoperative survival rate is still low (18,19).
Therefore, finding effective drugs to treat colorectal cancer is
crucial. Crocin is reported to have anti-myocardial ischemia and
anti-atherosclerotic properties, to regulate the immune system,
protect the liver and gallbladder, and regulate blood lipid levels
(20). In vitro experiments
show that crocin has a strong cytotoxic effect on tumor cells. For
example, crocin and its liposomal form can induce apoptosis in Hela
and MCF-7 cells, and the liposomal form of crocin has increased
cytotoxicity compared with crocin (21). Additionally, crocin inhibits the
proliferation of tongue squamous cell carcinoma cells and inhibits
their nucleic acid synthesis, as well as inducing apoptosis
(22). Cells treated with crocin
show extensive cytoplasmic vacuolar regions and cytoplasmic
reduction, but the sensitivity to crocin varies between cell lines
(23). Animal experiments show that
crocin can reverse tumor-like pathological changes in mice and is a
potential antitumor agent (24).
According to a previous report, it was found that crocin may have a
dose-dependent effect on tumors (25); therefore, the present study tested
the effect of low- and high-dose treatments of crocin on colon
cancer cells. The results showed that crocin inhibited the
proliferation of HCT116 cells. After obtaining the IC50
value, the cells were treated with high (271.18 µM) and low (135.6
µM) doses of crocin in the following experiments. Flow cytometry
showed that crocin induced apoptosis of HCT116 cells in a dose
dependent manner. This further demonstrated that crocin has an
inhibitory effect on the survival of colon cancer cells.
Chemokines and inflammatory factors released by
colon cancer cells are some of the important factors affecting the
progression of the disease (26). In
the present study, the quantity of common inflammatory factors
(IL-6, IL-1β and TNF-α) (27) and
chemokines (MIP2, MCP-1 and IL-8) (28,29)
secreted by HCT116 cells was examined. MIP2, MCP-1 and IL-8 are
reported to promote the aggregation of neutrophils to tumor sites,
and thus are deemed biomarkers for the chemotactic and metastatic
capability of cells (30–33). As stimulating factors, IL-6, IL-1β
and TNF-α further promote the transformation from inflammation to
colon cancer (12,13). The results showed that crocin
treatment reduced the levels of MIP2, MCP-1, IL-8, IL-6, IL-1β and
TNF-α in the supernatant from cultured HCT116 cells. It has been
demonstrated that activation of the STAT3 signaling pathway is
important for cell proliferation, migration and survival, and can
also lead to the release of chemokines and inflammatory factors
(17). The results of the present
study showed that crocin treatment reduced the expression of
P-STAT3 in HCT116 cells, suggesting that crocin may affect the
proliferation and apoptosis of HCT116 cells and that crocin may
also affect the release of chemokines and inflammatory factors from
HCT116 cells, by inhibiting the activity of the STAT3 signaling
pathway. A limitation of the present study is that only one cell
line was used. Further studies should extend the number of cell
lines used to confirm these observations.
In conclusion, the present study demonstrated that
crocin has pharmacological effects against the pathological
behavior of colon cancer cells, and its mechanism of action may be
related to the STAT3 signaling pathway. However, the exact
mechanism of action still requires further investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The final version of the manuscript has been read
and approved by all authors, and each author states that the
manuscript represents honest work. JW and TS collaborated to design
the study. JW, YK and TS were responsible for performing
experiments. JW and TS analyzed the data. All authors collaborated
to interpret results and develop the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee IA, Lee JH, Baek NI and Kim DH:
Antihyperlipidemic effect of crocin isolated from the fructus of
Gardenia jasminoides and its metabolite Crocetin. Biol Pharm Bull.
28:2106–2110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abe K and Saito H: Effects of saffron
extract and its constituent crocin on learning behaviour and
long-term potentiation. Phytother Res. 14:149–152. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun Y, Xu HJ, Zhao YX, Wang LZ, Sun LR,
Wang Z and Sun XF: Crocin exhibits antitumor effects on human
leukemia HL-60 cells in vitro and in vivo. Evid Based Complement
Alternat Med. 2013:6901642013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He SY, Qian ZY, Tang FT, Wen N, Xu GL and
Sheng L: Effect of crocin on experimental atherosclerosis in quails
and its mechanisms. Life Sci. 77:907–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nair SC, Kurumboor SK and Hasegawa JH:
Saffron chemoprevention in biology and medicine: A review. Cancer
Biother. 10:257–264. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsu JD, Chou FP, Lee MJ, Chiang HC, Lin
YL, Shiow SJ and Wang CJ: Suppression of the TPA-induced expression
of nuclear-protooncogenes in mouse epidermis by crocetin via
antioxidant activity. Anticancer Res. 19:4221–4227. 1999.PubMed/NCBI
|
|
7
|
Li K, Li Y, Ma Z and Zhao J: Crocin exerts
anti-inflammatory and anti-catabolic effects on rat intervertebral
discs by suppressing the activation of JNK. Int J Mol Med.
36:1291–1299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tamaddonfard E, Farshid AA, Eghdami K,
Samadi F and Erfanparast A: Comparison of the effects of crocin,
safranal and diclofenac on local inflammation and inflammatory pain
responses induced by carrageenan in rats. Pharmacol Rep.
65:1272–1280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thosani N, Guha S and Singh H: Colonoscopy
and colorectal cancer incidence and mortality. Gastroenterol Clin
North Am. 42:619–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang K and Karin M: Tumor-elicited
inflammation and colorectal cancer. Adv Cancer Res. 128:173–196.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chung KS, Cheon SY, Roh SS, Lee M and An
HJ: Chemopreventive effect of aster glehni on inflammation-induced
colorectal carcinogenesis in mice. Nutrients. 10(pii): E2022018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ray AL, Berggren KL, Restrepo Cruz S, Gan
GN and Beswick EJ: Inhibition of MK2 suppresses IL-1β, IL-6, and
TNF-α-dependent colorectal cancer growth. Int J Cancer.
142:1702–1711. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li CY, Huang WF, Wang QL, Wang F, Cai E,
Hu B, Du JC, Wang J, Chen R, Cai XJ, et al: Crocetin induces
cytotoxicity in colon cancer cells via p53-independent mechanisms.
Asian Pac J Cancer Prev. 13:3757–3761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim WH, An HJ, Kim JY, Gwon MG, Gu H, Lee
SJ, Park JY, Park KD, Han SM, Kim MK and Park KK: Apamin inhibits
TNF-α- and IFN-γ-induced inflammatory cytokines and chemokines via
suppressions of NF-κB signaling pathway and STAT in human
keratinocytes. Pharmacol Rep. 69:1030–1035. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edwards BK, Ward E, Kohler BA, Eheman C,
Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I,
Seeff LC, et al: Annual report to the nation on the status of
cancer, 1975–2006, featuring colorectal cancer trends and impact of
interventions (risk factors, screening, and treatment) to reduce
future rates. Cancer. 116:544–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Franko J, Shi Q, Goldman CD, Pockaj BA,
Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR and Sargent
DJ: Treatment of colorectal peritoneal carcinomatosis with systemic
chemotherapy: A pooled analysis of north central cancer treatment
group phase III trials N9741 and N9841. J Clin Oncol. 30:263–267.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
P Z and C L: Research progress of
anticancer active substances in Crocus sativus. Int J
Laboratory Med. 2:140–142. 2006.
|
|
21
|
Mousavi SH, Moallem SA, Mehri S,
Shahsavand S, Nassirli H and Malaekeh-Nikouei B: Improvement of
cytotoxic and apoptogenic properties of crocin in cancer cell lines
by its nanoliposomal form. Pharm Biol. 49:1039–1045. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun J, Xu XM, Ni CZ, Zhang H, Li XY, Zhang
CL, Liu YR, Li SF, Zhou QZ and Zhou HM: Crocin inhibits
proliferation and nucleic acid synthesis and induces apoptosis in
the human tongue squamous cell carcinoma cell line Tca8113. Asian
Pac J Cancer Prev. 12:2679–2683. 2011.PubMed/NCBI
|
|
23
|
Garcia-Olmo DC, Riese HH, Escribano J,
Ontañón J, Fernandez JA, Atiénzar M and García-Olmo D: Effects of
long-term treatment of colon adenocarcinoma with crocin, a
carotenoid from saffron (Crocus sativus L.): An experimental
study in the rat. Nutr Cancer. 35:120–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Magesh V, Singh JP, Selvendiran K,
Ekambaram G and Sakthisekaran D: Antitumour activity of crocetin in
accordance to tumor incidence, antioxidant status, drug
metabolizing enzymes and histopathological studies. Mol Cell
Biochem. 287:127–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang Y, Wang S, Zhang C, Xu Z, Shen J, Du
X, Zhang H, Zhang K and Zhang D: Experimental study of the
anti-atherosclerotic effect of demethylzeylasteral. Exp Ther Med.
13:2787–2792. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Itatani Y, Kawada K, Inamoto S, Yamamoto
T, Ogawa R, Taketo MM and Sakai Y: The role of chemokines in
promoting colorectal cancer invasion/metastasis. Int J Mol Sci.
17(pii): E6432016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen L, Zhou T, Wang J, Sang X, Lan L, Luo
L and Yin Z: Daphnetin reduces endotoxin lethality in mice and
decreases LPS-induced inflammation in Raw264.7 cells via
suppressing JAK/STATs activation and ROS production. Inflamm Res.
66:579–589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hao C, Wu B, Hou Z, Xie Q, Liao T, Wang T
and Ma D: Asiatic acid inhibits LPS-induced inflammatory response
in human gingival fibroblasts. Int Immunopharmacol. 50:313–318.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim KJ, Yoon KY, Yoon HS, Oh SR and Lee
BY: Brazilein suppresses inflammation through inactivation of
IRAK4-NF-κB pathway in LPS-induced Raw264.7 macrophage cells. Int J
Mol Sci. 16:27589–27598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang ZW, Wang JJ, Zhang JZ, Xue ZJ, Miao
J, Li L and Hu WX: Thrombolysis of deep vein thrombosis and
inhibiting chemotaxis of macrophage by MCP-1 blockage. Eur Rev Med
Pharmacol Sci. 21:1695–1701. 2017.PubMed/NCBI
|
|
31
|
Hol J, Wilhelmsen L and Haraldsen G: The
murine IL-8 homologues KC, MIP-2, and LIX are found in endothelial
cytoplasmic granules but not in Weibel-Palade bodies. J Leukoc
Biol. 87:501–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kadioglu A and Andrew PW: Susceptibility
and resistance to pneumococcal disease in mice. Brief Funct Genomic
Proteomic. 4:241–247. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gerber J, Pohl K, Sander V, Bunkowski S
and Nau R: Rifampin followed by ceftriaxone for experimental
meningitis decreases lipoteichoic acid concentrations in
cerebrospinal fluid and reduces neuronal damage in comparison to
ceftriaxone alone. Antimicrob Agents Chemother. 47:1313–1317. 2003.
View Article : Google Scholar : PubMed/NCBI
|