Introduction
Rheumatoid arthritis (RA) is characterized by
chronic arthromeningitis, persistent systemic inflammation and
production of autoantibodies, which include rheumatoid factors and
cyclic citrullinated peptides. RA can also lead to complications,
such as intraarticular cartilage damage, joint dysfunction and
cardiovascular and pulmonary conditions (1,2). The
incidence of RA in China is 0.5–1%, with 5–50 new cases per 100,000
annually (3).
The main pathological features of RA are abnormal
proliferation of synovial cells, infiltration of inflammatory
cells, formation of rheumatoid vasospasm, erosion of cartilage and
bone, and ultimately destruction of joints (4,5).
Abnormal proliferation of synovial cells in RA causes the cells to
proliferate from the original 1–3 layers to 5–6 layers or more.
Fibroblast-like synoviocytes (FLS) are the most important type of
synovial cells in the arthritic joint (6). There are two ways in which FLS interact
with immune cells in RA patients. One is through direct contact
between FLS and immune cells and the other is through FLS exposure
to immune cell-secreted growth factors and inflammatory cytokines.
Both these actions stimulate FLS to produce large amounts of
chemokines, angiogenic and proinflammatory growth factors, and
matrix metalloproteinases and cathepsins to degrade extracellular
matrices and cartilage (7). It would
therefore be beneficial to unravel the molecular mechanism of RA
disease development through research into the regulation mechanism
of inflammation and proliferation in FLS.
MicroRNA (miR)-155 has been shown to be highly
expressed in the plasma of RA patients and is a potential biomarker
for the diagnosis of RA (8,9). miR-155, may affect the progression of
RA by regulating the inflammatory response of monocytes (10) and T cells (11) in RA patients (12). However, the expression of miR-155 in
the synovial tissue of RA patients and its effects on proliferation
and the secretion of inflammatory cytokines by FLS are yet unknown.
In the present study, the expression of miR-155 was examined in the
synovial tissue of RA patients, and the effect of its expression on
the secretion of inflammatory cytokines and the proliferation of
FLS cells was explored. The results of the present study indicated
that miR-155 expression was increased in the synovial tissue of RA
patients, and directly inhibited the expression of FOXO3a. As
miR-155 regulated FLS proliferation and inflammatory cytokine
secretion, it may be a useful target for the development of new RA
treatments.
Materials and methods
Patients and tissues
A total of 89 synovial tissue specimens were
obtained from RA patients who received total knee replacement
between January 2016 and October 2017 in The Affiliated Hospital of
Inner Mongolia Medical University (Huhot, China). There were 53
male patients and 36 female patients, aged between 40 and 70 years.
Specimens from 49 patients, who underwent amputation, were
collected as controls. All patients in the control group had no
history of RA. Of these patients 36 were male and 13 female and
their ages ranged between 45 and 73 years. Exclusion criteria were
other rheumatic diseases, autoimmune diseases, tumor, liver and
kidney insufficiency, pregnancy or lactation and mental
disorders.
The present study was performed with the approval of
The Affiliated Hospital of Inner Mongolia Medical University. All
aspects of the study complied with the Declaration of Helsinki
(13). In addition, all participants
signed informed consent forms.
Isolation and culture of FLS
FLS were isolated, cultured and characterized from
surgically removed synovial tissue. The detailed experimental
methods can be found in Zhang et al (14) FLS cells were cultured in DMEM (Thermo
Fisher Scientific, Inc.) which was supplemented with 10% FBS (cat.
no. 10100-147; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2.
Reverse transcription-quantitative PCR
(RT-qPCR)
Trizol® (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from tissues or
cells. The extracted RNA was reverse transcribed to cDNA using a
PrimeScript™ RT Master Mix reverse transcription kit (cat. no.
RR036B, Takara Biotechnology Co., Ltd.) which was performed
according to the manufacturer's protocol. Reaction mixture (20 µl)
was prepared according to the SYBR Green qPCR Master Mix kit (cat.
no. 638320, Takara Biotechnology Co., Ltd.) instructions and
amplified using an ABI 7500 fluorescence qPCR instrument (Applied
Biosystems; Thermo Fisher Scientific Inc.). Thermocycling
conditions were: 95°C for 30 sec; 40 cycles of 90°C for 5 sec and
65°C for 30 sec. The sequences of the PCR primers were as follows:
miR-155 forward, 5′-ACACTCCAGCTGGGTTAATGCTAATCGTGATA-3′ and
reverse, 5′-TGGTGTCGTGGAGTCG-3′; forkhead box protein O3a (FOXO3a)
forward, 5′-CGGACAAACGGCTCACTCT-3′ and reverse,
5′-GTCGGAGATTCGTAGCTGGA-3′; tumor necrosis factor-α forward,
5′-CCTCTCTCTAATCAGCCCTCTG-3′ and reverse,
5′-GAGGACCTGGGAGTAGATGAG-3′; interleukin (IL)-6 forward,
5′-ACTCACCTCTTCAGAACGAATTG-3′ and reverse,
5′-CCATCTTTGGAAGGTTCAGGTTG-3′; IL-1β forward,
5′-ATGATGGCTTATTACAGTGGCAA-3′ and reverse,
5′-GTCGGAGATTCGTAGCTGGA-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′; and GAPDH forward,
5′-CTCGCCTAGAGTGAGCTCC-3′ and reverse,
5′-AACTGCTGCGTTGACGGGTATG-3′. U6 was used as a reference gene for
miR-155 and GAPDH was used as a reference for all other genes. The
2−ΔΔCq method was used to calculate quantitative gene
expression (15).
Western blotting
RIPA lysis buffer (cat. no. P0013K; Beyotime
Institute of Biotechnology) was used to lyse FLS and synovial
tissue, and a BCA Protein Assay kit (cat. no. P0010S; Beyotime
Institute of Biotechnology) was used to measure lysate protein
concentration. A total of 50 µg protein from tissue or cell lysates
was separated using SDS-PAGE on a 10% gel and then transferred to a
PVDF membrane. The membrane was blocked with 5% skim milk powder
for 1 h at room temperature. The primary antibodies used were
anti-FOXO3a (cat. no. ab70314; 1:5,000) or anti-GAPDH (cat. no.
ab9484; 1:3,000). The secondary antibodies used were horseradish
peroxidase-conjugated goat anti-rabbit (cat. no. ab6721; 1:1,000),
or goat anti-mouse (cat. no. ab205719; 1:1,000). All antibodies
were supplied by Abcam. Membranes were incubated with primary
antibodies overnight at 4°C and with secondary antibodies for 1 h
at room temperature. The BeyoECL Plus kit (cat. no. P0018S;
Beyotime Institute of Biotechnology) was used to visualize protein
bands and densitometry was performed using the Beckman Coulter
Immunoassay system (UniCel DxI 800; Beckman Coulter, Inc.).
Cell transfection
Small interfering (si)-FOXO3a RNA (forward,
5′-CCCAUGCUATUGUUGUCACUTT-3′ and reverse,
5′-AGUGAUTCGAUGAAUGGGTT-3′) and si-negative control (NC) (forward,
5′-ACCGCAUAGUGUAACUUUATT-3′ and reverse,
5′-GAAAGUUAGACUAUGCGGCTT-3′) were designed and synthesized by
Shanghai Shenggong Biology Engineering Technology Service, Ltd.
miR-155-mimic (5′-UUAAUGCUAAUCGUGAUAGGGU-3′), miR-155-inhibitor
(5′-AAUUACGAUUAGCACUAUCCCA-3′) and miR-155-NC
(5′-GCAUUUGAGAGCCAUUAUGGUA-3′) were also synthesized by Shanghai
Shenggong Biology Engineering Technology Service, Ltd. si-RNA (50
nM), si-NC (50 nM), miR-155-mimic (50 nM), miR-155-inhibitor (50
nM) and miR-155-NC (50 nM) were directly transfected into cells
using Lipofectamine™ 2000 transfection reagent (cat. no. 11668019;
Invitrogen; Thermo Fisher Scientific Inc.). Wild type or mutant
3′-untranslated regions of FOXO3a were first cloned into the
plasmid psiCHECK2 (Promega Corporation) and then transfected into
cells as si-RNA. pLV-FOXO3a lentivirus (cat. no. sc-425192; Santa
Cruz Biotechnology, Inc.) and its supporting no-load lentivirus
(pLV-FOXO3a) were added directly to the FLS cell culture medium,
and the number of lentiviral particles used for transfection was 2
fold the number of FLS cells. There was a 72 h interval between
transfection and subsequent experimentation.
Dual-luciferase reporter assay
TargetScan (http://www.targetscan.org) was used to predict the
target genes of miR-155. The Dual-Lucy Assay kit (cat. no. D00100;
Beijing Solarbio Science & Technology Co., Ltd.) was used to
detect the activity of luciferase following the manufacturer's
protocol. Briefly, cells were collected after 48 h of transfection
and then lysed for 5 min on ice before centrifugation (12,000 × g
for 1 min at room temperature) to collect the cell supernatant.
Five volumes of firefly luciferase reaction solution or
Renilla luciferase reaction solution were added to the cell
lysate and the enzyme activity was detected.
Cytokine detection
After 48-h incubation with transfection reagents,
serum-free medium was added for 24 h. The culture media were then
collected and centrifuged at 1,200 × g for 10 min at room
temperature. A human IL-1β ELISA kit (cat. no. ab46631) was used to
determine IL-1β concentration in medium, an IL-6 kit (cat. no.
ab46027) to determine IL-6 concentration and a TNF-α kit (cat. no.
ab181421) to determine TNF-α concentration. All kits were supplied
by Abcam and used according to the manufacturer's instructions.
MTT assay
Cells at a density of 2×103 cells/well
were seeded in a 96-well culture plate containing DMEM supplemented
with 10% FBS. The viability of FLS cells was assessed by MTT assay.
After 4 h of culture, MTT (10 µl per well, 10 mg/ml) was added to
the cells and incubated. Cell supernatant was removed and 100 µl
DMSO was added. After 30 min, optical density was measured using a
plate reader (ELx808; Bio-Tek Instruments, Inc.).
Statistical analysis
Data are presented as the mean ± SD and were
analyzed using SPSS 20.0 (IBM Corp.). Student's t-test was used to
compare differences between two groups and one-way ANOVA with
Tukey-Kramer post-hoc test was used to compare differences between
multiple groups. The correlation between two groups was analyzed by
Pearson's correlation coefficient. P<0.05 indicated statistical
significance.
Results
Expression of miR-155 and FOXO3a in
synovial tissue
RT-qPCR was used to determine the expression of
miR-155 and FOXO3a mRNA in 89 RA synovial tissues and 49 normal
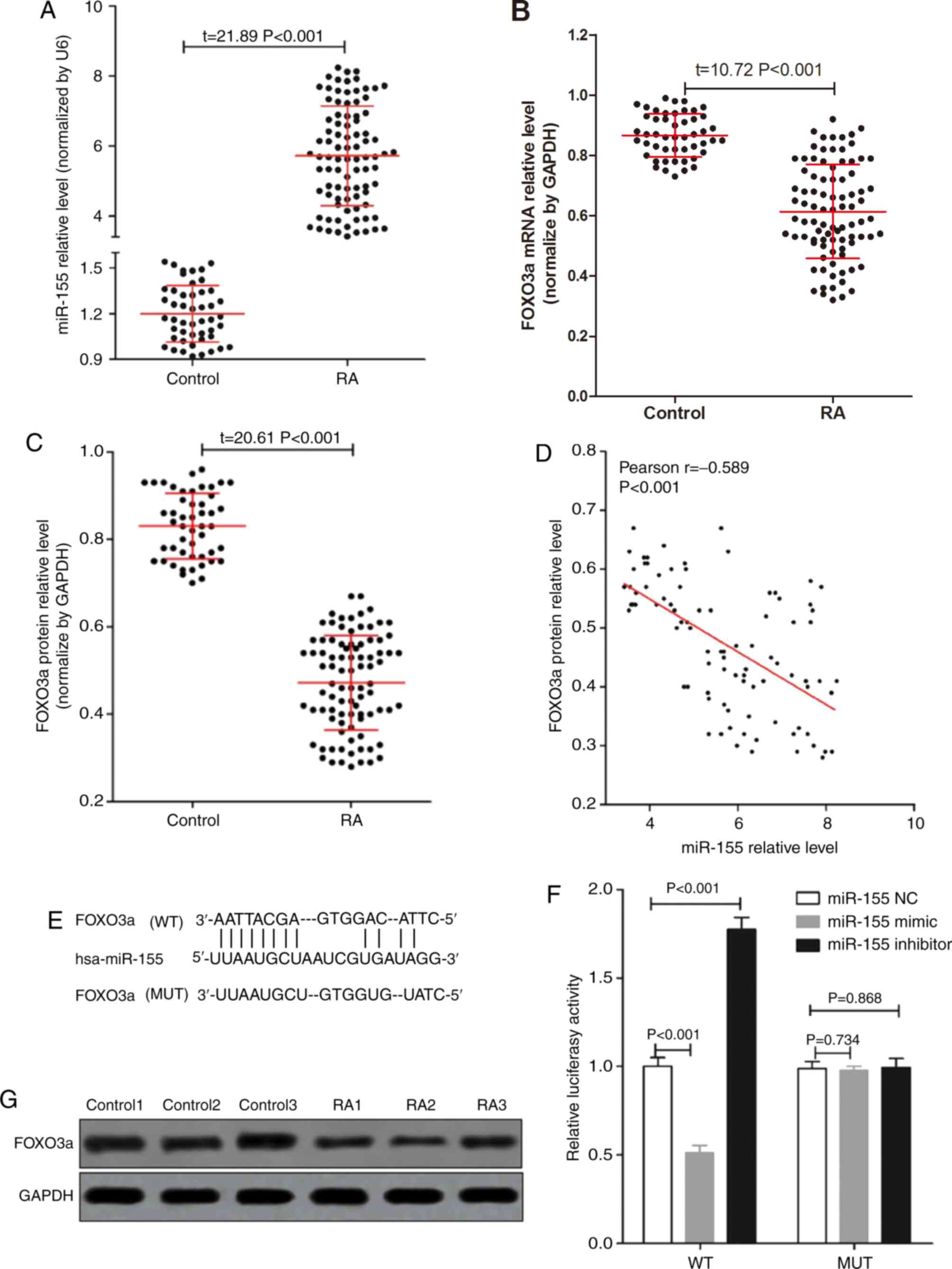
synovial tissues. As seen in Fig.
1A, the expression of miR-155 in RA synovial tissues was
significantly higher than that in the control group. The expression
of FOXO3a mRNA in RA synovial tissues was significantly lower than
that in the control group (Fig. 1B).
The expression of FOXO3a was also measured by western blotting and
the results indicated that the expression level of FOXO3a protein
in RA synovial tissues was significantly lower than that in the
control group (Fig. 1C and F).
miR-155 levels were negatively correlated with FOXO3a protein
expression (Fig. 1D) in RA synovial
tissues.
The results of a dual luciferase reporter system
(Fig. 1E and G) indicated that
FOXO3a was a target gene of miR-155 in FLS cells, and that a
miR-155-mimic could inhibit the expression of FOXO3a while a
miR-155-inhibitor could promote FOXO3a expression. These results
suggested that altered expression of miR-155 and FOXO3a in RA
patients' synovial tissue may have a role in the development of
RA.
miR-155 and FOXO3a are associated with
inflammation in synovial tissue
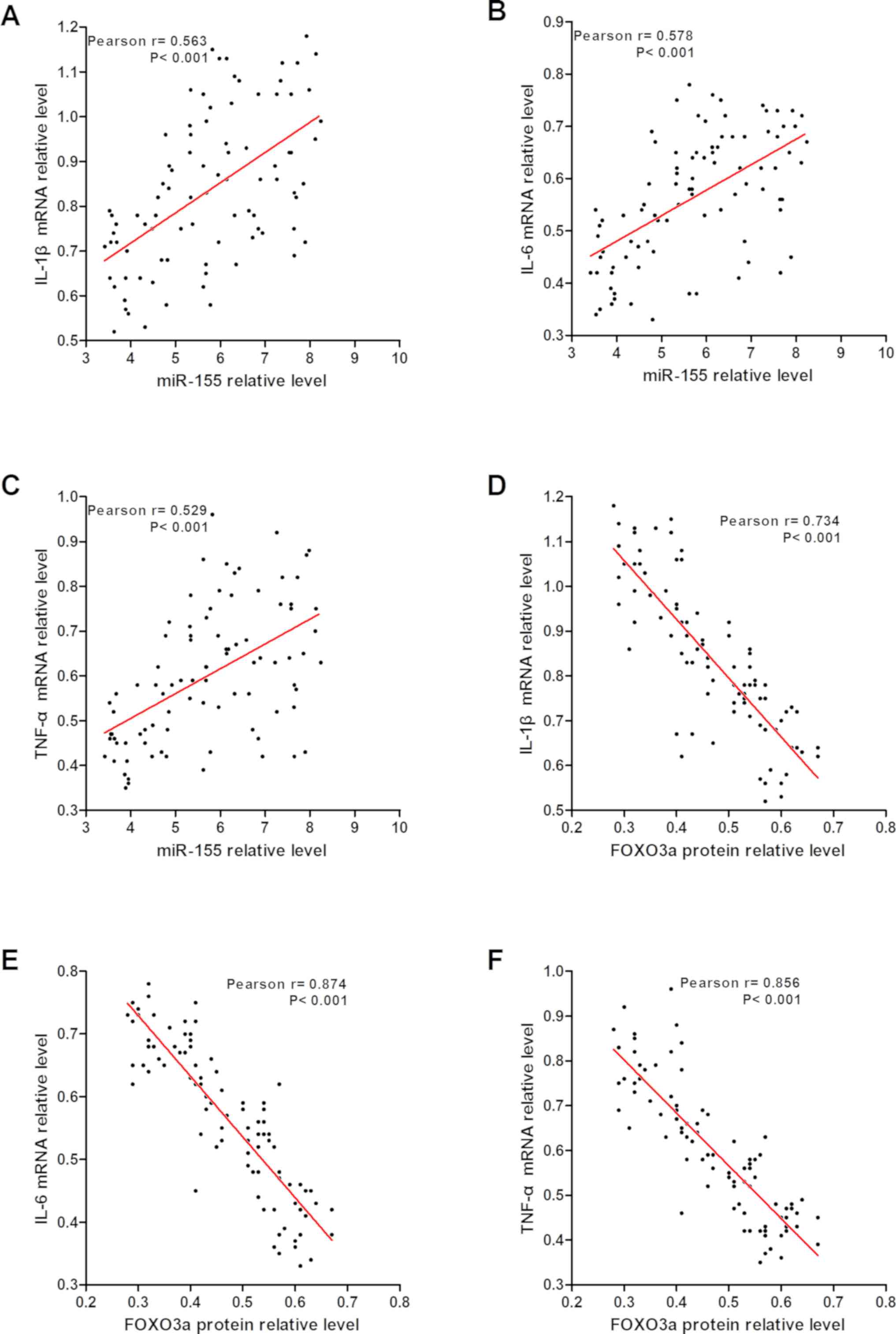
The relationship between the expression of miR-155
and FOXO3a in synovial tissue of RA patients and the expression of
inflammatory cytokines was analyzed (Fig. 2), and the results suggested that the
expression of IL-1β, IL-6 and TNF-α mRNA in synovial tissue were
positively correlated with miR-155 expression and negatively
correlated with FOXO3a expression. This suggested that miR-155 may
regulate inflammation in RA synovial tissue by targeting
FOXO3a.
miR-155 promotes inflammatory cytokine
secretion in FLS cells by targeting FOXO3a
As presented in Fig.
3A, miR-155-mimic increased the expression of miR-155, and
miR-155-inhibitor decreased the expression of miR-155. As described
in Fig. 3B, miR-155-mimic and
si-FOXO3a could reduce the expression of FOXO3a protein compared
with si-NC, and miR-155-inhibitor and pLV-FOXO3a could promote the
expression of FOXO3a protein compared with NC and pLV-NC groups,
respectively. The results shown in Fig.
3C and D suggested that miR-155-mimic (vs. NC group) and
si-FOXO3a (vs. si-NC group) could reduce the secretion of IL-1β,
IL-6 and TNF-α by FLS cells, and that miR-155-inhibitor (vs. NC
group) and pLV-FOXO3a (vs. si-NC group) could promote the secretion
of IL-1β, IL-6 and TNF-α by FLS cells targeting FOXO3a. This
indicated that miR-155 may promote inflammatory cytokine secretion
in FLS cells by targeting FOXO3a.
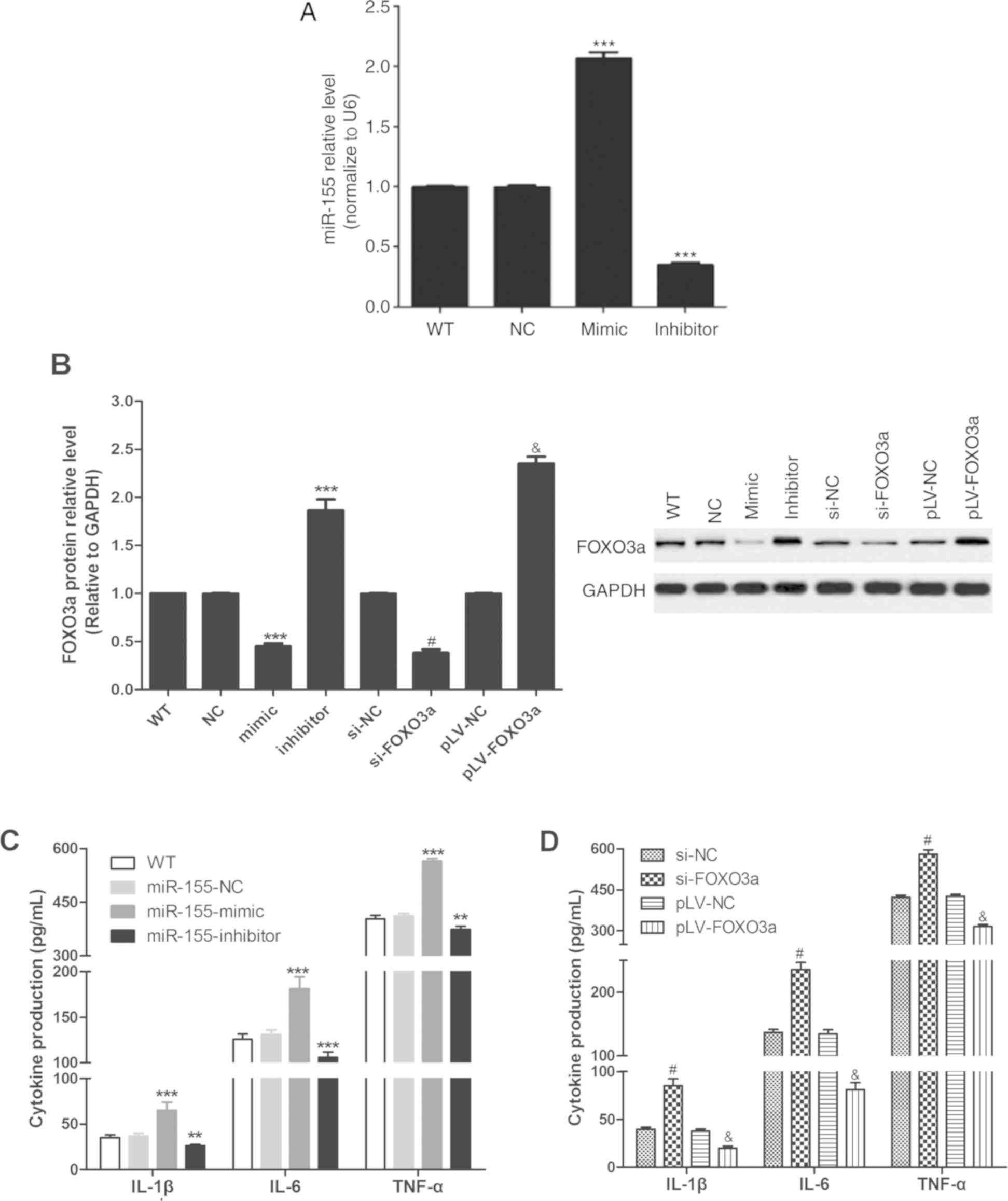 | Figure 3.miR-155 and FOXO3a regulate the
secretion of inflammatory cytokines by FLS cells. (A) Reverse
transcription-quantitative PCR was used to detect the expression of
miR-155. (B) Western blot was used to determine the expression of
FOXO3a protein in FLS cells after transfection. (C) Cytokine
release by FLS in response to miR-mimic and inhibitor. (D) Cytokine
release by FLS in response to FOXO3a knockdown and overexpression.
All experiments were performed in three independent replicates.
**P<0.01 and ***P<0.001 vs. miR-155-NC group,
#P<0.001 vs. si-FOXO3a group and
&P<0.001 vs. pLV-NC group. FLS, fibroblast-like
synoviocyte; FOXO3a, forkhead box protein O3a; interleukin, IL; LV,
lentivirus; miR, microRNA; NC, negative control; si, small
interfering; TNF-α, tumor necrosis factor-α; WT, cells without any
treatment. |
miR-155 promotes FLS cells
proliferation by targeting FOXO3a
The effect of miR-155 expression on the
proliferation of FLS was investigated, and the results indicated
that miR-155-mimic (vs. NC group) and si-FOXO3a (vs. si-NC group)
could promote FLS proliferation, and miR-155-inhibitor (vs. NC
group) and pLV-FOXO3a (vs. si-NC group) could suppress FLS
proliferation (Fig. 4). This
indicated that miR-155 regulated FLS cell proliferation by
targeting FOXO3a.
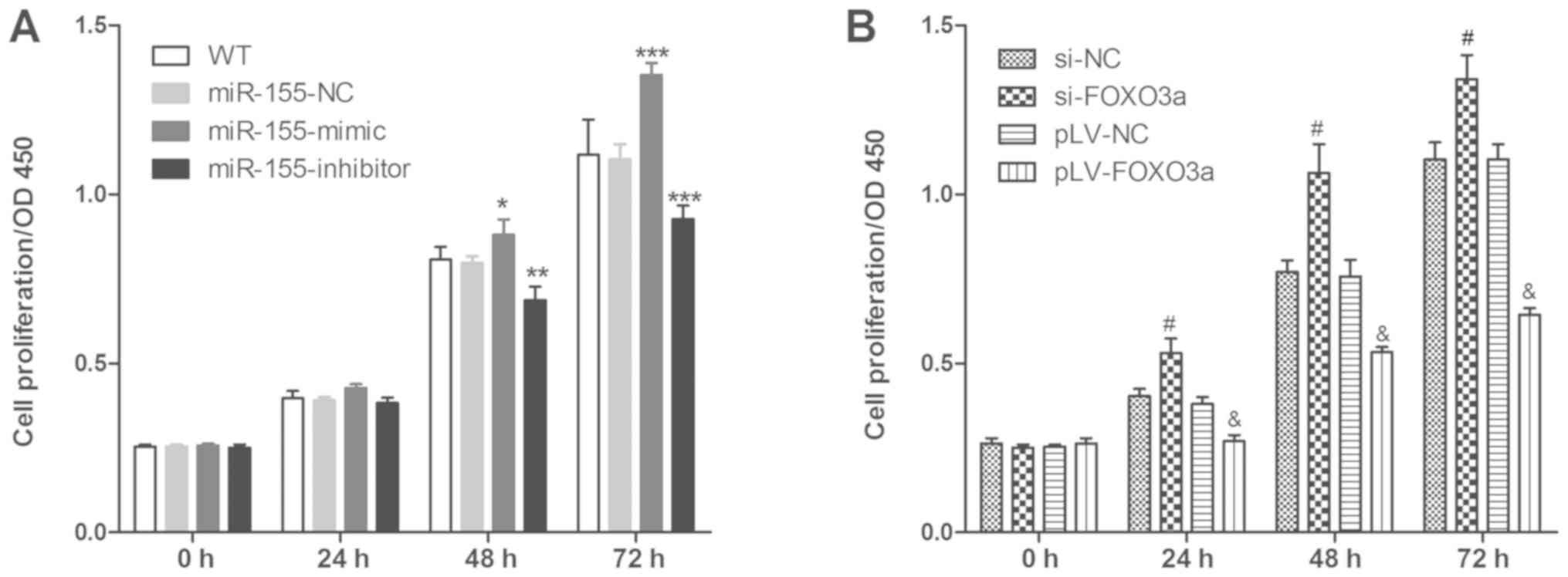 | Figure 4.miR-155 and FOXO3a regulate FLS cell
proliferation. MTT assay was used to detect FLS cell proliferation
after (A) treatment influencing miR-155 expression and (B)
treatment influencing FOXO3a expression. Three independent
experiments were performed. *P<0.05, **P<0.01 and
***P<0.001 vs. miR-155-NC group, #P<0.001 vs. si-NC
group group and &P<0.001 vs. pLV-NC group.
FOXO3a, forkhead box protein O3a; FLS, fibroblast-like synoviocyte;
LV, lentivirus; miR, microRNA; NC, negative control; MUT, mutant;
si, small interfering RNA; WT, cells without any treatment. |
Discussion
miRNAs are a class of evolutionarily conserved,
endogenous, non-coding single-stranded RNAs of approximately 20–24
nucleotides in length that regulate gene expression at the
post-transcriptional level through cleavage of complementary mRNA
or by inhibition of mRNA translation (7,16).
Previous studies confirmed that these miRNAs not only participate
in regulation of inflammatory responses in RA patients, but also
regulate proliferation, apoptosis, invasion and secretion of
inflammatory cytokines in FLS (17,18). In
a previous study, miR-17 suppressed TNF-α signaling by interfering
with tumor necrosis factor receptor-associated factor 2 and
inhibitor of apoptosis cIAP2 association (19). An additional study suggested that
miR-29a inhibited the proliferation of FLS and induced their
apoptosis in RA patients (20).
Further studies showed that miR-20a negatively regulated the
expression of the NACHT LRR an PYD domain containing protein 3
inflammatory corpuscles in FLS of RA patients by targeting
thioredoxin interacting protein (21) and that miR-140-5p inhibited synovial
fibroblast proliferation and inflammatory cytokine secretion
through targeting toll-like receptor 4 (22). This led to the hypothesis that miRNAs
play an important role in the development of RA and are a potential
target for the treatment of RA patients.
Large numbers of infiltrating inflammatory cells and
overexpression of inflammatory cytokines are some of the main
causes of RA disease progression (23,24). In
the present study, increased levels of miR-155 were found in
synovial tissue of RA patients and were negatively correlated with
FOXO3a protein expression. As miR-155 is a non-coding RNA, it
cannot directly regulate the biological behavior of cells, and may
participate indirectly through regulation of target gene expression
(25). FOXO3a is a target gene of
miR-155, and miR-155 can regulate glucose metabolism (26), and proliferation, migration, invasion
(27,28) and apoptosis (29) of cancer cells though inhibition of
FOXO3a expression. The miR-155/FOXO3a axis is known to play an
important role in the pathogenesis of inflammatory disease
(30). A dual luciferase reporter
gene system was used in the present study to verify that miR-155
targeted the inhibition of FOXO3a expression in FLS.
RA is a chronic, systemic disease characterized by
inflammatory synovitis, and multiple inflammatory factors are
involved in the pathological progression of RA, such as IL-1β, IL-6
and TNF-α (31,32). Previous studies confirmed that
miR-155, which is highly expressed in the circulation of RA
patients, could affect the peripheral blood inflammatory response
of RA patients by regulating peripheral blood mononuclear cells
(10) and T cell phenotypes
(11), and that inhibition of
miR-155 expression in the peripheral blood of patients with RA
would help to improve the condition (11,12). The
results of the present study indicate that miR-155 levels are
positively correlated with inflammatory cytokine release (IL-1β,
IL-6 and TNF-α) by synovial tissue of RA patients. It has been
reported that treatment with an IL-1β antibody may significantly
alleviate RA symptoms (33). An
additional study indicated that inhibition of IL-1 secretion is a
possible method for the treatment of RA (34). Further research suggests that
anti-IL-6 receptor-related drugs can be used to treat RA, either
alone or in combination with other drugs (35), and that inhibition of the
biosynthesis of TNF-α could improve RA (36).
Synovial thickening is one of the typical lesions of
RA (37). Activated FLS cells have
tumor-like proliferation characteristics, resulting in a large
increase in the number of cells and synovial thickening (38). The results of the present study also
suggested that miR-155 regulated FLS proliferation and inflammatory
cytokine secretion in FLS by targeting FOXO3a in vitro. The
FOXO family is an important family of transcriptional regulator
proteins involved in many cellular functions. Scientists have
identified four family members, FOXO1, FOXO3a, FOXO4 and FOXO6
(39). Although each FOXO family
member has its own role, FOXO3a has been extensively studied due to
its important regulatory effects on cell proliferation, apoptosis,
metabolism, and oxidative stress (39). It has been reported that abnormal
expression of FOXO3a is closely related to the progression of
various types of cancer (40),
fibrosis (41) and other diseases
(41,42). FOXO3a plays an important role in both
inflammation and regulation of cell proliferation. FOXO3a regulates
inflammation by NF-κB, T cells and autoinflammation (43,44);
however, down-regulation of FOXO3a expression may promote breast
cancer cell proliferation (45) and
the AKT/FOXO3a signaling pathway plays an important role in the
proliferation of human neural cells (46), primary macrophages (47) and fibroblasts (47). These findings indicate that miR-155
may be a potential target for the development of inhibitors of
inflammation in patients with RA.
An important limitation of the present study is that
all tissue was taken from RA patients who had received total knee
replacement and these findings may not be applicable to all forms
of RA.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science
Foundation of Inner Mongolia [2016MS (LH) 0808].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XSZ conceived and designed the present study, and
contributed to writing the manuscript. YW and TYF analyzed
experimental data. YW, TYF, SSD, SLL, LZ and YLS performed the
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Affiliated Hospital of Inner Mongolia Medical
University. All participants signed informed consent forms.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Scott DL, Wolfe F and Huizinga TW:
Rheumatoid arthritis. Lancet. 376:1094–1108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Felson DT, Anderson JJ, Boers M,
Bombardier C, Furst D, Goldsmith C, Katz LM, Lightfoot R Jr, Paulus
H, Strand V, et al: American college of Rheumatology. Preliminary
definition of improvement in rheumatoid arthritis. Arthritis Rheum.
38:727–735. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeng XF, Zhu SL, Tan AC and Xie XP:
Disease burden and quality of life of rheumatoid arthritis in
China: A systematic review. Chin J Evidence-Based Med. 13:300–307.
2013.
|
|
4
|
Feldmann M and Maini SR: Role of cytokines
in rheumatoid arthritis: An education in pathophysiology and
therapeutics. Immunol Rev. 223:7–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Firestein GS: Invasive fibroblast-like
synoviocytes in rheumatoid arthritis. Passive responders or
transformed aggressors? Arthritis Rheum. 39:1781–1790. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: Key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noss EH and Brenner MB: The role and
therapeutic implications of fibroblast-like synoviocytes in
inflammation and cartilage erosion in rheumatoid arthritis. Immunol
Rev. 223:252–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdul-Maksoud RS, Sediq AM, Kattaia A,
Elsayed W, Ezzeldin N, Abdel Galil SM and Ibrahem RA: Serum miR-210
and miR-155 expression levels as novel biomarkers for rheumatoid
arthritis diagnosis. Br J Biomed Sci. 74:209–213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mookherjee N and El-Gabalawy HS: High
degree of correlation between whole blood and PBMC expression
levels of miR-155 and miR-146a in healthy controls and rheumatoid
arthritis patients. J Immunol Methods. 400-401:106–110. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rajasekhar M, Olsson AM, Steel KJ,
Georgouli M, Ranasinghe U, Brender Read C, Frederiksen KS and Taams
LS: MicroRNA-155 contributes to enhanced resistance to apoptosis in
monocytes from patients with rheumatoid arthritis. J Autoimmun.
79:53–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spoerl D, Duroux-Richard I, Louis-Plence P
and Jorgensen C: The role of miR-155 in regulatory T cells and
rheumatoid arthritis. Clin Immunol. 148:56–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leah E: Rheumatoid arthritis: miR-155
mediates inflammation. Nat Rev Rheumatol. 7:4372011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
La Vaque TJ and Rossiter T: The ethical
use of placebo controls in clinical research: The Declaration of
Helsinki. Appl Psychophysiol Biofeedback. 2623–37. (61-65)2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang W, Chen L, Jiang Y and Shen Y:
miR-26a-5p regulates synovial fibroblast invasion in patients with
rheumatoid arthritis by targeting Smad 1. Med Sci Monit.
24:5178–5184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Qian K, Li C, Ma Y and Chen X:
Roles of microRNA-539 and osteopontin in rheumatoid arthritis. Exp
Ther Med. 15:2681–2687. 2018.PubMed/NCBI
|
|
16
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tavasolian F, Abdollahi E, Rezaei R,
Momtazi-Borojeni AA, Henrotin Y and Sahebkar A: Altered expression
of MicroRNAs in rheumatoid arthritis. J Cell Biochem. 119:478–487.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murata K, Yoshitomi H, Tanida S, Ishikawa
M, Nishitani K, Ito H and Nakamura T: Plasma and synovial fluid
microRNAs as potential biomarkers of rheumatoid arthritis and
osteoarthritis. Arthritis Res Ther. 12:R862010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Akhtar N, Singh AK and Ahmed S:
MicroRNA-17 suppresses TNF-α signaling by interfering with TRAF2
and cIAP2 association in rheumatoid arthritis synovial fibroblasts.
J Immunol. 197:2219–2228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J, Fei D, Xing J and Du J:
MicroRNA-29a inhibits proliferation and induces apoptosis in
rheumatoid arthritis fibroblast-like synoviocytes by repressing
STAT3. Biomed Pharmacother. 96:173–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li XF, Shen WW, Sun YY, Li WX, Sun ZH, Liu
YH, Zhang L, Huang C, Meng XM and Li J: MicroRNA-20a negatively
regulates expression of NLRP3-inflammasome by targeting TXNIP in
adjuvant-induced arthritis fibroblast-like synoviocytes. Joint Bone
Spine. 83:695–700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Guan SB, Lu Y and Wang F: MiR-140-5p
inhibits synovial fibroblasts proliferation and inflammatory
cytokines secretion through targeting TLR4. Biomed Pharmacother.
96:208–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Innala L, Sjöberg C, Möller B, Ljung L,
Smedby T, Södergren A, Magnusson S, Rantapää-Dahlqvist S and
Wallberg-Jonsson S: Co-morbidity in patients with early rheumatoid
arthritis-inflammation matters. Arthritis Res Ther. 18:332016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sharma AR, Sharma G, Lee SS and
Chakraborty C: miRNA-regulated key components of cytokine signaling
pathways and inflammation in rheumatoid arthritis. Med Res Rev.
36:425–439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ritchie W, Rasko JE and Flamant S:
MicroRNA target prediction and validation. Adv Exp Med Biol.
774:39–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim S, Lee E, Jung J, Lee JW, Kim HJ, Kim
J, Yoo HJ, Lee HJ, Chae SY, Jeon SM, et al: microRNA-155 positively
regulates glucose metabolism via PIK3R1-FOXO3a-cMYC axis in breast
cancer. Oncogene. 37:2982–2991. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kong W, He L, Coppola M, Guo J, Esposito
NN, Coppola D and Cheng JQ: MicroRNA-155 regulates cell survival,
growth and chemosensitivity by targeting FOXO3a in breast cancer. J
Biol Chem. 291:228552016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ling N, Gu J, Lei Z, Li M, Zhao J, Zhang
HT and Li X: microRNA-155 regulates cell proliferation and invasion
by targeting FOXO3a in glioma. Oncol Rep. 30:2111–2118. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Q, Li C, Zhu Z, Teng Y, Che X, Wang
Y, Ma Y, Wang Y, Zheng H, Liu Y and Qu X: miR-155-5p antagonizes
the apoptotic effect of bufalin in triple-negative breast cancer
cells. Anticancer Drugs. 27:9–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Min M, Peng L, Yang Y, Guo M, Wang W and
Sun G: MicroRNA-155 is involved in the pathogenesis of ulcerative
colitis by targeting FOXO3a. Inflamm Bowel Dis. 20:652–659. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choy EH and Panayi GS: Cytokine pathways
and joint inflammation in rheumatoid arthritis. N Engl J Med.
344:907–916. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Araki Y and Mimura T: The mechanisms
underlying chronic inflammation in rheumatoid arthritis from the
perspective of the epigenetic landscape. J Immunol Res.
2016:62906822016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qi J, Ye X, Ren G, Kan F, Zhang Y, Guo M,
Zhang Z and Li D: Pharmacological efficacy of anti-IL-1β scFv, Fab
and full-length antibodies in treatment of rheumatoid arthritis.
Mol Immunol. 57:59–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ruscitti P, Cipriani P, Cantarini L,
Liakouli V, Vitale A, Carubbi F, Berardicurti O, Galeazzi M,
Valenti M and Giacomelli R: Efficacy of inhibition of IL-1 in
patients with rheumatoid arthritis and type 2 diabetes mellitus:
Two case reports and review of the literature. J Med Case Rep.
9:1232015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshida Y and Tanaka T: Interleukin 6 and
rheumatoid arthritis. Biomed Res Int. 2014:6983132014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van Schouwenburg PA, Rispens T and Wolbink
GJ: Immunogenicity of anti-TNF biologic therapies for rheumatoid
arthritis. Nat Rev Rheumatol. 9:164–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyabe Y, Miyabe C, Iwai Y, Yokoyama W,
Sekine C, Sugimoto K, Harigai M, Miyasaka M, Miyasaka N and Nanki
T: Activation of fibroblast-like synoviocytes derived from
rheumatoid arthritis via lysophosphatidic acid-lysophosphatidic
acid receptor 1 cascade. Arthritis Res Ther. 16:4612014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Garcia-Carbonell R, Divakaruni AS, Lodi A,
Vicente-Suarez I, Saha A, Cheroutre H, Boss GR, Tiziani S, Murphy
AN and Guma M: Critical role of glucose metabolism in rheumatoid
arthritis fibroblast-like Synoviocytes. Arthritis Rheumatol.
68:1614–1626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Calnan DR and Brunet A: The FoxO code.
Oncogene. 27:2276–2288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qian Z, Ren L, Wu D, Yang X, Zhou Z, Nie
Q, Jiang G, Xue S, Weng W, Qiu Y and Lin Y: Overexpression of
FoxO3a is associated with glioblastoma progression and predicts
poor patient prognosis. Int J Cancer. 140:2792–2804. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nho RS, Hergert P, Kahm J, Jessurun J and
Henke C: Pathological alteration of FoxO3a activity promotes
idiopathic pulmonary fibrosis fibroblast proliferation on type i
collagen matrix. Am J Pathol. 179:2420–2430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Czyszek JA, Milkiewicz M, Elias E and
Milkiewicz PJG: Proapoptotic protein bim and its upstream activator
Foxo3A are overexpressed in primary biliary cirrhosis but not in
primary sclerosing cholangitis. Gut. 60 (Suppl 1):A2272011.
View Article : Google Scholar
|
|
43
|
Harada Y, Harada Y, Elly C, Ying G, Paik
JH, DePinho RA and Liu YC: Transcription factors Foxo3a and Foxo1
couple the E3 ligase Cbl-b to the induction of Foxp3 expression in
induced regulatory T cells. J Exp Med. 207:1381–1391. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin L, Hron JD and Peng SL: Regulation of
NF-kappaB, Th activation, and autoinflammation by the forkhead
transcription factor Foxo3a. Immunity. 21:203–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin H, Dai T, Xiong H, Zhao X, Chen X, Yu
C, Li J, Wang X and Song L: Unregulated miR-96 induces cell
proliferation in human breast cancer by downregulating
transcriptional factor FOXO3a. PLoS One. 5:e157972010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu Y, Peng H, Cui M, Whitney NP, Huang Y
and Zheng JC: CXCL12 increases human neural progenitor cell
proliferation through Akt-1/FOXO3a signaling pathway. J Neurochem.
109:1157–1167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nakao T, Geddis AE, Fox NE and Kaushansky
K: PI3K/Akt/FOXO3a pathway contributes to thrombopoietin-induced
proliferation of primary megakaryocytes in vitro and in vivo via
modulation of p27(Kip1). Cell Cycle. 7:257–266. 2008. View Article : Google Scholar : PubMed/NCBI
|