Introduction
Both endogenous and exogenous factors have been
demonstrated to cause skin aging. The endogenous factors are
largely influenced by a variety of intrinsic genetic and epigenetic
alterations, while the exogenous factors are characterized by
‘photoaging’ of the skin caused by ultraviolet (UV) rays in
sunlight (1). Photoaging mainly
impacts the conversion of synthetic collagen fibers to synthetic
elastic fibers, as well as inflammatory infiltration of the dermis
(2). UV-irradiation of skin induces
elevated matrix metalloproteinase (MMP) expression, leading to
degradation of fibrous connective tissue and reduction of collagen
synthesis, and these biological processes are important mechanisms
of skin photoaging (3-5). In
addition, tyrosinase is a key enzyme that initiates melanogenesis
and tyrosinase activity strongly correlates with melanin production
(6). All of these effects result in
collagen reduction and a decreased rate of epidermal turnover
during aging (7).
Platelet-rich plasma (PRP) is a highly concentrated
platelet plasma obtained from whole blood. In total, >1100
different proteins have been found in PRP, including immune system
messengers, various enzymes and growth factors (8,9). These
proteins have been demonstrated to participate in biological
processes, such as cellular proliferation and differentiation,
matrix remodeling and angiogenesis (8,9). PRP
proteins enhance wound healing and tissue regeneration. Among all
the proteins in PRP, growth factors are the most important
components (10-12).
Platelet-derived growth factor, transformation growth factor,
insulin-like growth factor, epidermal growth factor, fibroblast
growth factor and vascular endothelial growth factor have well
established roles in angiogenesis, cell migration, cell
proliferation and collagen deposition (8,13-15).
When PRP is implanted into damaged skin tissue, a
variety of high-concentration growth factors are activated and a
series of skin cell reactions occur. Cellulose, fibronectin and
vitronectin from PRP aggregate with the growth factors released by
platelets and function locally (8).
These proteins can also act as a scaffold for nascent cells and
tissues to promote the repair of damaged/aging skin. At the
molecular level, PRP injection induces DNA synthesis and promotes
the corresponding gene expression (16,17).
The aim of the present study was to evaluate the
effects of PRP injections on prevention of UV-B-induced photoaging
through clinical practice and the use of an in vitro model.
Furthermore, the aim of the present study was to elucidate the
molecular mechanisms underlying PRP injections to facilitate the
future clinical application of PRP injections as an anti-aging
therapy.
Materials and methods
Clinical study design
The present study was conducted at The Inner
Mongolia International Mongolian Hospital (Inner Mongolia, China)
between July 20 and September 20, 2018. In total, 30 females
between the ages of 30 and 50 years were recruited. Informed
written consent was obtained from all participants before
treatment. The individual patient also provided written informed
consent for the publication of the facial images. The present study
was approved by The Ethical Committee of Inner Mongolia
International Mongolian Hospital. Exclusion criteria included
unwilling patients and patients with abnormal renal function,
coagulopathy, acquired immune deficiency syndrome, hepatitis B or
other infectious diseases.
PRP was injected on the right sides of the faces of
the patients, and an equal volume of normal saline was injected on
the left side as a negative control. In total, ~1 ml PRP was
injected at multiple sites on the right side of each patient's face
at a depth of 2.0 mm. Injections were administered 3 times at
15-day intervals. Images using the noninvasive VISIA®
Complexion Analysis System (the VISIA® multi-point
positioning system; Canfield Scientific) were taken and computer
tomography (CT) detection of the injection sites was performed
before each injection and 2 weeks after the last injection. The 6th
generation VISIA® skin tester from Canfield Scientific
was used to detect skin texture. With advanced optical imaging,
RBX® and software technology, the VISIA® skin
tester automatically performed the quantitative evaluation for skin
thickness, pigmentation, pores, wrinkles, skin smoothness,
porphyrin, UV spots and brown spots. Using the reflectivity of the
facial skin, the shape trajectory of wrinkles or textures can be
reconstructed using the software algorithms.
Preparation of PRP
The method used for PRP preparation was as
previously described (18). Briefly,
whole blood was drawn into an anticoagulant tube and then
transferred to a new tube containing 3.2% (w/v) trisodium citrate
(9:1 v/v mixture). The blood sample was centrifuged at 110 x g for
15 min at room temperature and the resulting middle yellow PRP
layer was centrifuged for another 8 min at 1,400 x g at room
temperature to concentrate the platelets. The final concentration
of platelets in PRP was 1009.91±219.43x109/l.
Human organotypic skin explant
culture
An in vitro culture model of organotypic
human skin (hOSEC) was established, according to the method by
Frade et al (19). Excess
skin of the breast or abdomen, collected during orthopedic surgery
or breast surgery, was trimmed to remove the lower adipose tissue
and cut into 1x1 cm2 pieces. The skin sample was then
cultured in a 6-well plate containing metal mesh and DMEM with 10%
FBS (both Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin
and streptomycin for 7 days. These experiments were approved by The
Ethical Committee of Inner Mongolia International Mongolian
Hospital (reference no. B2018-013). The skin samples were collected
at The Inner Mongolia International Mongolian Hospital on July 12,
2018. The patient agreed to the use of her samples in scientific
research and written informed consent was provided.
UVB-induced photoaging and PRP
treatment
The hOSEC was irradiated with UVB light at a dosage
of 10 mJ/cm2 every other day for 3 days. Before UVB
irradiation, the medium was removed and PBS or PRP solution was
added to the explants. After UVB irradiation, the medium was
changed back to complete medium containing 10% FBS and 1%
penicillin and streptomycin, and the explants were cultured for
another 7 days.
Hematoxylin and eosin (HE) staining
and Masson's trichrome stain
The skin explants were fixed with 10% neutral
buffered formalin at room temperature for 24 h and then embedded
with paraffin and cut into 4 mm pieces. The sections were
deparaffinized at 65˚C for 4 h with gradient ethanol and then
stained with HE for 10 min or Masson's trichrome stain for 6 min at
room temperature. The images were captured of the sections using a
light microscope (magnification, x200; Olympus Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA samples were isolated from the cultured
skin tissue using TRIzol® reagent (Thermo Fisher
Scientific, Inc.). Briefly, ~50 mg tissue was lysed in 1 ml
TRIzol®, and then chloroform was added to the
homogenates and the samples were centrifuged at 14,000 x g for 10
min at 4˚C to obtain the RNA fractions (supernatants). Isopropanol
was added to the pellet RNA and then the samples were washed with
75% ethanol. The cDNA was synthesized from the RNA sample using
HiScript® III RT SuperMix for qPCR with +gDNA wiper
(Vazyme) according to manufacturer's protocol. The qPCR was
performed using a CFX96 system (Bio-Rad Laboratories, Inc.) with
ChamQ Universal SYBR® qPCR Master Mix (Vazyme). The
thermocycling conditions were as follows: Initial denaturation at
95˚C for 15 min, followed by 40 cycles of 95˚C for 10 sec and 60˚C
for 30 sec; 95˚C for 15 sec, 60˚C for 60 sec and 95˚C for 15 sec.
PCR primers were as follows: GAPDH: Sense
5'-TCAACAGCGACACCCACTCC-3', anti-sense 5'-TGAGGTCCACCACCCTGTTG3';
tropoelastin: Sense 5'-GCTGACGCTGCTGCAGCCTA-3', anti-sense
5'-CAGCAAAAGCTCCACCTACA-3'; fibrillin-1: Sense
5'-TGACTGGCCCACACGTGCATAG-3', anti-sense
5'-TGACATTGACCCCTTGTTGACAGGA-3'; MMP-1: Sense
5'-GGGAGATCATCGGGACAACTC-3', anti-sense 5'-GGGCCTGGTTGAAAAGCA-3';
p53: Sense 5'-ATCGTGGAGGCATGAGCAGA-3', anti-sense
5'-TCTGGAGTTTCTGCTGCTGCTA-3'; and Tyrosinase: Sense
5'-CTCCGCTGGCCATTTCCCTA-3', anti-sense 5'-GGTGCTTCATGGGCAAAATC-3'.
GAPDH was used as a housekeeping gene for normalization of gene
expression. The 2-ΔΔCq method was
used to quantify the relative gene expression (20).
Immunofluorescence
The skin graft was frozen in liquid nitrogen and
placed in a constant temperature freezer. A small amount of optimal
cutting temperature compound was added for cryosectioning
(thickness, 4 µm). The sections were dried with a cold air blower
and blocked with 5% BSA for 1 h at room temperature. Then, the
frozen sections were incubated with specific antibodies against
MMP-1 (1:800, R&D Systems, Inc.; cat. no. MAB901), tyrosinase
(1:800, Abcam; cat. no. ab738), tropoelastin (1:800, Abcam; cat.
no. ab21600) and fibrillin (1:800, Abcam; cat. no. ab53076) at room
temperature for 1 h. After washing, the frozen sections were
labeled with Alexa Fluor 488 goat anti-rabbit IgG (1:500,
Invitrogen; Thermo Fisher Scientific, Inc.; cat. no. A11008) at
room temperature for 1 h. Finally, the samples were imaged and
analyzed with a confocal laser scanning microscope (Carl Zeiss AG)
with x200 magnification.
Western blotting
The skin explants were irradiated 3 times with UVB
light at a dosage of 10 mJ/cm2 every other day. Then the
skin explants were washed twice with cold PBS and ground in RIPA
lysis buffer (containing protease inhibitor cocktail; Beyotime
Institute of Biotechnology). The whole cell lysates were incubated
at 4˚C for 30 min, followed by centrifugation (12,000 x g for 15
min at 4˚C). Protein samples were quantified using Bicinchoninic
Acid protein assay method and 30 µg protein for each sample was
separated by SDS-PAGE (4-18% gradient gel), transferred to a PVDF
membrane and blocked with 5% milk at room temperature for 30 min.
The membranes were then probed with specific antibodies against
MMP-1 (1:1,000, R&D Systems, Inc.; cat. no. MAB901), tyrosinase
(1:1,000; Abcam; cat. no. ab738), tropoelastin (1:1,000; Chemicon
International; Thermo Fisher Scientific, Inc.; cat. no. MAB2503)
and β-actin (1:2,000, Sigma-Aldrich; Merck KGaA; cat. no. A5441) at
4˚C overnight. The next day, the membranes were incubated with goat
horseradish peroxidase-conjugated anti-mouse IgG secondary antibody
(1:10,000; Thermo Fisher Scientific, Inc.; cat. no. G-21040) at
room temperature for 1 h. The protein bands were visualized with
SuperSignal™ West Pico PLUS Chemiluminescent Substrate kit (Thermo
Fisher Scientific, Inc.; cat. no. 34580) and analyzed using ImageJ
(version 1.52a; National Institutes of Health).
Statistical analysis
Numerical data (n=3) are presented as the mean ± SD
and were compared using unpaired Student's t-tests (GraphPad Prism
version 6.0; GraphPad Software, lnc.). One-way ANOVA followed by
Fisher's Least Significance Differences test were used for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Changes in skin biophysical parameters
and skin appearance upon PRP injections.
All 30 females (median age, 43 years) completed 3
PRP injections within 1 month and no adverse effects were observed
throughout the treatment. Data were collected before each injection
(0, 2 and 4 weeks) and 2 weeks after the last injection (week 6).
After 3 PRP injections, skin CT examination around the injection
sites showed that the translucency of the pigment ring decreased,
indicating a decrease in pigmentation. In addition, collagen was
increased and denser than that before treatment (Fig. 1). Baseline (week 0) skin pores,
texture, wrinkles and spots, which were assessed using the
VISIA® multi-point positioning system, were compared
with the follow-up measurements and are presented in Table I. Skin pore values continuously
decreased with the progress of PRP treatment. At week 0, the value
was 1094.26±351.42, but with continued PRP treatments, the value
significantly decreased to 907.21±362.89 (P=0.045) at the final
measurement. Wrinkle values measured 2 weeks after the last
treatment (20.72±6.07) at week 6 were also significantly lower than
that of week 0 (30.17±9.17; P<0.001). Similarly, the skin
texture at week 4 (507.23±247.02; P=0.03) and week 6
(496.52±265.47; P=0.02) was significantly lower than that at week 0
(673.45±317.23). Although the spot values also decreased, the
difference between week 0 and either of the 3 PRP treatments was
not significant.
 | Table ISkin biophysical parameter changes
upon platelet rich plasma injection. |
Table I
Skin biophysical parameter changes
upon platelet rich plasma injection.
| Week 0 | Week 2 | Week 4 | Week 6 |
|---|
| Parameter | Mean ± SD | Mean ± SD | P-valuea | Mean ± SD | P-valuea | Mean ± SD | P-valuea |
|---|
| Texture | 673.45±317.23 | 610.68±346.57 | 0.41 | 507.23±247.02 | 0.03 | 496.52±265.47 | 0.02 |
| Wrinkles | 30.17±9.17 | 27.19±10.01 | 0.18 | 24.66±8.34 | 0.01 | 20.76±6.07 | <0.001 |
| Spots | 223.65±71.93 | 214.73±52.67 | 0.53 | 203.18±42.32 | 0.15 | 197.24±49.57 | 0.07 |
| Pores |
1,094.26±351.42 |
1,021.93±379.44 | 0.46 | 958.23±401.87 | 0.16 | 907.21±362.89 | 0.045 |
In order to further verify the role of PRP in
improving human skin conditions, PRP injections were only performed
on one side of the patient's face and the other side of the face
was left untreated. With 3 rounds of PRP injections, the skin
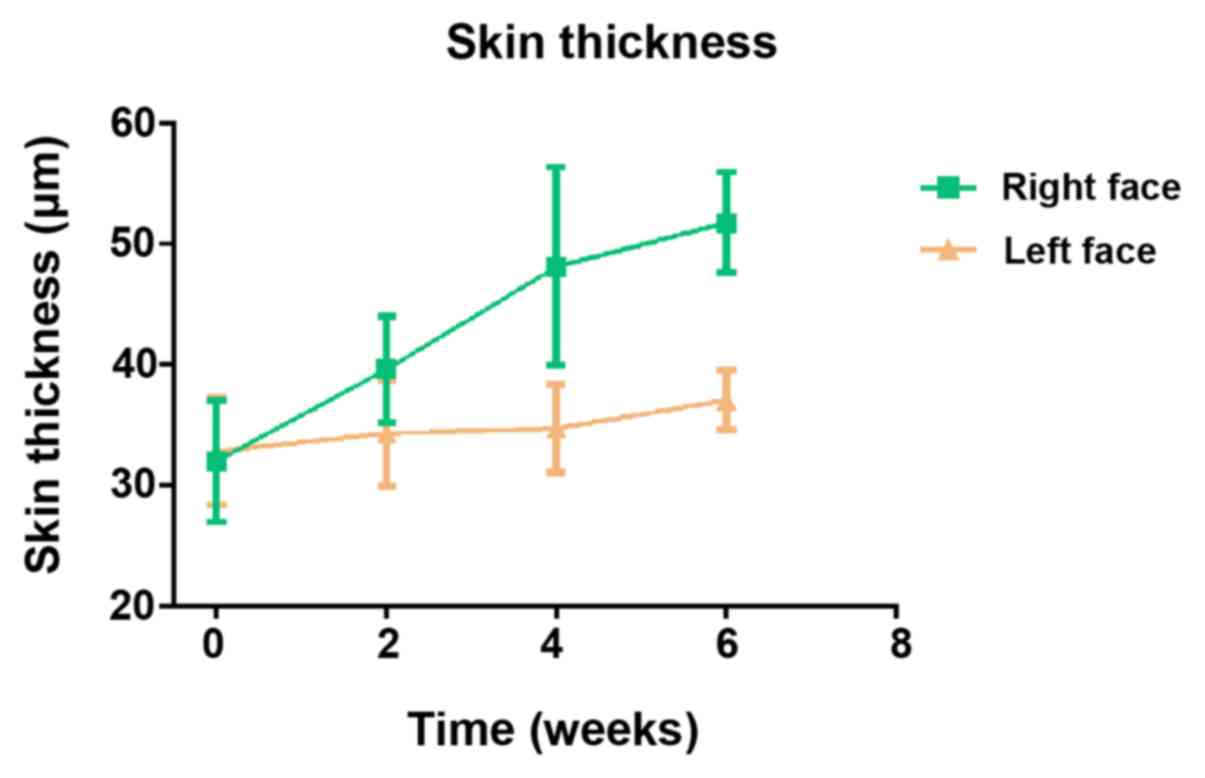
thickness markedly increased on the right-side face (with PRP
treatment), but the left-side face (without PRP treatment) showed
minimal changes (Fig. 2). Similarly,
with 3 rounds of PRP treatments, the right-side face showed better
skin texture, less wrinkles and relatively smooth and firm skin,
whereas the left-side face showed little changes (Figs. 3 and S1). Therefore, it was demonstrated that
PRP injections effectively improved human skin conditions.
PRP protects skin against photoaging
caused by UV light
A hOSEC was established using the method described
previously (19). To observe the
distribution of epidermal structures and dermal fibers, HE staining
and Masson's trichrome staining were used to detect collagen in the
human skin grafts. It was observed that the collagen fibers of skin
grafts without UVB treatment were arranged neatly and densely, and
the collagen staining was deep. After UVB irradiation, the collagen
fibers were denatured, broken and arranged disorderly, and the
collagen staining was light and showed markedly reduced collagen
content. However, with PRP treatment, collagen fibers of the skin
grafts were not altered after UVB irradiation (Fig. 4). These results suggested that PRP
can protect collagen fibers, delay collagen fiber changes, reduce
elastic fiber chain scission and resist skin photoaging caused by
UV rays.
PRP inhibits UVB-induced MMP-1 and
tyrosinase upregulation to protect skin against photoaging
In order to explore the potential molecular
mechanisms that mediate protection of PRP against photoaging, gene
expression changes of MMP-1, tyrosinase, fibrillin and tropoelastin
were measured after treatment with UVB and/or PRP. It was
identified that PRP significantly inhibited UVB induced-MMP-1 and
tyrosinase upregulation, but significantly restored the expression
of fibrillin and tropoelastin, which were downregulated by UVB
treatment (Fig. 5). These
observations were also made at both mRNA and protein levels
(Fig. 5A-C). These results suggested
that PRP may protect human skin against photoaging by restoring the
gene expressions of MMP-1, tyrosinase, fibrillin and
tropoelastin.
 | Figure 5PRP inhibits UVB-induced MMP-1 and
tyrosinase upregulation. (A) MMP-1, tyrosinase, fibrillin and
tropoelastin mRNA levels in human skin grafts after UVB and/or PRP
treatments were determined by reverse transcription-PCR using GAPDH
as an internal control. Data are presented as the mean ± SD of
three independent experiments. (B) MMP-1, tyrosinase, tropoelastin
and β-actin protein levels in human skin grafts after UVB and/or
PRP treatments were assessed by immunoblotting. Bar plots show the
quantified fold changes of the western blotting bands.
**P<0.01. (C) Distribution of MMP-1,
tyrosinase, fibrillin and tropoelastin in human skin grafts after
UVB and/or PRP were detected by immunofluorescence staining
(magnification, x200). PRP, platelet-rich plasma; MMP-1, matrix
metalloproteinase-1; UV, ultraviolet. |
Discussion
PRP has been widely applied for tissue repair in the
fields of plastic surgery, oral and maxillofacial surgery,
orthopedics and neurosurgery (8,12,15,18). As part of the diverse
functional factors contained in PRP, autologous PRP has the best
ratio of growth factors. The growth factor content in PRP is
consistent with that in the patient's body, and compensates for the
deficiencies of poor activity and low repair capacity of a single
growth factor (8). In addition,
there are no immunological problems and no risk of spreading
diseases in allogeneic transplantation (21). Furthermore, PRP forms a gel, which
protects platelets from damage and loss during injection, and
allows platelets to secrete growth factors for an extended period
to maintain a high concentration of growth factors (22,23). The
benefits of PRP treatment in skin anti-aging repair come not only
from the variety of high-concentration growth factors, but also
from its gelatin state, which has plasticity and good support for
skin wrinkles, cavities and skin relaxation (22,23). In
addition, PRP also contains a large number of cell adhesion
proteins, such as cellulose, fibronectin and vitronectin, that may
keep skin smooth and tight (24).
Physiologically, the growth factors in PRP have important roles in
reducing the rate of aging by restoring the declining DNA synthesis
that occurs with aging, resisting cell death and enhancing gene
expression for tissue repair (25,26). A
positive correlation between PRP and skin anti-aging has also been
reported in both pre-clinical and clinical practice (25-28).
Consistent with previous studies (17,26), the
present clinical study showed that PRP treatment improved skin
conditions, including increased skin thickness, enhanced collagen
content and reduced pigmentation. In addition, parameters assessed
using the VISIA system, such as wrinkles, texture and pores were
all decreased compared with pretreatment. Further evidence from the
hOSEC experiments provided insight for the potential molecular
mechanisms that explain how PRP treatments protect skin from
photoaging.
UV is the primary external stress that causes
oxidative stress in the skin. This reaction is initiated by
reactive oxygen species and eventually results in premature skin
aging by inhibiting transforming growth factor-β (TGF-β) activity,
inducing MMP expression and activating the mTOR signaling pathway,
culminating in the inhibition of autophagy (29,30).
TGF-β in PRP may compensate for the localized reduction of TGF-β
during photoaging. Furthermore, PRP is also reported to be involved
in autophagy (31). MMP-1 and
tyrosinase function in the degradation of fibrous connective tissue
and the reduction of collagen synthesis, and are also important
molecules in promoting skin photoaging (32-34).
The present study demonstrated that PRP could inhibit UV-induced
MMP-1 and tyrosinase upregulation to protect skin from photoaging.
PRP also induced the expression of fibrillin and tropoelastin, and
these factors have been reported to improve skin elasticity.
Therefore, PRP treatment directly implants a variety
of active growth factors into aging skin. These factors change gene
expression in skin cells, promote skin cell proliferation and
differentiation, and rearrange the structure of skin tissues. PRP
injections are effective in improving skin conditions and
protecting skin from photoaging, and thus have broad applications
in anti-aging skin repair.
Supplementary Material
Figure S1. Images show improved skin
conditions of the right.side face (PRP injected), measuring the
spots, red area, pore, texture, wrinkles, UV spots, porphyrins and
brown spots compared with the left.side face (without PRP
treatment) from week 0 to week 6. PRP, platelet.rich plasma; UV,
ultraviolet.
Acknowledgements
The authors would like to thank Dr Rina Wu
(Department of Dermatology, Inner Mongolia International Mongolian
Hospital, Hohhot, China); Dr Yaoxing Gao (Department of
Anesthesiology, The Affiliated Hospital of Inner Mongolia Medical
University, Hohhot, China); Dr Hao Li and Dr Peng Zhao (Department
of Dermatology, The Affiliated Hospital of Inner Mongolia Medical
University, Hohhot, China); and Dr Limin Yang (Department of
Molecular biology, Inner Mongolia Medical University, Hohhot,
China) for their valuable support of the present research.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TL and RD conceived and designed the study and wrote
the manuscript. RD performed the experiments, analyzed the data and
recorded the clinical characteristics of the patients. Both authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethical
Committee of Inner Mongolia International Mongolian Hospital.
Informed written consent was obtained from all participants before
treatment. The skin sample experiments were approved by The Ethical
Committee of Inner Mongolia International Mongolian Hospital
(reference no. B2018-013). The patient agreed to the use of her
samples in scientific research and written informed consent was
obtained.
Patient consent for publication
The patient provided written informed consent for
the publication of the facial images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alexiades-Armenakas MR, Dover JS and Arndt
KA: The spectrum of laser skin resurfacing: Nonablative,
fractional, and ablative laser resurfacing. J Am Acad Dermatol.
58:719–737. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Borrione P, Fagnani F, Di Gianfrancesco A,
Mancini A, Pigozzi F and Pitsiladis Y: The role of platelet-rich
plasma in muscle healing. Curr Sports Med Rep. 16:459–463.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ince B, Yildirim MEC, Dadaci M, Avunduk MC
and Savaci N: Comparison of the efficacy of homologous and
autologous platelet-rich plasma (PRP) for treating androgenic
alopecia. Aesthetic Plast Surg. 42(297)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Martinez-Zapata MJ, Martí-Carvajal AJ,
Solà I, Expósito JA, Bolíbar I, Rodríguez L, Garcia J and Zaror C:
Autologous platelet-rich plasma for treating chronic wounds.
Cochrane Database Syst Rev. CD006899:2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cervelli V, Nicoli F, Spallone D, Verardi
S, Sorge R, Nicoli M and Balzani A: Treatment of traumatic scars
using fat grafts mixed with platelet-rich plasma, and resurfacing
of skin with the 1540 nm nonablative laser. Clin Exp Dermatol.
37:55–61. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Iozumi K, Hoganson GE, Pennella R, Everett
MA and Fuller BB: Role of tyrosinase as the determinant of
pigmentation in cultured human melanocytes. J Invest Dermatol.
100:806–811. 1993.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fisher GJ, Datta SC, Talwar HS, Wang ZQ,
Varani J, Kang S and Voorhees JJ: Molecular basis of sun-induced
premature skin ageing and retinoid antagonism. Nature. 379:335–339.
1996.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Pavlovic V, Ciric M, Jovanovic V and
Stojanovic P: Platelet Rich Plasma: A short overview of certain
bioactive components. Open Med (Wars). 11:242–247. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang HL and Avila G: Platelet rich plasma:
Myth or reality? Eur J Dent. 1:192–194. 2007.PubMed/NCBI
|
|
10
|
Andia I, Sanchez M and Maffulli N: Joint
pathology and platelet-rich plasma therapies. Expert Opin Biol
Ther. 12:7–22. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Foster TE, Puskas BL, Mandelbaum BR,
Gerhardt MB and Rodeo SA: Platelet-rich plasma: From basic science
to clinical applications. Am J Sports Med. 37:2259–2272.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Frautschi RS, Hashem AM, Halasa B,
Cakmakoglu C and Zins JE: Current evidence for clinical efficacy of
platelet rich plasma in aesthetic surgery: A systematic review.
Aesthet Surg J. 37:353–362. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bielecki T, Dohan Ehrenfest DM, Everts PA
and Wiczkowski A: The role of leukocytes from L-PRP/L-PRF in wound
healing and immune defense: New perspectives. Curr Pharm
Biotechnol. 13:1153–1162. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Borrione P, Gianfrancesco AD, Pereira MT
and Pigozzi F: Platelet-rich plasma in muscle healing. Am J Phys
Med Rehabil. 89:854–861. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu W, Wang J and Yin J: Platelet-rich
plasma: A promising product for treatment of peripheral nerve
regeneration after nerve injury. Int J Neurosci. 121:176–180.
2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
El-Domyati M, Abdel-Wahab H and Hossam A:
Combining microneedling with other minimally invasive procedures
for facial rejuvenation: A split-face comparative study. Int J
Dermatol. 57:1324–1334. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Charles-de-Sá L, Gontijo-de-Amorim NF,
Takiya CM, Borojevic R, Benati D, Bernardi P, Sbarbati A and
Rigotti G: Effect of use of platelet-rich plasma (PRP) in skin with
intrinsic aging process. Aesthet Surg J. 38:321–328.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kamakura T, Kataoka J, Maeda K, Teramachi
H, Mihara H, Miyata K, Ooi K, Sasaki N, Kobayashi M and Ito K:
Platelet-rich plasma with basic fibroblast growth factor for
treatment of wrinkles and depressed areas of the skin. Plast
Reconstr Surg. 136:931–939. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Frade MA, Andrade TA, Aguiar AF, Guedes
FA, Leite MN, Passos WR, Coelho EB and Das PK: Prolonged viability
of human organotypic skin explant in culture method (hOSEC). An
Bras Dermatol. 90:347–350. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Marques LF, Stessuk T, Camargo IC, Sabeh
Junior N, dos Santos L and Ribeiro-Paes JT: Platelet-rich plasma
(PRP): Methodological aspects and clinical applications. Platelets.
26:101–113. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Del Fabbro M, Panda S and Taschieri S:
Adjunctive use of plasma rich in growth factors for improving
alveolar socket healing: A systematic review. J Evid Based Dent
Pract. 19:166–176. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ali M, Mohamed A, Ahmed HE, Malviya A and
Atchia I: The use of ultrasound-guided platelet-rich plasma
injections in the treatment of hip osteoarthritis: A systematic
review of the literature. J Ultrason. 18:332–337. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Qian Y, Han Q, Chen W, Song J, Zhao X,
Ouyang Y, Yuan W and Fan C: Platelet-rich plasma derived growth
factors contribute to stem cell differentiation in musculoskeletal
regeneration. Front Chem. 5(89)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ramaswamy Reddy SH, Reddy R, Babu NC and
Ashok GN: Stem-cell therapy and platelet-rich plasma in
regenerative medicines: A review on pros and cons of the
technologies. J Oral Maxillofac Pathol. 22:367–374. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Maisel-Campbell AL, Ismail A, Reynolds KA,
Poon E, Serrano L, Grushchak S, Farid C, West DP and Alam M: A
systematic review of the safety and effectiveness of platelet-rich
plasma (PRP) for skin aging. Arch Dermatol Res. Oct 18, 2019 2019
(Epub ahead of print). doi.org/10.1007/s0040. PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cakin MC, Ozdemir B, Kaya-Dagistanli F,
Arkan H, Bahtiyar N, Anapali M, Akbas F and Onaran I: Evaluation of
the in vivo wound healing potential of the lipid fraction from
activated platelet-rich plasma. Platelets. 1–. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Caruana A, Savina D, Macedo JP and Soares
SC: From platelet-rich plasma to advanced platelet-rich fibrin:
Biological achievements and clinical advances in modern surgery.
Eur J Dent. 13:280–286. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Anitua E, Pino A, Jaen P and Orive G:
Plasma rich in growth factors enhances wound healing and protects
from photo-oxidative stress in dermal fibroblasts and 3D skin
models. Curr Pharm Biotechnol. 17:556–570. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Aseichev AV, Azizova OA and Zhambalova BA:
Effect of UV-modified fibrinogen on platelet aggregation in
platelet-rich plasma. Bull Exp Biol Med. 133:41–43. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Addor FAS: Beyond photoaging: additional
factors involved in the process of skin aging. Clin Cosmet Investig
Dermatol. 11:437–443. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Aghajanova L, Houshdaran S, Balayan S,
Manvelyan E, Irwin JC, Huddleston HG and Giudice LC: In vitro
evidence that platelet-rich plasma stimulates cellular processes
involved in endometrial regeneration. J Assist Reprod Genet.
35:757–770. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yan S, Yang B, Shang C, Ma Z, Tang Z, Liu
G, Shen W and Zhang Y: Platelet-rich plasma promotes the migration
and invasion of synovial fibroblasts in patients with rheumatoid
arthritis. Mol Med Rep. 14:2269–2275. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shin MK, Lee JW, Kim YI, Kim YO, Seok H
and Kim NI: The effects of platelet-rich clot releasate on the
expression of MMP-1 and type I collagen in human adult dermal
fibroblasts: PRP is a stronger MMP-1 stimulator. Mol Biol Rep.
41:3–8. 2014.PubMed/NCBI View Article : Google Scholar
|