Introduction
The routine treatment of orthopedic trauma (OT)
frequently involves debridement, washing, dressing changes,
antibiotic treatment to control infection and flap surgery after
the granulation tissue has grown plump. For certain cases of OT
with large wounds and seriously injured soft tissues, however, the
dressing change is required more frequently if the conventional
treatment is used. In addition, the wound surface exposure time is
long, the necrotic tissue and toxins are absorbed and the healing
time is prolonged. Furthermore, the risk of infection increases
(1,2). Trauma, a period of inactivity and
vascular wall injury are risk factors for lower-limb deep venous
thrombosis (DVT) in patients with OT (3). Patients with long-term wound disunion
require a long period of inactivity due to the prolonged course of
the condition. Thus, lower-limb DVT is more likely to occur
(4). Therefore, accelerating the
wound healing process and promoting the recovery of patients with
OT may have a significant impact to reduce infection and lower-limb
DVT.
Vacuum sealing drainage (VSD) refers to the use of
continuous vacuum suction to seal the wound. It enables an easier
discharge of the exudate and provides a better clearance effect
compared with conventional treatment (5). Application of a VSD technique may
completely remove exudate, necrotic fluid and bacteria from the
wound surface through a continuous vacuum, removing local dead
space, reducing tissue edema and making granulation tissue easier
to grow. At the same time, vacuum aspiration may deform wound
cells, increase the synthesis of proteins and biological
macromolecules between cells and accelerate cell proliferation. In
addition, excess fluid between tissues may be further extracted,
thus reducing tissue edema, promoting local blood circulation and
facilitating wound healing. A previous study has indicated that VSD
may promote wound healing in OT and improve the survival rate of
transplanted skin (6). In the
present study, VSD was used for the treatment of patients with OT.
The effects to reduce infection and lower-limb DVT were observed
and reported below.
Materials and methods
General information.
The clinical data of 76 patients with OT admitted to
The Second Affiliated Hospital of Soochow University (Suzhou,
China) from January 2016 to January 2019 were retrospectively
analyzed and categorized into a control group (CG; n=37) and an
experimental group (EG; n=39) according to the treatment
administered. The general patient information exhibited no
differences between the two groups and the groups were comparable
(P>0.05; Table I). The present
study was approved by the ethics committee of The Second Affiliated
Hospital of Soochow University (Suzhou, China). The inclusion
criteria were as follows: i) Age, 18-70 years; ii) lumbosacral or
lower-limb trauma; iii) primary suture not practical or possible,
and iv) signed informed consent form. The exclusion criteria were
as follows: i) Serious infection secondary to fresh trauma, ii)
infectious diseases as complications and iii) serious
complications.
 | Table IComparison of general information. |
Table I
Comparison of general information.
| Item | Experimental group
(n=39) | Control group
(n=37) | χ2/t
value | P-value |
|---|
| Sex | | | 0.043 | 0.836 |
|
Male | 22 (56.41%) | 20 (54.05%) | | |
|
Female | 17 (43.59%) | 17 (45.95%) | | |
| Age (years) | 34-49
(41.65±7.21) | 35-48
(42.37±7.39) | 0.431 | 0.669 |
| Wound type | | | 0.164 | 0.686 |
|
Fresh | 27 (69.23%) | 24 (64.86%) | | |
|
Old | 12 (30.77%) | 13 (35.14%) | | |
| Trauma site | | | 0.018 | 0.895 |
|
Lower
limb | 29 (74.36%) | 28 (75.68%) | | |
|
Lumbosacral
portion | 10 (25.64%) | 9 (24.32%) | | |
| Cause | | | 0.676 | 0.879 |
|
Traffic | 17 (43.59%) | 18 (48.65%) | | |
|
Crushing | 13 (33.33%) | 11 (29.73%) | | |
|
Falling | 7 (17.95%) | 5 (13.51%) | | |
|
Others | 2 (5.13%) | 3 (8.11%) | | |
Methods
The patients in each group underwent debridement and
repair of damaged blood vessels, nerves and tendons. For patients
with fracture, fracture reduction and fixation were performed. All
patients were treated with antibiotics to control infection.
Routine dressing changes once every 1-2 days were applied to
patients in the CG. If the wound was large and deep, a drainage
strip was placed. After the granulation tissue had grown plump,
wound suture, skin grafting or flap transfer surgery was performed
according to the wound condition. Patients in the EG underwent VSD
treatment (7). After routine
debridement, according to the size and shape of the wound, a vacuum
therapeutic sponge adsorption pad and isolation pad of a suitable
size was made with sterile scissors (Jiangsu Suzhong Pharmaceutical
Group, Co., Ltd.) to cover the wound surface to ensure complete
coverage, and the isolation pad was placed between the adsorption
pad and the wound surface. Depending on the amount of exudate on
the wound surface and the depth of the wound, the adsorption sponge
was able to be completely inserted into the wound surface, with the
outer surface covered with the adsorption pad. The wound surface
was closed with a medical biological semipermeable membrane (Xiamen
Shanxing Hanfang Biotechnology Co., Ltd.) tightly attached to the
skin, with the outer edge of the membrane covering the normal skin
outside of the wound edge by at least >3 cm. Medical tape was
used to seal the wound again to ensure that the wound was closed.
One end of the silicone tube (Dongguan Ruixiang Precision Silicone
Products Co., Ltd.) was connected to the dressing and the other end
was connected to the vacuum suction device (Suzhou Oris Medical
Supplies Co., Ltd.) to ensure a good seal and no air leakage. The
vacuum pressure was set between -450 and -125 mmHg. At the same
time, an antibacterial agent was selected after a pathogenic test
for anti-infection treatment. If the wound was deep and large, the
dressing was replaced 2-3 times depending on the patient's
situation. The dressing maintenance time was up to 10 days. Wound
suture and skin grafting were performed when the granulation tissue
was plump. If the granulation tissue was not plump and depending on
the patient's situation, a skin flap transfer was performed.
Outcome measures
i) The dressing change frequency (the numbers of
dressing change during wound healing), dressing maintenance time
(duration of dressing in the wound after dressing change), 2nd
operation time (the time between the first operation and the second
operation) and duration of hospital stay (complete hospital stay)
were recorded; ii) with respect to efficacy, 'cure' was defined as
the healing of all wounds within two weeks with good epidermal
coverage. ‘Improvement’ was defined as a significantly reduced
wound area, the survival of most wound skin tissues, decreased
secretions and good growth of fresh and healthy granulation tissue,
although the dressing was still required to be replaced. In cases
of improvement, the wound healed after skin grafting. The treatment
was deemed ineffective when the wound area did not decrease or
expand, the wound skin was necrotic, the secretions increased and
there was no healthy granulation. The total effective rate was
calculated as 100% minus the ineffective rate. Wound healing and
evaluation were evaluated by the same attending physician with
>5 years of clinical experience to reduce errors. iii) Wound
healing time was recorded. iv) Venous blood (3 ml) was sampled
prior to treatment, at 5 days after VSD treatment and at 10 days
after VSD treatment. The levels of serum interleukin-6 (IL-6) and
tumor necrosis factor-α (TNF-α) were determined by ELISA. All kits
were purchased from R&D Systems. The presence of infection
and/or lower-limb DVT was observed. The signs of lower-limb DVT
included the presence of limb swelling, sharp pain, shank and
femoral triangle tenderness, dark-red skin, elevated skin
temperature, varicosity and positive results in the straight-leg
and ankle-extension tests diagnosed using ultrasound and
venography. The manifestations of infection were a red and swollen
wound, heat pain and wound sinus after abscess formation diagnosed
by bacterial culture and pathological tissue examination.
Statistical analysis
Data were analyzed with SPSS statistical software
(version 23.0; IBM Corp.). The measurement data were expressed as
the mean ± standard deviation. Independent-samples t-test was used
for the analysis of differences in measurement data. Enumeration
data were expressed as n (%) and the χ2 test was adopted
for the analysis of differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparability of clinical data
In EG, there were 22 males and 17 females, with an
average age of 41.65±7.21 years; In CG, there were 20 males and 17
females, with an average age of 42.37±7.39 years. There were no
significant differences detected in sex, average age, wound type,
trauma region or cause of trauma between the two groups
(P>0.05). The clinical data were comparable between the two
groups (Table I).
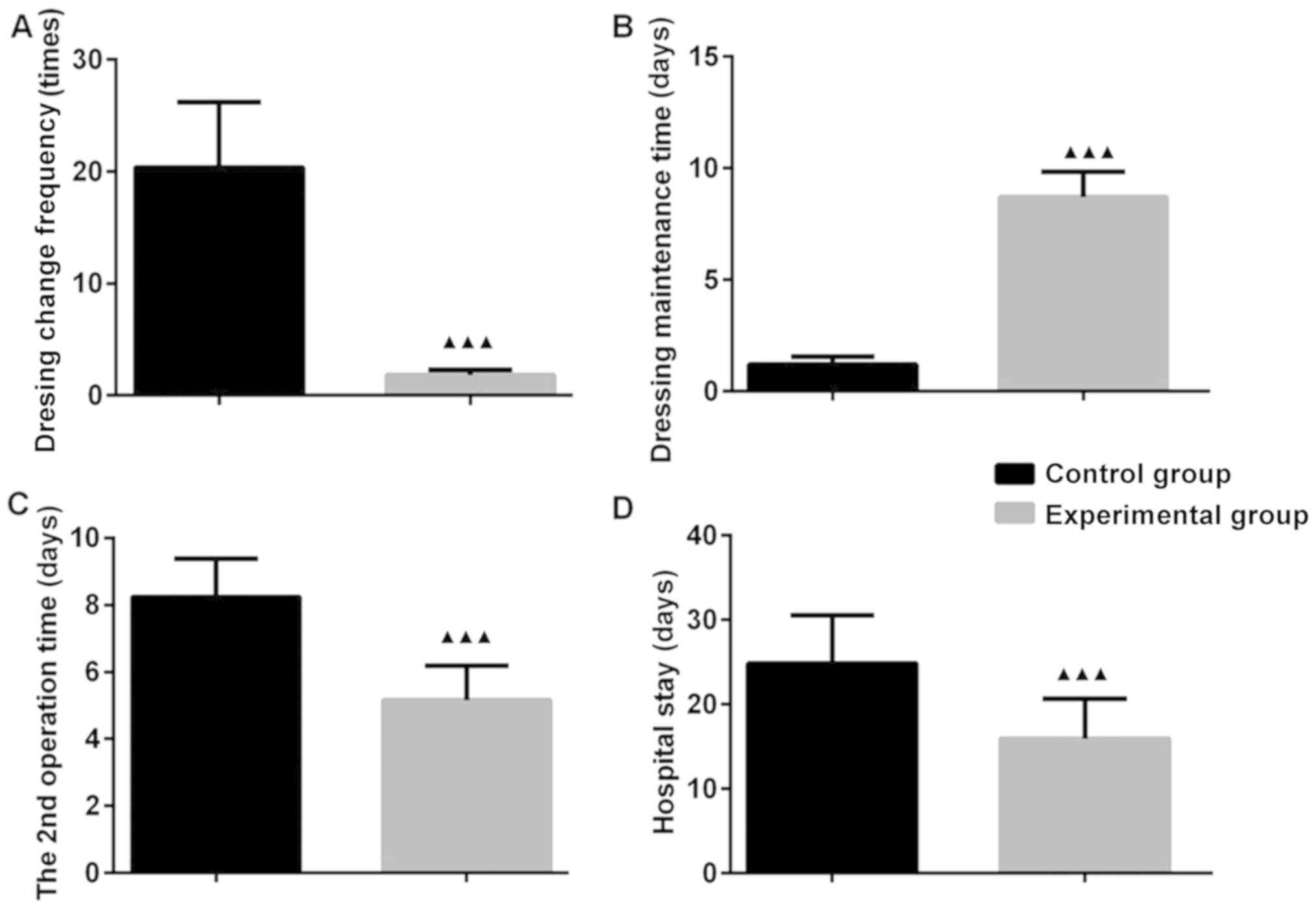
Acceleration of recovery time
The dressing change frequency in the EG was less
than that in the CG (P<0.05). The 2nd operation time and
hospital stay were shorter in the EG than in the CG (P<0.05).
The dressing maintenance time was longer in the EG than in the CG
(P<0.05). These results indicate that VSD markedly reduced the
dressing change frequency, increased the dressing maintenance time
and shortened the 2nd operation time and hospital stay. Thus, the
recovery of patients with OT was accelerated (Fig. 1).
Improvement of therapeutic effect
The total effective rate in the EG was higher than
that in the CG (P<0.05; Table
II). This implies that VSD is able to promote wound healing and
improve the clinical efficacy of treatment.
 | Table IIComparison of efficacy. |
Table II
Comparison of efficacy.
| Efficacy | Experimental group
(n=39) | Control group
(n=37) | t value | P-value |
|---|
| Cure | 26 (66.67) | 19 (51.35) | | |
| Improvement | 12 (30.77) | 10 (27.03) | | |
| Ineffectiveness | 1 (2.56) | 8 (21.62) | | |
| Total effective
rate | 38 (97.44) | 29 (78.38) | 4.906 | 0.01 |
Shortening of wound healing time
The wound healing time in the EG was shorter than
that in the CG (P<0.05; Table
III). This suggests that VSD is able to shorten the wound
healing time and accelerate the recovery of patients with OT.
 | Table IIIComparison of wound healing time. |
Table III
Comparison of wound healing time.
| Item | Experimental group
(n=39) | Control group
(n=37) | χ2/t
value | P-value |
|---|
| Wound healing
duration (weeks) | | | 8.902 | 0.031 |
|
1 | 18 (46.15) | 9 (24.32) | | |
|
2 | 15 (38.46) | 11 (29.73) | | |
|
3 | 5 (12.82) | 15 (40.54) | | |
|
>3 | 1 (2.56) | 2 (5.41) | | |
| Average healing
time (weeks) | 1.72±0.73 | 2.23±0.85 | 2.81 | 0.006 |
Reduction of the serum levels of
inflammatory factors
With the progression of the treatment, the levels of
serum IL-6 and TNF-α in the two groups decreased at days 5 and 10
after treatment, respectively, but the levels in the EG were lower
than those in the CG (P<0.05). This result indicates that VSD is
able to decrease the serum levels of inflammatory factors, reduce
wound inflammation and promote wound healing (Fig. 2).
Reduction of the incidence of
post-operative infection and lower-limb venous thrombosis
There were 5 cases of lower-limb venous thrombosis
in the CG, and no case lower-limb venous thrombosis in the EG was
observed. The incidence of post-operative infection and lower-limb
venous thrombosis in the EG were lower than those in the CG
(P<0.05). This implies that VSD may reduce the risk of
post-operative infection and lower-limb venous thrombosis (Table IV).
 | Table IVComparison of the incidence of
post-operative infection and lower limb deep venous thrombosis. |
Table IV
Comparison of the incidence of
post-operative infection and lower limb deep venous thrombosis.
| Event | Experimental group
(n=39) | Control group
(n=37) | χ2
value | P-value |
|---|
| Post-operative
infection | 3 (7.69) | 10 (27.03) | 5.006 | 0.025 |
| Lower limb venous
thrombosis | 0 (0) | 5 (13.51) | 3.656 | 0.018 |
Discussion
The number of patients with OT has increased with
socioeconomic developments. The repair of severely traumatized
tissue has become the emphasis and may cause difficulty in the
treatment of OT. In certain patients with OT with various types of
injury, including skin soft tissue injury, nerve injury and
vascular injury, the trauma is difficult to treat due to serious
contamination, a deep and large wound and a large amount of
exudates. Routine dressing changes may cause considerable pain and
a psychological burden in such patients. In addition, the
therapeutic effect is frequently unsatisfactory, the dressing
requires to be frequently replaced, wound healing is slow and
treatment is more difficult once infection occurs (8). A clinical study in 1,196 patients with
OT reported an incidence of infection of 2.01% (9). Open wound, wound drainage, wound
contamination and a long hospital stay may increase the risk of
wound infection (10). If primary
suture cannot be performed in patients with OT, the recovery from
trauma may be difficult and a large wound may remain. Furthermore,
the connective tissue is exposed during healing without protective
epithelial tissue. Delayed nonunion may occur if the wound is not
properly treated. This will confer a greater psychological and
economic burden to the patients. In the present study, VSD was used
for the treatment of OT. The results suggested that VSD is able to
reduce the dressing change frequency, shorten the operation time
and hospital stay, accelerate the wound healing process and reduce
post-operative infection and lower-limb DVT.
The materials used in VSD are polyethylene/ethanol
hydrated seaweed salt foam and polyurethane film, which produce a
semipermeable membrane. During treatment, the VSD material is
tailored so that its shape and area match those of the wound.
Thereafter, a tailored VSD dressing is used to completely cover the
wound. A drainage tube is connected and direct contact between the
drainage tube and the wound is avoided. After connecting the vacuum
suction device, negative pressure is established so that the
exudate may be suctioned out. Thereby, the bacteria, toxin and pus
on the wound surface may be removed (11). Furthermore, VSD is also able to
effectively clear the dead cavity and eliminate the survival
environment for pathogens. Thus, a good microenvironment is
provided for wound repair, which effectively prevents infection and
promotes wound healing (12). The
continuous suction of exudate during VSD treatment may obviate
frequent dressing changes and reduce the patient's pain (13). In addition, a relatively sealed,
clean and dry negative-pressure environment is formed after the
wound is covered with the VSD dressing. This contributes to the
dilation of the capillaries and accelerates and promotes the growth
of granulation tissue (14).
Negative pressure helps in controlling local edema, removing
inflammatory mediators, reconstructing blood vessels, forming
granulation and reducing the biological load of the wound (15). According to relevant studies
(16,17), VSD has achieved good results in the
treatment of scald wounds and avulsion injuries of limb skin and
soft tissues. It may effectively promote wound healing and improve
the quality of healing. The results of the present study indicated
that the dressing change frequency in the EG was less than that in
the CG. The 2nd operation time and hospital stay were shorter in
the EG than in the CG. A previous study has also suggested that VSD
may effectively shorten the hospital stay and reduce the dressing
change frequency compared with traditional pressure dressings in
the treatment of degloved skin injury (18). This result is similar to that of the
present study and indicates that VSD is able to markedly reduce the
dressing change frequency, accelerate wound healing and shorten the
2nd operation time and hospital stay in the treatment of OT without
primary suture. The effect was better than that of routine dressing
changes. In the present study, the total effective rate in the EG
was 97.44%, which was higher than the rate of 78.38% in the CG. A
previous study reported a total effective rate of 98% in 60
patients with OT and infected wounds treated with VSD (19). This rate was higher than that in the
traditional dressings group (75%). These results are consistent
with those of the present study, which indicated that the wound
healing time in the VSD group was markedly shorter than that in the
routine dressings group. Kaushik et al (20) reported that the complete wound
closure time and hospital stay were 12.5 and 17.3 days,
respectively, after the contaminated trauma wound was treated with
negative-pressure wound therapy. These were markedly shorter than
the corresponding durations of 21.4 and 23.8 days in the
conventional gauze dressing group, respectively. These results are
consistent with those of the present study and imply that VSD is
able to accelerate wound healing and shorten the wound healing
time.
Trauma may cause an inflammatory response. The
release of inflammatory factors into the blood may lead to an
abnormal increase in serum inflammatory factors. Upregulation of
serum IL-6 and TNF-α expression is observed in patients with OT.
Such upregulation is closely linked to the severity of the trauma
(21). The results of the present
study indicated that the expression of serum IL-6 and TNF-α
decreased in the two groups with the progression of the treatment,
but that the downregulation was more obvious in the EG. This
indicates that VSD was able to markedly reduce the degree of
inflammatory response and improve the condition of patients with
OT. Post-operative infection is a common complication in OT. The
post-operative infection rate of patients with open wounds was
reported to be up to 25-35% (22).
In the present study, the post-operative infection rate in the EG
was 7.69%, which was significantly lower than that in the CG
(27.03%). The reason may be that VSD promotes blood circulation in
the wound. In addition, the degree of wound edema is improved;
wound bacteria and their products are effectively eliminated;
necrotic tissue and abscess are removed; the wound is kept clean;
and bacterial proliferation is inhibited. The sealed environment
formed by the VSD dressing is able to temporarily isolate the wound
from the external environment, thus avoiding wound exposure.
Furthermore, the risk of infection is reduced (23). Lower-limb DVT is one of the serious
complications in patients with OT. The manifestations are limb
swelling, pain, varicosity, exposure and muscle tenderness
(24). Patients with OT are usually
subjected to violent forces and experience serious traumatic
extrusion. After the trauma, a long period of inactivity is
required. This leads to poor venous circulation, serious vessel
congestion or damaged vascular wall, an activated coagulation
mechanism, platelet aggregation and changes in hemodynamics. As a
result, lower-limb DVT frequently occurs (25). The present study indicated that the
incidence of lower-limb DVT was lower in the EG than in the CG. The
reason may be that VSD is able to effectively shorten the wound
healing time, reduce the period of inactivity and lower the
occurrence and risk of lower-limb DVT; however, the specific
mechanisms require to be further investigated.
There are certain limitations to this study. All
patients were treated with antibiotics, but no statistical analyses
were performed on the effect of different antibiotics on wound
healing. This is required to be further observed in a future
study.
In conclusion, VSD in the treatment of OT may reduce
the dressing change frequency, shorten the operation time and
hospital stay, accelerate wound healing and reduce post-operative
infection and lower-limb venous thrombosis. Therefore, the VSD
treatment method is worthy of promotion and implementation in
clinic.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LZ, YZ and YS conceived the study and designed the
experiments. YL and PH contributed to the data collection, PZ and
ZL performed the data analysis and interpreted the results. LZ and
YZ wrote the manuscript. YS contributed to the critical revision of
the article. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent. The
present study was approved by the Ethics Committee of the Second
Affiliated Hospital of Soochow University (Suzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mannino BJ, Pullen MW and Gaines R:
Preventing seal leak during negative pressure wound therapy near
external fixators: A Technical tip. J Orthop Trauma. 31:e101–e102.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chagas MQ, Costa AM, Mendes PH and Gomes
SC: Analysis of surgical site infections in pediatric patients
after orthopedic surgery: A case-control study. Rev Paul Pediatr.
35:18–24. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Walker ME, Tsay C, Broer PN, Zhu VZ,
Sturrock T, Ng R, Scoutt LM, Thomson JG and Kwei SL: A prospective,
randomized-controlled pilot study comparing closed suction versus
negative pressure drains for panniculectomy patients. J Plast
Reconstr Aesthet Surg. 71:438–439. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Webb LX: The impact of negative pressure
wound therapy on orthopaedic infection. Orthop Clin North Am.
48:167–179. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Davis J, Caruso DM, Foster KN and Matthews
MR: A novel approach to sealing the denuded dermis of the abdominal
wall with a negative pressure wound device after a decompressive
laparotomy. J Burn Care Res. 39:838–842. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mohsin M, Zargar HR, Wani AH, Zaroo MI,
Baba PU, Bashir SA, Rasool A and Bijli AH: Role of customised
negative-pressure wound therapy in the integration of
split-thickness skin grafts: A randomised control study. Indian J
Plast Surg. 50:43–49. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu F, Luo Q and Liang Z: The principle of
vacuum-assisted closure and wound repair. Chin J Inj Repair Wound
Healing. 3:487–492. 2008.
|
|
8
|
Engelhardt M, Rashad NA, Willy C, Müller
C, Bauer C, Debus S and Beck T: Closed-incision negative pressure
therapy to reduce groin wound infections in vascular surgery: A
randomised controlled trial. Int Wound J. 15:327–332.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Robert N: Negative pressure wound therapy
in orthopaedic surgery. Orthop Traumatol Surg Res. 103:S99–S103.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bcc R and Wenke JC: An effective negative
pressure wound therapy-1 compatible local antibiotic delivery
device. J Orthop Trauma. 31(1)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Green T, Kavros S, Springer S, Drez D Jr,
McCabe M and Gremillion J: Team approach: Complex dermal
wound-healing utilizing negative-pressure wound therapy (NPWT) in
orthopaedic trauma. JBJS Rev. 6(e1)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nam D, Sershon RA, Levine BR and Della
Valle CJ: The use of closed incision negative-pressure wound
therapy in orthopaedic surgery. J Am Acad Orthop Surg. 26:295–302.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chang FS, Chou C, Hu CY and Huang SH:
Suture technique to prevent air leakage during negative-pressure
wound therapy in fournier gangrene. Plast Reconstr Surg Glob Open.
6(e1650)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shim HS, Choi JS and Kim SW: A role for
postoperative negative pressure wound therapy in multitissue hand
injuries. BioMed Res Int. 2018(3629643)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bazaliński D, Więch P, Kaczmarska D,
Sałacińska I and Kózka M: Use of controlled negative pressure in
management of phlegmon caused by fulminant complication of pressure
wound: A case report. Medicine (Baltimore).
97(e11319)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Angspatt A, Laopiyasakul T, Pungrasmi P
and Suwajo P: The role of negative-pressure wound therapy in
latissimus dorsi flap donor site seroma prevention: A cohort study.
Arch Plast Surg. 44:308–312. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Philip B, McCluskey P and Hinchion J:
Experience using closed incision negative pressure wound therapy in
sternotomy patients. J Wound Care. 26:491–495. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Masters JPM, Achten J, Cook J, Dritsaki M,
Sansom L and Costa ML: Randomised controlled feasibility trial of
standard wound management versus negative-pressure wound therapy in
the treatment of adult patients having surgical incisions for hip
fractures. BMJ Open. 8(e020632)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Saku I, Kanda S, Saito T, Fukushima T and
Akiyama T: Wound management with negative pressure wound therapy in
postoperative infection after open reconstruction of chronic
Achilles tendon rupture. Int J Surg Case Rep. 37:106–108.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kaushik D, Joshi N, Kumar R, Gaba S, Sapra
R and Kumar K: Negative pressure wound therapy versus gauze
dressings for the treatment of contaminated traumatic wounds. J
Wound Care. 26:600–606. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Novelli G, Daleffe F, Birra G, Canzi G,
Mazzoleni F, Boni P, Maino C, Giussani C, Sozzi D and Bozzetti A:
Negative pressure wound therapy in complex cranio-maxillofacial and
cervical wounds. Int Wound J. 15:16–23. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ludolph I, Fried FW, Kneppe K, Arkudas A,
Schmitz M and Horch RE: Negative pressure wound treatment with
computer-controlled irrigation/instillation decreases bacterial
load in contaminated wounds and facilitates wound closure. Int
Wound J. 15:978–984. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bi H, Li J, Xue C and Marks M: early
closure of infected laparotomy wound with negative-pressure wound
therapy: Safety and efficacy in 42 consecutive cases. Am Surg.
84:938–946. 2018.PubMed/NCBI
|
|
24
|
Bazaliński D, Więch P, Barańska B and
Binkowska-Bury M: Use of negative pressure wound therapy in a
chronic leg wound with coexisting rheumatoid arthritis: A case
study. J Int Med Res. 46:2495–2499. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Izadpanah K, Hansen S, Six-Merker J,
Helwig P, Südkamp NP and Schmal H: Factors influencing treatment
success of negative pressure wound therapy in patients with
postoperative infections after osteosynthetic fracture fixation.
BMC Musculoskelet Disord. 18(247)2017.PubMed/NCBI View Article : Google Scholar
|