Introduction
Acute coronary syndrome (ACS) presents a major cause
of mortality and economic burden in the world and accounts for
>2.5 million hospitalizations annually worldwide (1,2).
Approximately 15% of patients with ACS experience recurrent
cardiovascular events within one year (3). The etiology and pathogenesis of this
disease are complex and the morbidity increases with age as well as
with the presence of risk factors such as hypertension and smoking
(4,5). Numerous studies suggested that innate
immunity and inflammatory responses have important roles in the
occurrence and development of ACS (6-8).
α1-antitrypsin (AAT) deficiency was first identified
by paper electrophoresis in 1963 by Laurel and Eriksson (9). AAT is an acute-phase protein that is
mainly produced in the liver and it is expressed in neutrophils,
monocytes, macrophages, alveolar macrophages, intestinal epithelial
cells, cancer cells and corneal cells (9). It is also a serine protease inhibitor
which circulates in healthy individuals and is usually increased in
most inflammatory diseases such as ACS (10). The normal AAT plasma level is 1.04
g/l or ~20 µM (11) and this may
increase by 3-5-fold when an inflammatory reaction occurs (12).
An increase in AAT expression may also contribute to
activation of signaling events that initiate the production and
release of pro-inflammatory cytokines and adhesion molecules, such
as lipopolysaccharides, TNF-α, IL-1 and IL-6, which are released by
neutrophils, monocytes, macrophages and alveolar macrophages that
may in turn activate innate immunity and inflammatory responses
(13-15).
A number of studies have suggested that AAT is associated with the
development of chronic hepatitis, liver cirrhosis (16), chronic obstructive pulmonary disease
(17), atherosclerotic diseases
(18), tumors (19) and autoimmune diseases (20).
Plasma AAT levels have been demonstrated to
correlate with both the presence and severity of coronary stenosis
in patients with stable angina pectoris (SA) (21). However, at present, available
studies on the association between plasma AAT concentrations and
the severity of ACS are sparse and insufficient (21). Thus, it remains elusive whether
plasma AAT is correlated with ACS. The AAT concentration is likely
to be different in patients with ACS as they can express differing
pathological features. It remains elusive whether an increase or a
decrease in plasma AAT is an independent predictor for the severity
of coronary atherosclerosis in patients with ACS. Accordingly, it
was hypothesized in the present study that decreases in plasma AAT
levels in patients with ACS may be a predictor for the risk and
severity of the disease. In order to test this hypothesis, plasma
AAT levels were first compared between patients with SA and
controls. It was further investigated whether there was a
correlation between AAT levels and Gensini scores in patients with
ACS.
Patients and methods
Study population
A total of 117 cases (36 control subjects, 35
patients with SA and 46 ACS patients) who underwent coronary
angiography between March 2017 and April 2018 at the Affiliated
Hospital of Youjiang Medical University for Nationalities (Baise,
China) were enrolled.
Exclusion criteria
The exclusion criteria were as follows: rheumatic
heart disease, dilated cardiomyopathy, congenital heart disease,
patients undergoing intravenous thrombolysis, coronary stenting and
coronary artery bypass grafting, systemic or local severe
infection, auto-immunologic and blood system diseases, severe
kidney or liver disease and malignancies.
Coronary angiography (CAG)
Coronary artery disease (CAD) was defined as
patients with ≥50% of luminal stenosis in at least one major
coronary vessel and major branches (the left main, left anterior
descending, left circumflex and right coronary arteries) based on
the result of CAG, which was determined as agreed by two
experienced cardiologists. According to the number of diseased
vessels based on the results of the CAG, patients were classified
into 1-vessel, 2-vessel and multiple-vessel disease groups. If the
left main coronary artery was affected by 2-vessel disease and it
was combined with the right coronary artery, this was referred to
as multiple-vessel disease.
The severity of coronary lesions was assessed by
determining the Gensini score (GS) (22), which was calculated according to the
severity of stenosis as follows: 1 point for <25% stenosis, 2
points for 26-50% stenosis, 4 points for 51-75% stenosis, 8 points
for 76-90% stenosis and 32 points for complete occlusion. The
scores were then multiplied by a coefficient representing the
importance of the lesion's position in the coronary artery system.
Control subjects also underwent a CAG and were confirmed to be free
of coronary artery stenosis. In addition, these patients did not
exhibit any clinical or electrocardiographic evidence of myocardial
infarction or CAD.
Clinical data and laboratory
tests
Clinical data, including age, sex, body mass index
(BMI), smoking status (smokers were defined as smoking at least one
cigarette per day for >1 year), drinking status (drinkers were
defined as consuming at least one alcoholic drink per day for a
minimal period of six months), hypertension, diabetes, total
cholesterol (TC), triglycerides (TG), high-density lipoprotein
cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C),
very low-density lipoprotein cholesterol (VLDL-C), apolipoprotein
A1, apolipoprotein B, lipoprotein a, homocysteine, hemoglobin A1C
(HbA1C), uric acid (UA), platelets (PLT), high-sensitivity
C-reactive protein (hs-CRP), aspartate aminotransferase (AST),
alanine aminotransferase (ALT) and γ-glutamyl transferase (GGT)
were obtained from all participants.
AAT protein assay
Blood was collected aseptically from the caudal vein
by venipuncture into one of three vacutainer tubes containing
either sodium heparin, sodium citrate or EDTA. These tubes were
immediately centrifuged at 375 x g for 15 min at 4˚C and samples
were prepared or analyzed within 45 min of collection. Aliquots
were frozen at -80˚C for determination of AAT by ELISA using a
commercially available kit purchased from Enzyme-linked
Biotechnology Co., Ltd. (cat. no. ml057793). The intra- and
inter-assay coefficient of variation for the ELISA kit for AAT was
determined to be <10 and <15%, respectively. The detection
range of the ELISA was 125-4,000 µg/ml. AAT levels in the plasma
were determined according to the manufacturer's protocol.
Statistical analysis
All data were analyzed with SPSS 22.0 (IBM Corp.)
and GraphPad Prism 5.0 (GraphPad Software, Inc.). The data were
first tested for normality using the Kolmogorov-Smirnov test and if
they were normally distributed, the variables were expressed as the
mean ± standard deviation and were compared by t-test or one-way
ANOVA. For ANOVA, Fisher's least significant difference method was
used as the post hoc test. Otherwise, the data with a non-normal
distribution were expressed as the medians (interquartile range)
and were compared by the Kruskal-Wallis test. Categorical variables
were expressed as n (%) and were compared with the χ2
test. A two-tailed P<0.05 was considered to indicate statistical
significance.
Results
Characteristics of the study
participants
The basic biochemical parameters and clinical
characteristics of all subjects are summarized in Table I. A total of 36 patients with SA, 46
patients with ACS and 35 healthy controls were enrolled in the
present study.
 | Table ICharacteristics of the participants
enrolled in the study. |
Table I
Characteristics of the participants
enrolled in the study.
| Characteristics | Controls (n=36) | SA (n=35) | ACS (n=46) | χ2
(F) | P-value |
|---|
| Male sex | 22 (61.1) | 24 (68.6) | 32 (69.6) | 0.731 | 0.694 |
| Smoking | 7 (19.4) | 5 (14.3) | 14 (30.4) | 3.231 | 0.199 |
| Drinking | 19 (52.8) | 25 (71.4) | 36
(78.3)a | 6.280 | 0.043 |
| Hypertension | 2 (5.6) | 17
(48.6)a | 27
(58.7)a | 25.696 | <0.001 |
| Diabetes | 0 (0) | 10
(28.6)a | 5
(10.9)b | 13.220 | 0.001 |
| Smoking
(n)c | 1.72±0.74 | 2.17±0.95 | 3.13±1.07 | 0.595 | 0.553 |
| Age (years) | 57.17±9.49 | 62.57±6.20 | 59.43±9.47 | 3.170 | 0.046 |
| SBP (mmHg) | 126.72±1.44 | 133.69±3.22 |
142.54±2.90a,b | 9.058 | <0.001 |
| DBP (mmHg) | 78.78±1.41 | 82.20±1.76 |
85.48±1.75a | 4.118 | 0.019 |
| MAP (mmHg) | 94.76±1.17 | 99.36±2.07 |
104.5±1.97a,b | 7.421 | 0.001 |
| BMI
(kg/m2) | 23.14±0.44 | 23.47±0.45 | 24.61±0.63 | 2.179 | 0.118 |
| AG (mmol/l) | 4.96±0.11 |
7.21±0.40a |
7.03±0.40a | 12.443 | <0.001 |
| PG (mmol/l) | 6.98±0.23 |
10.50±0.67a |
9.50±0.64a | 9.363 | <0.001 |
| HbA1C (%) | 5.21±0.16 | 5.71±0.28 |
5.80±0.13a | 2.749 | 0.068 |
| UA (µmol/l) | 308.33±13.81 |
379.23±16.77a |
319.69±14.30b | 6.164 | 0.003 |
| TC (mmol/l) | 4.63±0.19 | 4.24±0.25 | 4.11±0.15 | 1.959 | 0.146 |
| TG (mmol/l) | 1.94±0.18 | 2.04±0.38 | 1.47±0.09 | 1.973 | 0.144 |
| LDL-C (mmol/l) | 2.63±0.15 | 2.40±0.16 | 2.66±0.15 | 0.814 | 0.446 |
| HLDL-C
(mmol/l) | 1.21±0.07 | 1.22±0.04 | 1.17±0.04 | 0.224 | 0.800 |
| VLDL-C
(mmol/l) | 0.63±0.04 | 0.61±0.04 | 0.68±0.04 | 0.779 | 0.461 |
| Apolipoprotein A1
(g/l) | 1.49±0.15 | 1.42±0.05 | 1.37±0.04 | 0.584 | 0.560 |
| Apolipoprotein B
(g/l) | 0.94±0.09 | 0.83±0.04 | 0.81±0.04 | 0.516 | 0.599 |
| Lipoprotein a
(nmol/l) | 47.28±18.58 | 225.56±44.82 | 341.80±51.00 | 2.614 | 0.079 |
| Homocysteine
(µmol/l) | 12.14±0.48 |
14.60±0.61a |
15.23±0.77a | 5.980 | 0.003 |
| hs-CRP (mg/l) | 2.85±0.91 | 5.20±1.64 |
8.12±1.62a | 3.356 | 0.038 |
| PLT
(x109/l) | 203.39±12.42 | 202.23±11.08 | 228.93±9.14 | 2.116 | 0.125 |
| AST (U/l) | 29.01±6.91 | 27.89±7.21 | 28.91±10.38 | 0.203 | 0.816 |
| ALT (U/l) | 28.94±6.54 | 29.03±7.04 | 26.8±8.37 | 1.197 | 0.306 |
| GGT (U/l) | 30.72±6.39 | 31.29±7.37 | 32.87±10.97 | 0.677 | 0.510 |
| Gensini score | 0 |
30.97±5.45a |
32.74±2.73a | 19.295 | <0.001 |
As for the SA patients, 11 (31.4%) were females and
24 (68.6%) were males, with a mean age of 62.57±6.20 years (range,
44-75 years). Among the ACS patients, 14 (30.4%) were females and
32 (69.6%) were males, with a mean age of 59.43±9.47 years (range,
31-79 years). With respect to the healthy controls, 14 (38.9%) were
females and 22 (61.1%) were males, with a mean age of 57.17±9.49
years (range, 28-79 years). There were no statistically significant
differences between the controls and patient groups in terms of
sex, smoking, BMI, TC, TG, HDL-C, VLDL-C, apolipoprotein A1,
apolipoprotein B, lipoprotein a, PLT and HbA1c. Compared with the
SA group, the ACS group had a larger proportion of individuals with
hypertension, but less cases of diabetes. Significantly higher
values for systolic blood pressure, diastolic blood pressure, mean
arterial blood pressure, homocysteine, admission glucose,
postprandial blood glucose and hs-CRP were observed in the ACS
group when compared to the controls. There were significant
differences between the SA and ACS groups with respect to systolic
blood pressure, mean arterial blood pressure and UA. No significant
differences in AST, ALT and GGT levels were present between the
groups.
Associations between plasma AAT
protein levels and different types of CAD
A comparison of the plasma levels of AAT protein
between the different types of CAD and the control group was
performed. AAT was determined by ELISA of the plasma of all 117
patients. The results indicated that the plasma concentrations of
AAT in the SA group were significantly higher than those in the ACS
group [867.34 (588.48-1,156.53) vs. 491.33 (242.02-827.93) ng/ml;
P<0.05; Table II]. These
results show that the levels of AAT were higher in the control
group, indicating that the levels of this metabolite decreased with
the severity of the pathology of CHD.
 | Table IIPlasma levels of AAT protein in
patients with different types of coronary artery disease. |
Table II
Plasma levels of AAT protein in
patients with different types of coronary artery disease.
| Group | n | AAT (ng/ml) |
|---|
| Control | 36 | 1,264.98
(1,033.88-1,711.67) |
| SA | 35 | 867.34
(588.48-1,156.53)a |
| ACS | 46 | 491.33
(242.02-827.93)a,b |
| Z | 48.647 | |
| P-value | <0.001 | |
Association between the levels of AAT
and the number of diseased vessels
A comparison of the expression levels of AAT between
different groups according to the number of coronary lesions was
also made. The concentrations of AAT in the plasma were 784.19
(421.73-1,205.71), 776.97 (505.33-925.44) and 531.67
(258.85-984.59) ng/ml in the single, double and multi-lesion
groups, respectively (P>0.05, Table III). The lowest expression of AAT
was in the multi-vessel lesion group, followed by the double-vessel
lesion group and the highest expression was in the single-vessel
lesion group, but the inter-group differences did not reach
statistical significance (P>0.05).
 | Table IIIAssociation between plasma levels of
AAT in patients with coronary heart disease and the number of
diseased vessels. |
Table III
Association between plasma levels of
AAT in patients with coronary heart disease and the number of
diseased vessels.
| Number of
vessels | n | AAT (ng/ml) |
|---|
| 1 | 20 | 784.19
(421.73-1,205.71) |
| 2 | 26 | 776.97
(505.33-925.44) |
| >2 | 35 | 531.67
(258.85-984.59) |
| Z | 2.347 | |
| P-value | 0.309 | |
Association between AAT levels and the
severity of coronary artery stenosis
A comparison of the expression levels of AAT between
different groups according to the degree of coronary lesions was
also made. The concentrations of AAT in the plasma were 400.92
(217.47-990.27), 789.79 (531.67-1,213.33) and 599.09
(361.26-984.59) ng/ml in the mild, moderate and severe stenosis
group, respectively. Most of the patients had severe stenosis
(62.96% of the total), but the highest AAT levels were in the
moderate stenosis group. However, there were no statistically
significant differences among these groups (Table IV).
 | Table IVAssociation between the plasma levels
of AAT in patients with coronary artery disease and the degree of
coronary artery stenosis. |
Table IV
Association between the plasma levels
of AAT in patients with coronary artery disease and the degree of
coronary artery stenosis.
| Severity of
stenosis | n | AAT (ng/ml) |
|---|
| Mild | 7 | 400.92
(217.47-990.27) |
| Moderate | 23 | 789.79
(531.67-1,213.33) |
| Severe | 51 | 599.09
(361.26-984.59) |
| Z | 3.092 | |
| P-value | 0.213 | |
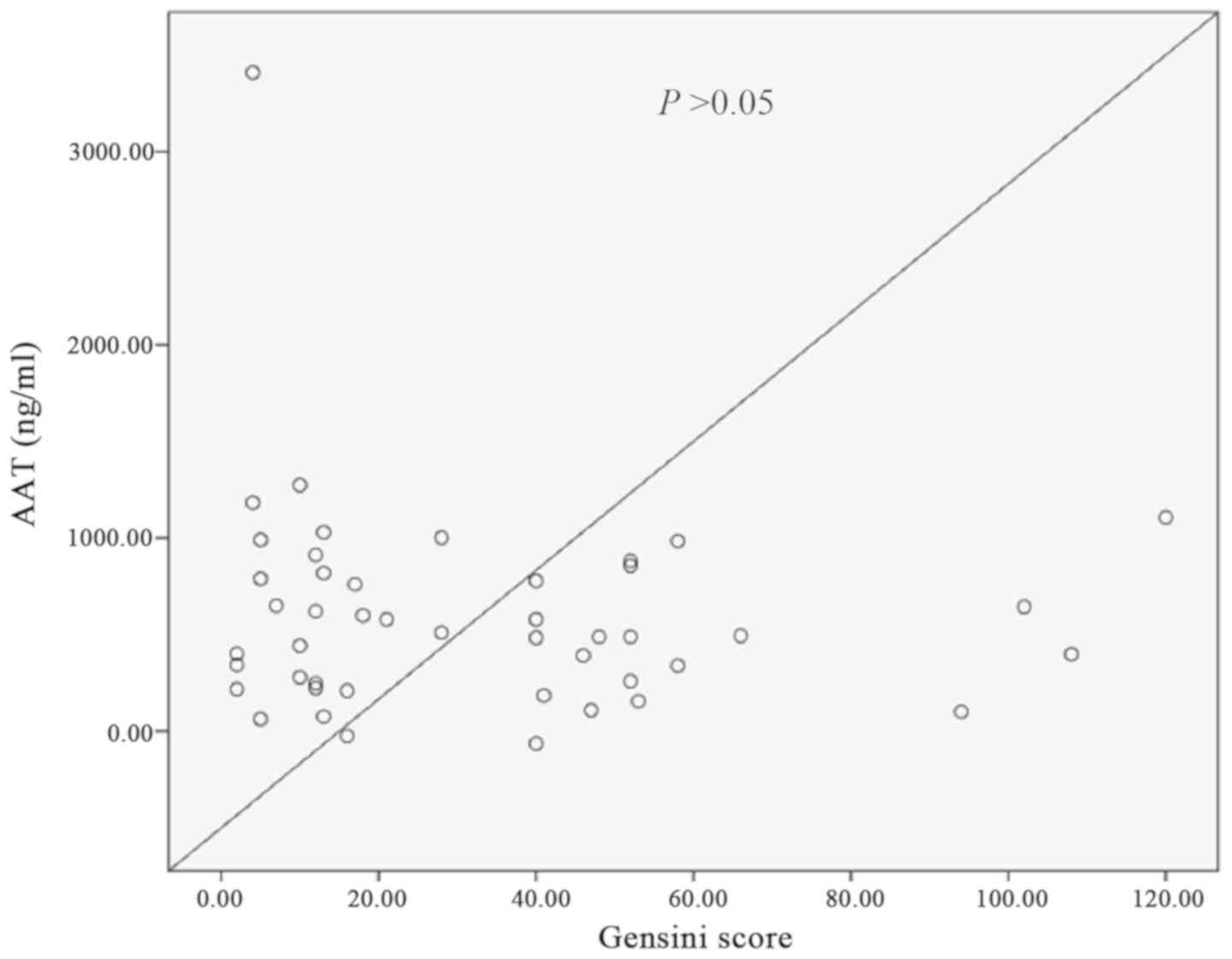
Correlation between AAT levels and
coronary Gensini scores in ACS patients
There was no clear correlation between AAT and the
coronary Gensini scores in patients with ACS and this did not reach
statistical significance (Fig.
1).
Discussion
CAD is considered to be a chronic inflammatory
disease of the blood vessels, which is a disorder influenced by a
combination of multiple factors (23,24).
To date, several risk factors have been identified to be associated
with CAD (25,26), including age, smoking, diabetes,
hypertension, obesity and dyslipidemia as well as a high-fat or
high-cholesterol diet. The results of the present study are
consistent with those of other studies, suggesting that patients
with CAD were older and had higher blood glucose, blood pressure,
UA and homocysteine (P<0.05). However, these risk factors are
only able to partially explain the occurrence and development of
CAD. The molecular mechanisms of the pathogenesis of CAD remain to
be fully elucidated. Studies have also indicated that inflammatory
factors have an important function in the molecular mechanisms
associated with the pathogenesis of CAD, particularly in cases of
ACS (27,28).
ACS is a syndrome of coronary atherosclerosis,
erosion, thrombosis and other factors leading to obstruction and
poor blood flow. ACS is commonly encountered at cardiology
departments. Inflammatory factors cause atherosclerotic plaques to
develop and become unstable, leading to thrombosis and resulting in
obstruction of the coronary arteries (29). Studies have indicated that ACS is an
inflammation-mediated atherosclerotic disease and that inflammation
and immune responses have an important role at all stages of
atherosclerosis (30).
Hs-CRP is an acute phase-reactive protein
synthesized in the liver (31-33).
Only a small amount of acute phase-reactive protein is present in
the serum of healthy humans. However, during acute myocardial
infarction, development of tumors and periods of infection,
hepatocytes are stimulated to synthesize and secrete inflammatory
factors, resulting in severe symptoms and increases in the serum
concentration of hs-CRP (34). The
present results indicated that hs-CRP levels in the ACS group were
significantly higher than those in the SA and control groups
(P<0.05). This further confirms that ACS is an
inflammation-mediated disease.
AAT is produced mainly in the liver. It is a serine
protease inhibitor synthesized by hepatocytes, which may also be
synthesized by monocytes, alveolar macrophages and epithelial cells
(35). Its molecular weight is 52
kDa and the concentration of AAT in the human body varies with the
inhibition of protease phenotypes (36,37).
AAT is able to inhibit >80-90% of protease activity in normal
plasma (38,39). It is one of the most important
members of a family of protease inhibitors in the human body. It is
able to inhibit numerous serine-centered proteases, particularly
neutrophil elastase, as well as trypsin, chymotrypsin, urokinase,
renin, collagenase, fibrinolytic enzyme and thrombin-releasing
enzyme (38).
AAT is able to inhibit protease-induced tissue
damage during the inflammatory response. As AAT is an acute-phase
reactive protein, an increase in plasma AAT levels in patients with
ACS may be the result of the body being in an inflammatory state.
In 1983, Gilutz et al (40)
first confirmed a rise in plasma AAT levels in patients with acute
myocardial infarction. Subsequently, Brunetti et al
(41) detected elevated AAT levels
in the plasma of patients with unstable angina pectoris. In 2015,
Zhao et al (21) first
indicated that the plasma concentrations of AAT in patients with
stable angina pectoris were significantly higher than those in a
healthy control population and positively correlated with the
severity of coronary artery stenosis.
However, the results of the present study were
opposite to these findings. The plasma AAT levels of patients with
ACS were lower than those in the SA and healthy control groups
(P<0.001). Furthermore, AAT levels were not correlated with the
coronary Gensini score. In addition, there was no significant
association between AAT levels and the number of diseased vessels
or the disease severity. AAT is an acute phase-reactive protein and
inflammation tends to increase its levels, but the plasma levels
cannot always simultaneously reflect this. In addition, as an acute
phase-reactive protein, AAT is able to inhibit serine proteases and
endogenous inhibitors of neutrophil elastase, which may, in turn,
inhibit protease-induced tissue damage during the inflammatory
response. Thus, when it is deficient, its function will
disappear.
The major limitation of the present study is the
small sample size. This shortcoming will be remedied in the next
study by our group; it is now possible to recruit more patients
with ACS, as a Chest Center has been established at our
hospital.
In conclusion, the plasma levels of AAT protein may
contribute to the occurrence and development of CAD, particularly
that of ACS. However, there were no significant associations the
plasma levels of AAT protein and the number of coronary vessels
affected or degree of stenosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Baise Science and Technology Cooperation Project Foundation of
Guangxi Province, China (grant no. 20150819) and a self-financing
research project by the Guangxi Zhuang Autonomous Region Health and
Family Planning Commission (grant no. 22016420).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, DH and BL were involved in the acquisition,
analysis and interpretation of the data. YL, DH, SRS and BL also
contributed to the design and conception of the study. WL, ZH, JG
and XP conceived the study and participated in its design and
coordination. ZH, SRS and JG drafted the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Youjiang Medical University
for Nationalities (Baise, China), in accordance with the
Declaration of Helsinki. All participants provided written informed
consent to participate in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Makki N, Brennan TM and Girotra S: Acute
coronary syndrome. J Intensive Care Med. 30:186–200.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Grech ED and Ramsdale DR: Acute coronary
syndrome: Unstable angina and non-ST segment elevation myocardial
infarction. BMJ. 326:1259–1261. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wachira JK and Stys TP: Cardiovascular
disease and bridging the diagnostic gap. S D Med. 66:366–369.
2013.PubMed/NCBI
|
|
4
|
Ofori-Asenso R, Zomer E, Chin KL, Markey
P, Si S, Ademi Z, Curtis AJ, Zoungas S and Liew D: Prevalence and
impact of non-cardiovascular comorbidities among older adults
hospitalized for non-ST segment elevation acute coronary syndrome.
Cardiovasc Diagn Ther. 9:250–261. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dai X, Busby-Whitehead J and Alexander KP:
Acute coronary syndrome in the older adults. J Geriatr Cardiol.
13:101–108. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mao X, Zhu R, Zhang F, Zhong Y, Yu K, Wei
Y, Sun H, Xu W, Luo Q, Wang Y, et al: IL-37 Plays a beneficial role
in patients with acute coronary syndrome. Mediators Inflamm.
2019(9515346)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vroegindewey MM, Oemrawsingh RM, Kardys I,
Asselbergs FW, van der Harst P, Oude Ophuis AJ, Etienne Cramer G,
Maas A, Hong Kie The S, Wardeh AJ, et al: The temporal pattern of
immune and inflammatory proteins prior to a recurrent coronary
event in post-acute coronary syndrome patients. Biomarkers.
24:199–205. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wyss CA, Neidhart M, Altwegg L, Spanaus
KS, Yonekawa K, Wischnewsky MB, Corti R, Kucher N, Roffi M, Eberli
FR, et al: Cellular actors, Toll-like receptors, and local cytokine
profile in acute coronary syndromes. Eur Heart J. 31:1457–1469.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Laurell CB and Eriksson S: The
electrophoretic pattern α1-globulin pattern of serum in
α1-antitrypsin deficiency. 1963. COPD. 10 (Suppl 1):S3–S8.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ma H, Lu Y, Li H, Campbell-Thompson M,
Parker M, Wasserfall C, Haller M, Brantly M, Schatz D, Atkinson M
and Song S: Intradermal alpha1-antitrypsin therapy avoids fatal
anaphylaxis, prevents type 1 diabetes and reverses hyperglycaemia
in the NOD mouse model of the disease. Diabetologia. 53:2198–2204.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Reeves EP, Dunlea DM, McQuillan K, O'Dwyer
CA, Carroll TP, Saldova R, Akepati PR, Wormald MR, McElvaney OJ,
Shutchaidat V, et al: Circulating truncated alpha-1 antitrypsin
glycoprotein in patient plasma retains anti-inflammatory capacity.
J Immunol. 202:2240–2253. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen YH, Wu KJ, Wu KL, Wu KL, Tsai HM,
Chen ML, Chen YW, Hsieh W, Lin CM and Wang Y: Recombinant
adeno-associated virus-mediated expression of methamphetamine
antibody attenuates methamphetamine-induced hyperactivity in mice.
Sci Rep. 7(46301)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Knoell DL, Ralston DR, Coulter KR and
Wewers MD: Alpha 1-antitrypsin and protease complexation is induced
by lipopolysaccharide, interleukin-1beta, and tumor necrosis
factor-alpha in monocytes. Am J Respir Crit Care Med. 157:246–255.
1998.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Abecassis A, Schuster R, Shahaf G, Ozeri
E, Green R, Ochayon DE, Rider P and Lewis EC: α1-antitrypsin
increases interleukin-1 receptor antagonist production during
pancreatic islet graft transplantation. Cell Mol Immunol.
11:377–386. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gottlieb PA, Alkanani AK, Michels AW,
Lewis EC, Shapiro L, Dinarello CA and Zipris D: α1-Antitrypsin
therapy downregulates toll-like receptor-induced IL-1β responses in
monocytes and myeloid dendritic cells and may improve islet
function in recently diagnosed patients with type 1 diabetes. J
Clin Endocrinol Metab. 99:E1418–E1426. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang D, Huang J, Luo D, Feng X and Liu Y
and Liu Y: Glycosylation change of alpha-1-acid glycoprotein as a
serum biomarker for hepatocellular carcinoma and cirrhosis. Biomark
Med. 11:423–430. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hennawy MG, Elhosseiny NM, Sultan H,
Abdelfattah W, Akl Y, Sabry NA and Attia AS: The effect of
α1-antitrypsin deficiency combined with increased
bacterial loads on chronic obstructive pulmonary disease
pharmacotherapy: A prospective, parallel, controlled pilot study. J
Adv Res. 7:1019–1028. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dichtl W, Moraga F, Ares MP, Crisby M,
Nilsson J, Lindgren S and Janciauskiene S: The carboxyl-terminal
fragment of alpha1-antitrypsin is present in atherosclerotic
plaques and regulates inflammatory transcription factors in primary
human monocytes. Mol Cell Biol Res Commun. 4:50–61. 2000.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhao Z, Ma J, Mao Y, Dong L, Li S and
Zhang Y: Silence of α1-Antitrypsin inhibits migration and
proliferation of triple negative breast cancer cells. Med Sci
Monit. 24:6851–6860. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Song S: Alpha-1 antitrypsin therapy for
autoimmune disorders. Chronic Obstr Pulm Dis. 5:289–301.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao H, Liu H, Chai L, Xu P, Hua L, Guan
XY, Duan B, Huang YL and Li YS: Plasma α1-antitrypsin: A neglected
predictor of angiographic severity in patients with stable angina
pectoris. Chin Med J (Engl). 128:755–761. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gensini GG: A more meaningful scoring
system for determining the severity of coronary heart disease. Am J
Cardiol. 51(606)1983.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Khera AV and Kathiresan S: Genetics of
coronary artery disease: Discovery, biology and clinical
translation. Nat Rev Genet. 18:331–344. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Khera AV, Emdin CA, Drake I, Natarajan P,
Bick AG, Cook NR, Chasman DI, Baber U, Mehran R, Rader DJ, et al:
Genetic risk, adherence to a healthy lifestyle, and coronary
disease. N Engl J Med. 375:2349–2358. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huxley RR, Hirakawa Y, Hussain MA,
Aekplakorn W, Wang X, Peters SA, Mamun A and Woodward M: Age- and
Sex-specific burden of cardiovascular disease attributable to 5
major and modifiable risk factors in 10 asian countries of the
western pacific region. Circ J. 79:1662–1674. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Foody J, Huo Y, Ji L, Zhao D, Boyd D, Meng
HJ, Shiff S and Hu D: Unique and varied contributions of
traditional CVD risk factors: A systematic literature review of CAD
risk factors in China. Clin Med Insights Cardiol. 7:59–86.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xu Y, Ye J, Wang M, Liu J, Wang Z, Jiang
H, Ye D, Zhang J and Wan J: The expression of interleukin-25
increases in human coronary artery disease and is associated with
the severity of coronary stenosis. Anatol J Cardiol. 23:151–159.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Peikert A, Kaier K, Merz J, Manhart L,
Schafer I, Hilgendorf I, Hehn P, Wolf D, Willecke F, Sheng X, et
al: Residual inflammatory risk in coronary heart disease: Incidence
of elevated high-sensitive CRP in a real-world cohort. Clin Res
Cardiol. 109:315–323. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Blaum C, Brunner FJ, Kroger F, Braetz J,
Lorenz T, Goßling A, Ojeda F, Koester L, Karakas M, Zeller T, et
al: Modifiable lifestyle risk factors and C-reactive protein in
patients with coronary artery disease: Implications for an
anti-inflammatory treatment target population. Eur J Prev Cardiol:
Nov 10, 2019 doi: 10.1177/2047487319885458 (Epub ahead of
print).
|
|
30
|
Zorlu C and Koseoglu C: Comparison of the
relationship between inflammatory markers and contrast-induced
nephropathy in patients with acute coronary syndrome after coronary
angiography. Angiology. 71:249–255. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Toniatti C, Demartis A, Monaci P, Nicosia
A and Ciliberto G: Synergistic trans-activation of the human
C-reactive protein promoter by transcription factor HNF-1 binding
at two distinct sites. EMBO J. 9:4467–4475. 1990.PubMed/NCBI
|
|
32
|
Majello B, Arcone R, Toniatti C and
Ciliberto G: Constitutive and IL-6-induced nuclear factors that
interact with the human C-reactive protein promoter. EMBO J.
9:457–465. 1990.PubMed/NCBI
|
|
33
|
Taylor AW, Ku NO and Mortensen RF:
Regulation of cytokine-induced human C-reactive protein production
by transforming growth factor-beta. Immunol. 145:2507–2513.
1990.PubMed/NCBI
|
|
34
|
Zhang Y, Shao T, Yao L, Yue H and Zhang Z:
Effects of tirofiban on stent thrombosis, Hs-CRP, IL-6 and sICAM-1
after PCI of acute myocardial infarction. Exp Ther Med.
16:3383–3388. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Janciauskiene SM, Bals R, Koczulla R,
Vogelmeier C, Kohnlein T and Welte T: The discovery of
α1-antitrypsin and its role in health and disease. Respir Med.
105:1129–1139. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gooptu B, Dickens JA and Lomas DA: The
molecular and cellular pathology of α1-antitrypsin deficiency.
Trends Mol Med. 20:116–127. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Stockley RA and Turner AM: α-1-Antitrypsin
deficiency: Clinical variability, assessment, and treatment. Trends
Mol Med. 20:105–115. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fregonese L and Stolk J: Hereditary
alpha-1-antitrypsin deficiency and its clinical consequences.
Orphanet J Rare Dis. 3(16)2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ferrarotti I, Thun GA, Zorzetto M,
Ottaviani S, Imboden M, Schindler C, von Eckardstein A, Rohrer L,
Rochat T, Russi EW, et al: Serum levels and genotype distribution
of α1-antitrypsin in the general population. Thorax. 67:669–674.
2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gilutz H, Siegel Y, Paran E, Cristal N and
Quastel MR: Alpha 1-antitrypsin in acute myocardial infarction. Br
Heart J. 49:26–29. 1983.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Brunetti ND, Correale M, Pellegrino PL,
Cuculo A and Biase MD: Acute phase proteins in patients with acute
coronary syndrome: Correlations with diagnosis, clinical features,
and angiographic findings. Eur J Intern Med. 18:109–117.
2007.PubMed/NCBI View Article : Google Scholar
|