Introduction
Non-small cell lung cancer (NSCLC) is a malignant
tumor, accounting for >85% of all newly diagnosed lung cancer
cases (1). There were ~730,000 new
patients with lung cancer and ~610,000 lung cancer-related deaths
in China in 2015(2). There are no
obvious symptoms or efficient routine screening methods available
for the early diagnosis of NSCLC; thus, at diagnosis, the majority
of patients with NSCLC are at advanced stages and miss the best
time for effective surgical treatment (3). Currently, the existing regular or low
dose CT screening method is insufficient, and the specificity of
serum tumor antigen markers to NSCLC is low, which limits their
clinical applications (4).
Cytotoxic chemotherapy is still an important method used for the
treatment of metastatic NSCLC (5,6);
however, the prognosis following the treatment remains inadequate.
This may be attributed to the lack of effective prognostic
biomarkers that can be used to monitor the progression and
metastasis of NSCLC and help clinicians develop more appropriate
treatment strategies for the patients (7). Therefore, it remains a priority to
identify efficient prognostic biomarkers and to develop effective
treatment strategies for NSCLC.
MicroRNAs (miRNAs/miRs) are small non-coding RNAs
that are widely present in animal and plant cells (8). miRNAs are involved in numerous
biological processes, including cell proliferation, migration and
apoptosis, through post-transcriptional regulation (7). With the development of targeted and
immune therapies, miRNAs have gradually attracted increasing
attention (9,10), and emerging evidence has indicated
that abnormally expressed miRNAs may be involved in the occurrence
and development of different types of cancer, in addition to being
closely associated with the prognosis of patients (11-13).
At present, it has been confirmed that miR-1225-5p serves a role in
several types of cancers (14-17).
For example, the downregulated expression levels of miR-1225-5p in
thyroid cancer tissues and cell lines was reported to regulate
tumor cell proliferation, apoptosis, migration and invasion by
targeting sirtuin-3(15). In
addition, a previous study by Zhang et al (17) also illustrated the tumor suppressive
role of miR-1225-5p in osteosarcoma, in which the overexpression of
miR-1225-5p suppressed osteosarcoma cell proliferation, migration
and invasion. However, to the best of our knowledge, the expression
levels and functional role of miR-1225-5p in NSCLC remains
unclear.
The present study aimed to investigate the
expression levels of miR-1225-5p in NSCLC, in addition to analyzing
its prognostic value and biological role. The expression levels of
miR-1225-5p in NSCLC tissues and cell lines were analyzed using
reverse transcription-quantitative PCR (RT-qPCR), and Kaplan-Meier
survival curves and Cox regression analyses were performed to
evaluate the prognostic value of miR-1225-5p in NSCLC. In addition,
the effects of miR-1225-5p on cell proliferation, migration and
invasion were investigated. The findings of the present study may
indicate a novel biomarker and therapeutic target for NSCLC, and
the potential dysregulation of miR-1225-5p in NSCLC progression may
assist to further uncover the mechanisms underlying NSCLC
pathogenesis.
Materials and methods
Patient studies
NSCLC tissues and adjacent normal tissues (at least
3 cm from the edge of the tumor) were collected from 118 patients
at Shouguang People's Hospital (Shouguang, China) from May 2011 to
April 2014. The patients included 52 females and 66 males with a
meanage of 59.88±11.39 years (range, 38-84 years). All collected
tissues were stored at -80˚C for subsequent experiments. The
inclusion criteria were as follows: i) All patients were
pathologically diagnosed with NSCLC during surgery; ii) None of the
patients had received any cancer treatment prior to the operation;
and iii) The electronic medical records of the patients were
complete. The following exclusion criteria were also used: i)
Patients with a history of other types of cancer other than NSCLC;
ii) Patients who had received any kind of antitumor therapy; iii)
Patients with incomplete records of clinicopathological
characteristics; and iv) Patients who had died from causes other
than NSCLC. The demographic and clinicopathological characteristics
of the patients are summarized in Table
I. The personal information of each patient was kept
confidential and each patient provided written informed consent.
The study was approved by the Ethics Committee of Shouguang
People's Hospital. Each patient was followed up for a 5-year period
following surgery to record survival information. The cases who
died from other unrelated events were excluded from the study.
 | Table IAssociation between miR-1225-5p
expression levels and the clinicopathological features of patients
with non-small cell lung cancer. |
Table I
Association between miR-1225-5p
expression levels and the clinicopathological features of patients
with non-small cell lung cancer.
| | miR-1225-5p
expression level | |
|---|
| Clinicopathological
characteristic | Total (n=118) | Low (n=64) | High (n=54) | P-value |
|---|
| Sex | | | | 0.940 |
|
Female | 52 | 28 | 24 | |
|
Male | 66 | 36 | 30 | |
| Age, years | | | | 0.959 |
|
≤60 | 44 | 24 | 20 | |
|
>60 | 74 | 40 | 34 | |
| Tumor size, cm | | | | 0.185 |
|
≤3 | 71 | 35 | 36 | |
|
>3 | 47 | 29 | 18 | |
| Smoking
history | | | | 0.691 |
|
Negative | 46 | 26 | 20 | |
|
Positive | 72 | 38 | 34 | |
| Lymph node
metastasis | | | | 0.012 |
|
Negative | 53 | 22 | 31 | |
|
Positive | 65 | 42 | 23 | |
| TNM stage | | | | 0.002 |
|
I-II | 54 | 21 | 33 | |
|
III-IV | 64 | 43 | 21 | |
Cell culture and transfection
NSCLC cell lines, A549, HCC827, H1299 and NCI-H460,
and a normal human bronchial epithelial cell line, NHBE, were
obtained from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. All cell lines were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), and maintained at 37˚C in
an atmosphere of 5% CO2 in a cell incubator.
For the cell transfections, 4x104 A549
and H1299 cells/well were seeded into six-well plates and cultured
in a humidified incubator at 37˚C with 5% CO2. Following
24 h of incubation, the cells were transfected with 50 nM
miR-1225-5p mimic, 100 nM miR-1225-5p inhibitor, 50 nM mimic
negative control (NC) and 100 nM inhibitor NC (all purchased from
Shanghai GenePharma Co., Ltd.) using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol, to regulate the expression levels of
miR-1225-5p in NSCLC cells. The sequences used for cell
transfection were as follows: miR-1225-5p mimic,
5'-GUGGGUACGGCCCAGUGGGGGG-3', miR-1225-5p inhibitor,
5'-CCCCCCACUGGGCCGUACCCAC-5', mimic NC, 5'-UUCUCCGAACGUGUCACGU-3'
and inhibitor NC. 5'-CAGUACUUUUGUGUAGUACAA-3'. Cells transfected
with transfection reagent alone were set as the mock group.
Following 6 h of incubation at 37˚C, the culture medium was
replaced, and subsequent cell experiments were performed at 48 h
post-transfection.
RT-qPCR
Total RNA was extracted from NSCLC tissues (50 mg)
and cells (1x106 cells) using 1 ml TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
reverse transcribed into cDNA using a PrimeScript RT reagent kit
(Takara Bio, Inc.) at 42˚C for 30 min and 85˚C for 10 min. qPCR was
subsequently performed using the SYBR Green PCR Master Mix (Thermo
Fisher Scientifc, Inc.) on a 7300 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95˚C for 10 min; followed by 40 cycles of 95˚C for
20 sec, 60˚C for 15 sec and 72˚C for 20 sec. The following primer
sequences were used for the qPCR: miR-1225-5p, forward
5'-GCCGAGGTGGGTACGGCCCA-3', reverse 5'-CTCAACTGGTGTCGTGGA-3'; and
U6, forward 5'-CTCGCTTCGGCAGCACA-3', reverse
5'-AACGCTTCACGAATTTGCGT-3'. U6 was used as the internal reference
gene. The relative expression levels of miR-1225-5p were quantified
using the 2-ΔΔCq method (18) and normalized to U6.
Cell viability assay
H1299 and A549 cells were seeded into 96-well plates
at a density of 2x105 cells/well and cultured in an
incubator at 37˚C for 24, 48 or 72 h. Following the incubation, 10
µl MTT was added to each well for 4 h at 37˚C. Subsequently, 150 µl
DMSO was added to the wells and incubated for 1 h at 37˚C to
dissolve the purple formazan. The optical density was measured at
570 nm on a microplate reader.
Cell migration and invasion
assays
Transwell chambers (Corning, Inc.) were used to
analyze the migratory and invasive abilities of NSCLC cells. The
chambers used for the invasion assay were precoated with Matrigel
(Corning, Inc.) at 37˚C for 1 h, whereas the chambers for the
migration assay were not precoated. A549 and H1299 cells (cell
density, 5x105 cells/well) in serum-free DMEM were
seeded into the upper chambers of the Transwell plates. The lower
chambers were filled with DMEM supplemented with 10% FBS. Following
48 h of incubation at 37˚C, the cells in the lower chambers were
fixed with 4% paraformaldehyde for 10 min at room temperature and
stained using 0.1% crystal violet at room temperature for 20 min.
The number of migratory or invasive cells in five randomly selected
fields were counted under an inverted light microscope at x200
magnification (Olympus Corporation).
Western blotting
Total protein was extracted from A549 and H1299
cells (1x107 cells) using 100 µl RIPA buffer (Thermo
Fisher Scientific, Inc.) and quantified using a BCA method. A total
of 20 µg protein/lane was separated by 10% SDS-PAGE and
subsequently transferred onto polyvinylidene fluoride membranes
(EMD Millipore). The membranes were blocked with 5% skimmed milk at
room temperature for 2 h then incubated at 4˚C overnight with the
following primary antibodies: Anti-proliferating cell nuclear
antigen (PCNA; rabbit; 1:1,000; cat. no. ab92552; Abcam), anti-Ki67
(rabbit; 1:1,000; cat. no. ab16667; Abcam) and anti-GAPDH (rabbit;
1:2,500; cat. no. ab9485; Abcam). Following the primary antibody
incubation, the membranes were washed with PBS +0.05% Tween-20 and
incubated with a horseradish peroxidase-conjugated anti-rabbit
secondary antibody (1:10,000; cat. no. 111-035-045; Jackson
ImmunoResearch Europe, Ltd.) at 37˚C for 2 h. Protein bands were
visualized using an ECL system (EMD Millipore) and analyzed using
ImageJ v1.46 software (National Institutes of Health).
Target gene prediction and
dual-luciferase reporter assay
The target genes of miR-1225-5p were predicted using
miRanda (http://www.microrna.org) (19). To confirm the interaction between
miR-1225-5p and Sox9 in NSCLC cells, 4x104 A549 and
H1299 cells/well were plated into six-well plates and cultured at
37˚C for 24 h. Subsequently, the wild-type (WT) or mutant (MUT)
3'-UTR of Sox9 was cloned into the luciferase reporter vector
PsiCheck2 (Promega Corporation). The MUT sequence was generated
using a site-directed mutagenesis kit (Promega Corporation). The WT
or MUT Sox9 luciferase plasmid (100 ng) was co-transfected into
A549 and H1299 cells with the miR-1225-5p mimic (50 nM),
miR-1225-5p inhibitor (100 nM) or the respective NCs (50 nM mimic
NC; 100 nM inhibitor NC) using Lipofectamine 2000, according to the
manufacturer's protocol. Following incubation for 48 h at 37˚C, the
relative luciferase activity was measured using a Dual-Luciferase
Reporter Assay System (Promega Corporation). Firefly luciferase
activity was normalized to Renilla luciferase activity.
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software (IBM Corp.) and data are presented as the mean ± SD. All
experiments were performed at least three times. Statistical
differences between groups were analyzed using a paired Student's
t-test or a one-way ANOVA followed by Tukey's post hoc test. The
association between miR-1225-5p expression levels and the
clinicopathological features of patients with NSCLC was determined
using a χ2 test. Kaplan-Meier survival, followed by
log-rank, and Cox regression analyses were adopted to determine the
prognostic value of miR-1225-5p. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression levels of miR-1225-5p are
downregulated in NSCLC tissues and cell lines
The expression levels of miR-1225-5p in NSCLC
tissues and cell lines were quantified using RT-qPCR, which
demonstrated that the expression levels of miR-1225-5p were
significantly lower in NSCLC tissues compared with the levels of
expression in adjacent normal tissues (P<0.001; Fig. 1A). Consistent with these findings,
the expression levels of miR-1225-5p in NSCLC cell lines were also
significantly lower compared with the control NHBE cells (all
P<0.001; Fig. 1B). Since A549
and H1299 cells showed the lowese miR-1225-5p expression, these
cells were used for subsequent cell experiments.
Association between miR-1225-5p
expression levels and the clinicopathological characteristics of
patients with NSCLC
Through determining the association between
miR-1225-5p expression levels and the clinicopathological data of
patients, it was identified that miR-1225-5p may be involved in
NSCLC development. The mean expression value (0.550) of miR-1225-5p
was used as the cut-off value to classify the patients into low
(n=64) and high (n=54) miR-1225-5p expression groups. miR-1225-5p
expression levels were discovered to be significantly associated
with lymph node metastasis (P=0.012) and TNM stage (P=0.002)
(Table I). By contrast, no
significant associations were identified between miR-1225-5p
expression levels and the other parameters, including sex, age,
tumor size and smoking history (all P>0.05).
Clinical significance of miR-1225-5p
expression levels for the prognosis of NSCLC
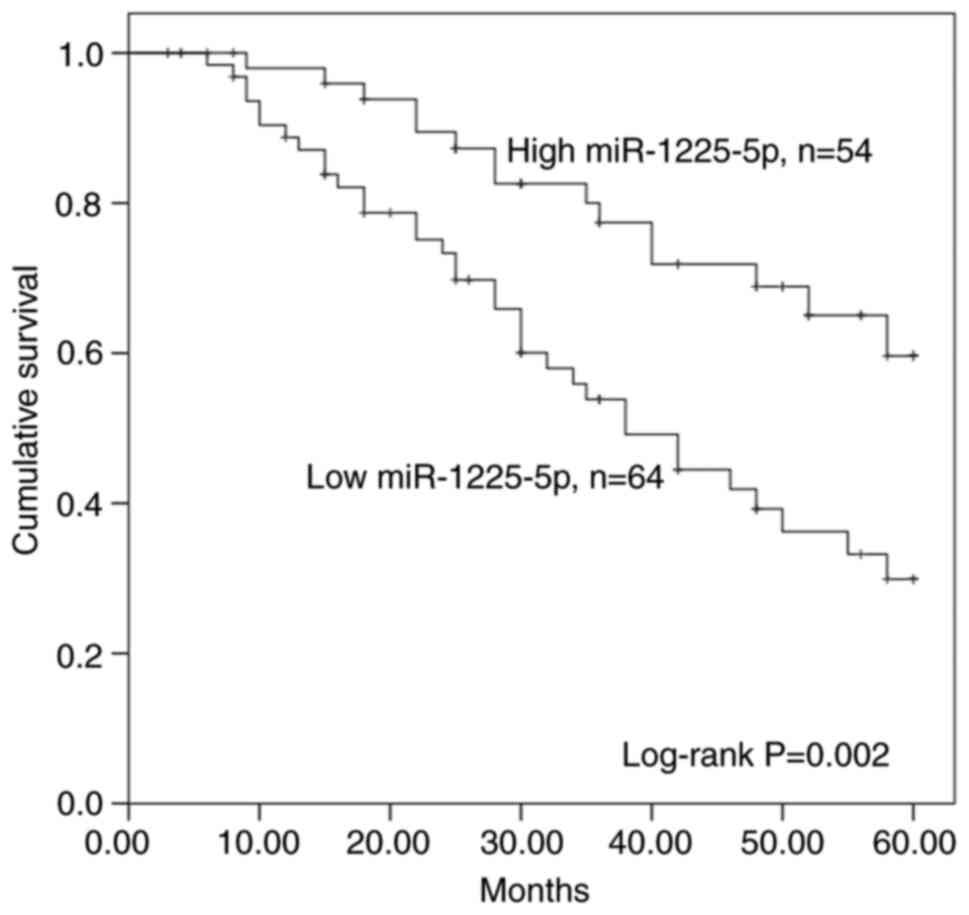
Kaplan-Meier survival curves and Cox regression
analyses was performed to determine the prognostic value of
miR-1225-5p. Patients with high expression levels of miR-1225-5p
exhibited a significantly increased overall survival compared with
those with low miR-1225-5p expression levels (P=0.002; Fig. 2). In addition, the
clinicopathological indicators and miR-1225-5p that might be
related with survival of patients were included in univariate and
multivariate Cox regression analysis. The results indicated that
miR-1225-5p expression levels and TNM stage were associated with
the prognosis of NSCLC as two independent prognostic factors
(miR-1225-5p: Hazard ratio=2.240, 95% CI=1.184-4.239; P=0.013; TNM
stage: Hazard ratio=2.059, 95% CI=1.109-4.187; P=0.032; Table II). However, other indicators,
including sex, age, tumor size, smoking history and lymph node
metastasis, showed no independent association with the overall
survival of patients with NSCLC (all P>0.05).
 | Table IIMultivariate Cox regression analysis
for miR-1225-5p expression levels in patients with non-small cell
lung cancer. |
Table II
Multivariate Cox regression analysis
for miR-1225-5p expression levels in patients with non-small cell
lung cancer.
| | Univariate Cox
analysis | Multivariate Cox
analysis |
|---|
| Variable | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| miR-1225-5p | 2.474 | 1.348-4.539 | 0.003 | 2.240 | 1.184-4.239 | 0.013 |
| Sex | 1.513 | 0.867-2.641 | 0.145 | 1.418 | 0.807-2.492 | 0.225 |
| Age | 1.090 | 0.612-1.942 | 0.771 | 1.244 | 0.676-2.290 | 0.483 |
| Tumor size | 1.556 | 0.891-2.715 | 0.120 | 1.520 | 0.851-2.715 | 0.157 |
| Smoking
history | 1.408 | 0.783-2.531 | 0.253 | 1.325 | 0.727-2.415 | 0.357 |
| Lymph node
metastasis | 1.896 | 0.921-2.954 | 0.053 | 1.788 | 1.012-3.859 | 0.068 |
| TNM stage | 2.159 | 1.238-4.215 | 0.021 | 2.059 | 1.109-4.187 | 0.032 |
miR-1225-5p inhibits NSCLC cell
proliferation
Cell proliferation assays were performed to
determine the function of miR-1225-5p in the progression of NSCLC.
Following the transfection with the miR-1225-5p mimic or
miR-1225-5p inhibitor, the expression levels of miR-1225-5p were
significantly upregulated or downregulated compared with the
corresponding NCs, respectively, in both A549 and H1299 cells (all
P<0.001; Fig. 3A). Furthermore,
the inhibition of miR-1225-5p significantly increased NSCLC cell
proliferation compared with cells transfected with inhibitor NC
(all P<0.05), whereas the overexpression of miR-1225-5p
significantly suppressed cell proliferation compared with the cells
transfected with mimic NC, in both A549 and H1299 cells (all
P<0.05; Fig. 3B). Furthermore,
compared to the cells with mimic NC transfection, both A549 and
H1299 cells with overexpression of miR-1225-5p showed significantly
downregulated protein expression levels of PCNA and Ki67, which are
two proliferation-related proteins (19), whereas the inhibition of miR-1225-5p
expression levels significantly upregulated the expression levels
of PCNA and Ki67 in both cell lines compared with cells transfected
with inhibitor NC (all P<0.05; Fig.
3C).
 | Figure 3Effects of miR-1225-5p on A549 and
H1299 cell proliferation, migration and invasion. (A) Reverse
transcription-quantitative PCR demonstrated that the expression
levels of miR-1225-5p were upregulated by the miR-1225-5p mimic but
downregulated by the miR-1225-5p inhibitor.
***P<0.001. (B) MTT assay demonstrated that
miR-1225-5p overexpression inhibited NSCLC cell proliferation,
whereas the inhibition of miR-1225-5p promoted tumor cell
proliferation. *P<0.05. (C) The expressional changes
of proliferation-related proteins PCNA and Ki67 in NSCLC cells with
deregulated miR-1225-5p. *P<0.05. (D) Transwell assay
results demonstrated that NSCLC cell migration was promoted by the
inhibition of miR-1225-5p but inhibited following the
overexpression of miR-1225-5p. Magnification x200. (E) Transwell
Matrigel assay demonstrated that the overexpression of miR-1225-5p
in NSCLC cells inhibited cell invasion, whereas the inhibition of
miR-1225-5p expression promoted the opposite results.
Magnification, x200. **P<0.01,
***P<0.001. miR, microRNA; NC, negative control;
NSCLC, non-small cell lung cancer; OD, optical density; PCNA,
proliferating cell nuclear antigen. |
Effect of miR-1225-5p on the migration
and invasion of NSCLC cells
Transwell assays were performed to determine the
influence of miR-1225-5p on NSCLC cell migration and invasion.
Compared with the cells transfected with mimic NC or inhibitor NC,
miR-1225-5p overexpression significantly decreased NSCLC cell
migration and invasion (all P<0.001), whereas the inhibition of
miR-1225-5p significantly induced NSCLC cell migration and invasion
(P<0.01 and P<0.001; Fig. 3D
and E).
Sox9 is a direct target of miR-1225-5p
in NSCLC cells
Through target gene prediction, a putative binding
site of miR-1225-5p was identified in the 3'-UTR of Sox9 (Fig. 4A). Subsequently, a dual-luciferase
reporter assay was performed to confirm the interaction between
miR-1225-5p and Sox9. The relative luciferase activity of the WT
Sox9-3'-UTR was significantly inhibited following the
overexpression of miR-1225-5p (all P<0.05), whereas it was
significantly increased following the inhibition of miR-1225-5p
when compared to the respective NCs, in both A549 and H1299 cells
(P<0.05 and P<0.01; Fig. 4B
and C). Conversely, no significant
differences were observed in the relative luciferase activity of
the MUT Sox9-3'-UTR across all groups in both cell lines (all
P>0.05). These findings indicated that miR-1225-5p may directly
bind to the 3'-UTR of Sox9 in NSCLC cells.
Discussion
The aberrant expression of miRNAs have been
identified to be closely associated to the occurrence of numerous
types of human malignant tumor, in which they were reportedly
involved in cell apoptosis, proliferation, the cell cycle and
metastasis by altering post-transcriptional gene expression
(20-22).
miRNAs have been identified to serve as both oncogenes or tumor
suppressor genes in tumorigenesis; for example, the abnormal
expression of miRNAs, such as miR-146a (23), miR-375(24) and miR-449c (25), was determined to be closely
associated with gastric tumorigenesis. In addition, a significant
association was reported between tumor budding and the
downregulated expression levels of miRNAs, such as miR-148a
(26) and miR-625-3p (27). miR-21, miR-144-3p and miR-148a were
also reported to be abnormally expressed in NSCLC tissues (28-30).
The results of the present study also confirmed the dysregulation
of miR-1225-5p in NSCLC cells. Clinically effective prognostic
biomarkers can not only indicate the progression and metastatic
status of potential cancers, but they can also help clinicians
develop more appropriate treatment strategies for patients with
cancer (31,32). Therefore, it is important to
identify further potential functional miRNAs for the treatment of
NSCLC.
miR-1225-5p, which was investigated in the current
study, has been considered as an important regulator in various
types of tumor. Previous studies have demonstrated that compared
with its expression levels in normal tissues, the expression levels
of miR-1225-5p were downregulated in osteosarcoma (17), thyroid cancer (15) and pancreatic cancer (33), in which it was discovered to be
associated with tumor grade and poor prognosis. In the present
study, the expression levels of miR-1225-5p in tumor tissues of
patients with NSCLC and NSCLC cell lines were significantly
downregulated compared with expression in adjacent normal lung
tissues and human bronchial epithelial cells. The low expression
levels were subsequently illustrated to be associated with lymph
node metastasis, TNM stage and poor prognosis, thus it was
suggested that miR-1225-5p may be a potential tumor suppressor
gene. These finding indicated that miR-1225-5p may serve an
important role in the development and progression of NSCLC tumors
and may be used clinically to predict the metastasis of NSCLC.
Given the abnormal expression of miR-1225-5p in
NSCLC tissues, the present study analyzed the clinical significance
of its diagnostic and prognostic values in NSCLC. Biomarkers can
provide clinicians with an effective guide during the early stages
of treatment of patients with NSCLC (34-36).
The prognostic value of miR-1225-5p was evaluated based on the
5-year survival information of patients with NSCLC. The
Kaplan-Meier survival curve illustrated that patients with low
miR-1225-5p expression levels had a worse overall survival compared
with patients with high expression levels. In addition, miR-1225-5p
was independently related to overall survival, suggesting that
miR-1225-5p may be a potential prognostic biomarker for NSCLC.
A previous study reported that the aberrant
expression levels of miR-145 in NSCLC mediated chemoresistance and
brain metastasis, and its downregulation was associated with the
poor prognosis of NSCLC (37). The
overexpression of miR-142-5p was discovered to downregulate the
expression levels of phosphatidylinositol 4,5-biphosphate 3-kinase
catalytic subunit α isoform at both the mRNA and protein levels,
thereby inhibiting the proliferation of NSCLC cells (38,39).
The results of the present study revealed that miR-1225-5p
inhibition promoted the proliferation, migration and invasion of
NSCLC cells, while miR-1225-5p overexpression inhibited tumor cell
proliferation, migration and invasion, which suggested that
miR-1225-5p may serve a tumor suppressive role in NSCLC
development. However, to the best of our knowledge, the mechanism
of action of miR-1225-5p in NSCLC remains unknown. Zhang et
al (17) discovered that
miR-1225-5p served a tumor suppressive role in osteosarcoma by
targeting Sox9. In addition, Sun et al (14) demonstrated that miR-1225 inhibited
laryngeal cancer cell proliferation and survival by targeting the
3'-UTR of dual-specificity protein phosphatase CDC14B, leading to
G1/S phase cell cycle arrest. Based on the existing
research and conclusions, the present study hypothesized that
miR-1225-5p may regulate proliferation and/or metastasis of NSCLC
cells by binding to the 3'-UTR region of target genes, thereby
exerting the anticancer effect of miR-1225-5p. Subsequently, a
dual-luciferase reporter assay was used to confirm the interaction
between miR-1225-5p and Sox9; the results revealed that miR-1225-5p
directly bound to the 3'-UTR of Sox9 in NSCLC cells. Sox9 was found
to be upregulated in NSCLC tissues, which was correlated with
histological stage and shorter survival time of patients with NSCLC
(40), and serves as an oncogene in
NSCLC progression (40). Thus,
these findings suggested that the tumor suppressive role of
miR-1225-5p in NSCLC progression may be achieved by targeting Sox9.
To further confirm the functional role and underlying mechanisms of
miR-1225-5p in NSCLC pathogenesis, further investigations with
in vivo experiments are required.
In conclusion, the findings of the present study
demonstrated that miR-1225-5p expression levels were downregulated
in NSCLC, which may serve as an independent prognostic biomarker.
Therefore, the current study provided a novel, potential
non-invasive biomarker to predict the survival outcomes of patients
with NSCLC. In addition, the aberrant expression levels of
miR-1225-5p in NSCLC progression may help to further elucidate the
pathogenesis of this malignancy. The overexpression of miR-1225-5p
was revealed to inhibit NSCLC cell proliferation, migration and
invasion, indicating that miR-1225-5p may be a potential
therapeutic target, and that methods to upregulate miR-1225-5p
expression levels may be novel approaches for NSCLC therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL and HL designed and conceived the study,
conducted the clinical studies, analyzed the clinical data and
wrote the manuscript. FZ conducted the in vitro experiments
and analyzed the cell experimental data. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
The personal information of each patient involved
remains confidential and each patient provided written informed
consent. The study was approved by the Ethics Committee of
Shouguang People's Hospital (Shouguang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gridelli C, Rossi A, Carbone DP, Guarize
J, Karachaliou N, Mok T, Petrella F, Spaggiari L and Rosell R:
Non-Small-Cell lung cancer. Nat Rev Dis Primers.
1(15009)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Denkçeken T and Pala E: Investigation of
key miRNAs and potential mechanisms in non-small cell lung cancer
development from chronic obstructive pulmonary disease. Gen Physiol
Biophys. 39:69–77. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rafei H, El-Bahesh E, Finianos A,
Nassereddine S and Tabbara I: Immune-Based therapies for non-small
cell lung cancer. Anticancer Res. 37:377–387. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hill A, Gupta R, Zhao D, Vankina R, Amanam
I and Salgia R: Targeted therapies in non-small-cell lung cancer.
Cancer Treat Res. 178:3–43. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Signorelli D, Giannatempo P, Grazia G,
Aiello MM, Bertolini F, Mirabile A, Buti S, Vasile E, Scotti V,
Pisapia P, et al: Patients selection for immunotherapy in solid
tumors: Overcome the naive vision of a single biomarker. Biomed Res
Int. 2019(9056417)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tutar Y: miRNA and cancer; computational
and experimental approaches. Curr Pharm Biotechnol.
15(429)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chaudhuri K and Chatterjee R: MicroRNA
detection and target prediction: Integration of computational and
experimental approaches. DNA Cell Biol. 26:321–337. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li M, Huo X, Davuljigari CB, Dai Q and Xu
X: MicroRNAs and their role in environmental chemical
carcinogenesis. Environ Geochem Health. 41:225–247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rak B, Marczewska JM and Wlodarski P: The
role of microRNAs in endometrial cancer and influence on future
therapy: Focusing on miRNA-21. Eur J Gynaecol Oncol. 37:599–603.
2016.PubMed/NCBI
|
|
11
|
Kumar S, Keerthana R, Pazhanimuthu A and
Perumal P: Overexpression of circulating miRNA-21 and miRNA-146a in
plasma samples of breast cancer patients. Indian J Biochem Biophys.
50:210–214. 2013.PubMed/NCBI
|
|
12
|
Mishra S, Yadav T and Rani V: Exploring
miRNA based approaches in cancer diagnostics and therapeutics. Crit
Rev Oncol Hematol. 98:12–23. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Loubaki L, Chabot D, Paré I, Drouin M and
Bazin R: miR-146a potentially promotes IVIg-mediated inhibition of
TLR4 signaling in LPS-activated human monocytes. Immunol Lett.
185:64–73. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sun P, Zhang D, Huang H, Yu Y, Yang Z, Niu
Y and Liu J: MicroRNA-1225-5p acts as a tumor-suppressor in
laryngeal cancer via targeting CDC14B. Biol Chem. 400:237–246.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang S, Chen X, Zhang Z and Wu Z:
MicroRNA-1225-5p inhibits the development and progression of
thyroid cancer via targeting sirtuin 3. Pharmazie. 74:423–427.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li D, Chi G, Chen Z and Jin X:
MicroRNA-1225-5p behaves as a tumor suppressor in human
glioblastoma via targeting of IRS1. Onco Targets Ther.
11:6339–6350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang W, Wei L, Sheng W, Kang B, Wang D
and Zeng H: miR-1225-5p functions as a tumor suppressor in
osteosarcoma by targeting sox9. DNA Cell Biol. 3:78–91.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The urihttp://microRNA.orgsimplemicroRNA.org resource:
Targets and expression. Nucleic Acids Res. 36:D149–D153. 2008.
|
|
20
|
Zhang Z, Li W, Jiang D, Liu C and Lai Z:
MicroRNA-139-5p inhibits cell viability, migration and invasion and
suppresses tumor growth by targeting HDGF in non-small cell lung
cancer. Oncol Lett. 19:1806–1814. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huang Y, Bao T, Li Z, Ji G and Zhang L:
Function of miR-200a in proliferation and apoptosis of non-small
cell lung cancer cells. Oncol Lett. 20:1256–1262. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang L, Zhao Y, Xiong W, Ye W, Zhao W and
Hua Y: MicroRNA-449a is downregulated in cervical cancer and
inhibits proliferation, migration, and invasion. Oncol Res Treat.
42:564–571. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rahman NA, Chan CM, Zakaria MI and Jaafar
MJ: Knowledge and attitude towards identification of systemic
inflammatory response syndrome (SIRS) and sepsis among emergency
personnel in tertiary teaching hospital. Australas Emerg Care.
22:13–21. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xu Y, Deng Y, Yan X and Zhou T: Targeting
miR-375 in gastric cancer. Expert Opin Ther Targets. 15:961–972.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen X, Wang A and Yue X: miR-449c
inhibits migration and invasion of gastric cancer cells by
targeting PFKFB3. Oncol Lett. 16:417–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xu Y, Chao L, Wang J and Sun Y: miRNA-148a
regulates the expression of the estrogen receptor through
DNMT1-mediated DNA methylation in breast cancer cells. Oncol Lett.
14:4736–4740. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Baltruskeviciene E, Schveigert D,
Stankevicius V, Mickys U, Zvirblis T, Bublevic J, Bublevic J,
Suziedelis K and Aleknavicius E: Down-Regulation of miRNA-148a and
miRNA-625-3p in colorectal cancer is associated with tumor budding.
BMC Cancer. 17(607)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Marin I, Ofek E, Bar J, Prisant N,
Perelman M, Avivi C, Lavy-Shahaf G, Onn A, Katz R and Barshack I:
miR-21, EGFR and PTEN in non-small cell lung cancer: An in situ
hybridisation and immunohistochemistry study. J Clin Pathol.
14(206420)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jiang W, Xu Z, Yu L, Che J, Zhang J and
Yang J: MicroRNA-144-3p suppressed TGF-beta1-induced lung cancer
cell invasion and adhesion by regulating the src-akt-erk pathway.
Cell Biol Int. 30(1002)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen Y, Min L, Ren C, Xu X, Yang J, Sun X,
Wang T, Wang F, Sun C and Zhang X: miRNA-148a serves as a
prognostic factor and suppresses migration and invasion through
wnt1 in non-small cell lung cancer. PLoS One.
12(e0171751)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li Y, Chen X, Xue W, Liang J and Wang L:
miR-874 inhibits cell proliferation, migration, and invasion of
glioma cells and correlates with prognosis of glioma patients.
Neuromolecular Med. 14(1007)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yu M, Yu HL, Li QH, Zhang L and Chen YX:
miR-4709 overexpression facilitates cancer proliferation and
invasion via downregulating NR3C2 and is an unfavorable prognosis
factor in colon adenocarcinoma. J Biochem Mol Toxicol.
33(e22411)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhong R, Li S, Fang K, Yang L and Wang L:
MicroRNA-1225 inhibit apoptosis of pancreatic cancer cells via
targeting JAK1. Cell Cycle. 18:990–1000. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Osmani L, Askin F, Gabrielson E and Li QK:
Current WHO guidelines and the critical role of immunohistochemical
markers in the subclassification of non-small cell lung carcinoma
(NSCLC): Moving from targeted therapy to immunotherapy. Semin
Cancer Biol. 52:103–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Aoki MN, Amarante MK, de Oliveira CE and
Watanabe MA: Biomarkers in non-small cell lung cancer: Perspectives
of individualized targeted therapy. Anticancer Agents Med Chem.
18:2070–2077. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Müller S, Janke F, Dietz S and Sültmann H:
Circulating microRNAs as potential biomarkers for lung cancer.
Recent Results Cancer Res. 215:299–318. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mizuno K, Mataki H, Seki N, Kumamoto T,
Kamikawaji K and Inoue H: MicroRNAs in non-small cell lung cancer
and idiopathic pulmonary fibrosis. J Hum Genet. 62:57–65.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang Z, Liu Z, Fang X and Yang H:
miR-142-5p suppresses tumorigenesis by targeting PIK3CA in
non-small cell lung cancer. Cell Physiol Biochem. 43:2505–2515.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Su YH, Zhou Z, Yang KP, Wang XG, Zhu Y and
Fa XE: MIR-142-5p and miR-9 may be involved in squamous lung cancer
by regulating cell cycle related genes. Eur Rev Med Pharmacol Sci.
17:3213–3220. 2013.PubMed/NCBI
|
|
40
|
Zhou CH, Ye LP, Ye SX, Li Y, Zhang XY, Xu
XY and Gong LY: Clinical significance of SOX9 in human non-small
cell lung cancer progression and overall patient survival. J Exp
Clin Cancer Res. 31(18)2012.PubMed/NCBI View Article : Google Scholar
|