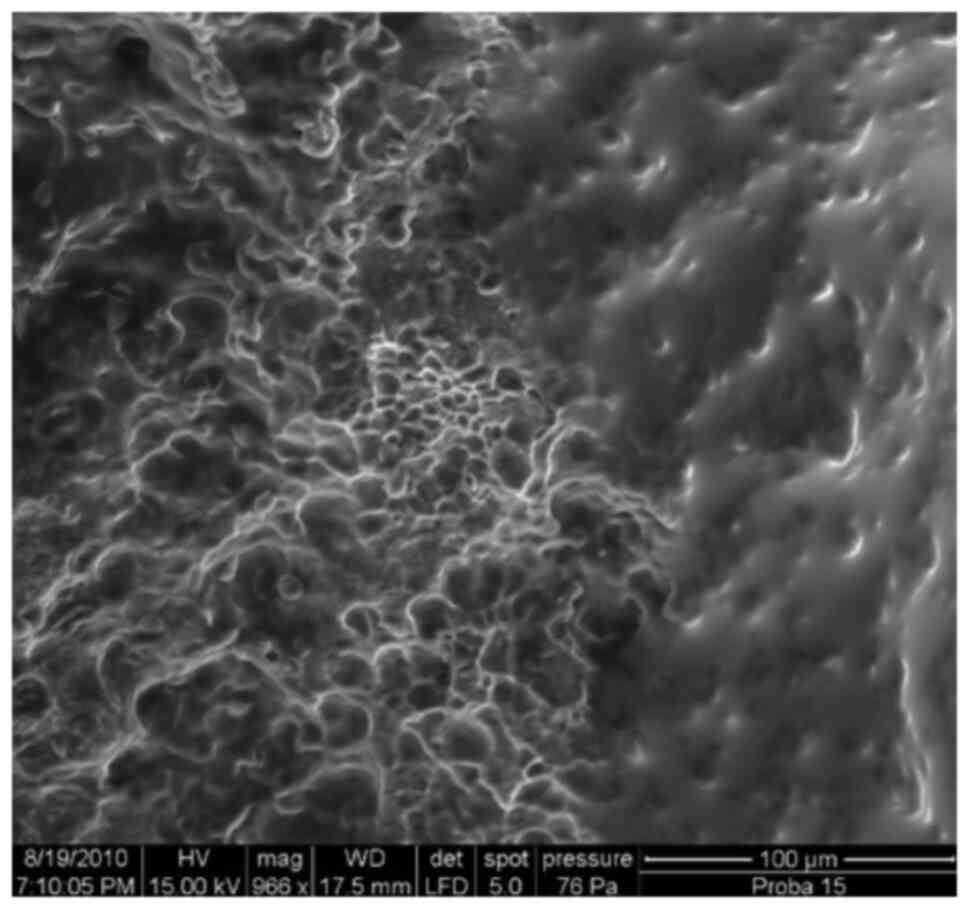
Introduction
Bacterial biofilm infections often involve
aggregates of bacteria heterogeneously distributed throughout a
tissue or on a surface (such as implanted medical devices).
Identification of a biofilm infection requires direct visualization
via microscopy, followed by characterization of the microbial
community by culturing or sequencing-based approaches (1).
During recent years, extensive research has led to a
better understanding of the etiology, pathogenesis and pattern of
progression of periodontal diseases. Scanning electron microscopy
(SEM) has contributed to this improvement, mainly with respect to
histology of periodontal tissues, the description of the morphology
and distribution of bacteria on the exposed root surface, analysis
of the host-parasite interactions on the gingival pocket wall, and
morphological evaluation of root treatment (2). Recently, it was suggested using SEM
photographs that the cemento-enamel junction could act as a ‘trap’
to biofilm and calculus (3).
Important progress has been made concerning the description of the
endodontic biofilm associated with apical lesions (4-6).
It was demonstrated that bacteria can live in biofilms and maintain
endodontic infections within periapical lesions (4,7).
SEM is an excellent, highly descriptive observation
method for any type of biofilm. Structure of bacterial biofilms may
be investigated using several variants of SEM (8). Low-vacuum SEM (so-called ‘Wet-SEM’)
has been used in studies that evaluated the results of Nd:YAG laser
irradiation used to remove the biofilm from periodontally-involved
roots. The laser irradiation at 70-100 mJ, 20 pps for 2 sec caused
surface cratering, areas of porosity, pitting, fissures, and
lava-like structures, that were observed under Wet-SEM (9). Low vacuum SEM micrographs were used to
observe the changes in the bacterial plasma-membrane of
drug-resistant S. aureus and P.
Aeruginosa bacterial cells treated with Macropin, a new
antimicrobial (10).
The formation of biofilms in different environments,
including clinical situations, has been studied intensively using a
great variety of microscopic techniques. SEM is a precious tool for
ultrastructural investigation of the general aspect of the biofilm
and its characteristics: Bacterial species, individual bacterial
cells, the glycocalyx and the presence of inorganic biofilm
components. There are various descriptions of SEM use in biofilm
assessment on implants, prosthetic devices, catheter, teeth or
other solid structures in order to establish the role of biofilms
in the persistence of infections (11-14).
The conventional SEM technique needs a complicated procedure:
Sample fixation in glutaraldehyde and/or in osmium tetroxide,
followed by dehydration and coverage (‘sputtering’) of the biofilm
with conductive metallic material (Gold, Palladium) or Carbon. The
low-vacuum SEM observation method differs from traditional
preparation protocols for SEM examination; the method is simple,
quick and offers sample protection.
Endodontic infection may lead to bone resorption,
development of periapical lesion and also extraradicular biofilm
infection, as reported in two studies on teeth with
therapy-resistant lesions (4,7) and on
teeth with pulp necrosis and chronic periapical lesions (15,16).
The endo-periodontal biofilm, situated on radicular areas of
confluence between the endodontic and periodontal pathology, raises
a special interest for researchers interested in SEM observation,
due to its development on rough mineralized surfaces, subject to
extension of plaque both from the root canal and from the
periodontal pocket.
Although the distribution and morphology of the
apical and radicular infections in periodontal pockets are well
studied, there is no data until now in the literature regarding the
morphology and the composition of the biofilm of combined
endodontic-periodontal lesions (EPL).
The aim of this study was assessment of the biofilm
on root surfaces of teeth with EPL with a modified protocol, using
a simplified histological method to prepare specimens examined
under low-vacuum SEM.
Patients and methods
Using aseptic surgical techniques and sterile
instruments, 25 teeth with EPL diagnosed clinically and
radiographically and with indication of extraction were extracted
under local anesthesia in the Department of Periodontology of the
‘Victor Babes’ University of Medicine and Pharmacy of Timisoara,
Romania. An informed consent was obtained from all patients and the
study was approved by the Research Ethics Committee of the ‘Victor
Babes’ University of Medicine and Pharmacy (ethics approval no.
12b/2009). In addition to severe EPL, the teeth had either deep
circular periodontal defects (pocket depth over 7 mm) with
increased mobility, or advanced furcation involvement, or extensive
carious destruction. All teeth were asymptomatic and had no
fistula. For each tooth, the following data were recorded: The type
of EPL (according to the Simon, Glick and Frank classification,
1971, based on the case history), the position of the periodontal
pocket, the presence/absence of vitality, the existence of a root
canal filling, the pocket depth, the clinical attachment level, the
gingival recession, the plaque index PlI (17), the bleeding on probing BOP, the
furcation involvement (18), and
the mobility (on the Miller scale). The clinical parameters were
used for statistical correlations (data published elsewhere). These
findings are included in Tables I
and SI.
 | Table IIdentification and clinical
characteristics of the teeth included in the study. |
Table I
Identification and clinical
characteristics of the teeth included in the study.
| Sample | Tooth | EPL type | Site (perio pocket)
position | Vitality | Endo tx. | PD | CAL | Rec. | PlI | BOP | Furcation
involvement | Mobility |
|---|
| EP1 | 1.1. | EI-PII | M, V | N | Y | 8 | 14 | 6 | 3 | 5/6 | - | II/III |
| EP2 | 1.1. | PI-EII | D | N | N | 10 | 12 | 2 | 2 | 4/6 | - | II |
| EP3 | 2.1. | PI-EII | M, D | N | N | 9 | 11 | 2 | 3 | 6/6 | - | II/III |
| EP4 | 1.8. | PI-EII | V, D | Y | N | 8 | 8 | 0 | 2 | 6/6 | 1 | - |
| EP5 | 1.2. | EI-PII | D | N | N | 12 | 13 | 1 | 3 | 3/6 | - | II |
| EP6 | 4.6. (M) | EI-PII | M, V, L | N | N | 13 | 14 | 1 | 2 | 4/6 | 3 | I/II |
| EP7 | 4.1. | PI-EII | Circ. | Y | N | 7 | 14 | 7 | 2 | 6/6 | - | III |
| EP8 | 1.6. | EI-PII | M | N | N | 10 | 12 | 2 | 3 | 6/6 | 2 | II/III |
| EP9 | 2.5. | PI-EII | Circ. | N | Y | 12 | 12 | 0 | 2 | 4/6 | - | - |
| EP10 | 4.6. (D) | PI-EII | D, V, L | Y (1/2) | N | 14 | 16 | 2 | 1 | 3/6 | 3 | I |
| EP11 | 3.7. (M) | EI-PII | M, V | N | Y | 12 | 13 | 1 | 1 | 2/6 | 1 | I |
| EP12 | 4.5. | PI-EII | D | N | Y | 6 | 8 | 2 | 1 | 3/6 | - | I/II |
| EP13 | 1.7. | PI-EII | Circ. | Y | N | 15 | 15 | 0 | 2 | 4/6 | - | - |
| EP14 | 1.7. | PI-EII | M | N | Y | 7 | 11 | 4 | 1 | 2/6 | - | I/II |
| EP15 | 1.7. | PI-EII | M, D | Y | N | 14 | 15 | 1 | 2 | 5/6 | 2 | II |
| EP16 | 4.5. | PI-EII | Circ. | N | Y | 13 | 15 | 2 | 3 | 6/6 | - | III |
| EP17 | 3.8. | EI-PII | M | N | N | 12 | 13 | 1 | 2 | 3/6 | 2 | I/II |
| EP18 | 4.8. | PI-EII | M | Y | N | 10 | 14 | 4 | 2 | 3/6 | 1 | II |
| EP19 | 3.7. (M) | EI-PII | M | N | N | 11 | 11 | 0 | 2 | 3/6 | 3 | I/II |
| EP20 | 1.4. | PI-EII | Circ. | N | N | 10 | 13 | 3 | 3 | 5/6 | - | II/III |
| EP21 | 3.1. | PI-EII | Circ. | N | N | 12 | 14 | 2 | 3 | 4/6 | - | II/III |
| EP22 | 3.5. | EI-PII | Circ. | N | Y | 15 | 15 | 0 | 2 | 6/6 | - | III |
| EP23 | 4.3. | PI-EII | Circ. | N | Y | 14 | 14 | 0 | 3 | 5/6 | - | III |
| EP24 | 3.7. | PI-EII | D, M | N | N | 11 | 14 | 4 | 3 | 4/6 | 2 | III |
| EP25 | 4.5. | PI-EII | Circ. | N | Y | 11 | 12 | 1 | 2 | 3/6 | - | II |
After extraction, the samples were carefully and
gently rinsed with sterile saline solution, in order to avoid the
disruption of the biofilm and to remove any biological material
that could possibly come in contact with the root during the
extraction (e.g., blood). The samples were prepared according to
the protocol described by Noiri and Ebisu (5), modified for examination in low-vacuum
SEM. For all further manipulation of the samples, delicate pliers
were used only on the coronal third of the roots, in order not to
disrupt the biofilm. The samples were introduced in vials for
fixation in modified Karnovsky solution (glutaraldehyde 2.5%,
paraformaldehyde 4%, sodium cacodylate 0.1 M at pH 7.2-7.4); the
transportation of the vials to the laboratory took utmost care to
prevent as much as possible the samples to touch the walls of the
vials. In the Department of Histology of the ‘Victor Babes’
University of Medicine and Pharmacy of Timisoara, the samples were
dehydrated in series of ethanol (70, 95 and 100%), changed every 15
min. Because the prolonged immersion in 100% ethanol could
irreversibly modify the aspect of the biofilm through extreme
dehydration, only 3 samples at a time were dehydrated and then
immediately examined under low-vacuum SEM in the laboratory of the
Department of Mechanics and Material Resistance of the Politehnica
University of Timisoara using the SEM Inspect S (FEI), under
pressures of 80-250 Pa and acceleration voltage of 15 kV. For
samples with higher conductivity, the pressure used was 80 Pa,
while for those with lower conductivity the pressure used was 150
Pa, as the conductivity is known to increase with the density of
the examined biologic material.
Before SEM examination, all samples underwent a
preliminary examination under a light microscope. Using sterile
instruments, the samples were fixed in a special device with
clamps, first in a vertical position to examine the apical part.
After the examination of the apical part, each sample was inclined
and then fixed horizontally for examination of the root surface
corresponding to the periodontal pocket. The primary examination
was performed under magnification x50-x80, in order to localize the
apical foramen or to select the main apical foramen in case there
was more than one. The magnifications x200, x500 and x800 were used
for the examination of the external radicular surface, for the
areas of cemental and dentinal resorptions, and for the detection
of the presence of the bacterial film. Finally, the magnifications
x1,000-x20,000 were used for the detection and characterization of
the morphology of the microorganisms. The bacteria included in the
biofilm, as well as the solitary microorganisms on the hard
surfaces were morphologically categorized in cocci, rods, motile
(spirochete, spyrils) and filaments. Through graphic delimitation
of specific areas of pictures and using the Print screen function,
the objects of interest were identified. The chronological list of
these areas under increasing magnification was saved on a single
Word document and registered under the tag of each sample, to make
sure the identification of each object of interest can be re-traced
at any time later.
For the SEM topographical examination of the
biofilm, target zones on the apical surface of each sample were
defined as follows: The internal wall of the cemental cone (its
biofilm mostly seen as an extension of the root canal infection,
sometimes in the presence of root canal filling materials); the
near-foraminal (peri-foraminal, juxta-foraminal) zone (present in
any typical chronic apical infection); the ‘transition’ zone
between the near-foraminal and the periodontal pocket zone (of
great interest in the hypothesis that it harbors biofilm with mixed
morphology: Endodontic and periodontal); the periodontal pocket
zone (harboring typical periodontal biofilm).
The ‘transition’ zone was considered to be limited
apically by the marks of the former apical lesion (cemental
resorptive lacunae for the cases of EPL with primary endodontic
onset and for the very rare cases of simultaneous EPL) and
coronally by the apical limit of the calculus deposits typical for
periodontal pockets. For EPL with primary periodontal onset and no
marks of resorbtion available, the ‘transition zone’ was considered
to begin at 2 mm coronally to the crest of the cemental cone.
As the understanding of the radicular biofilm
morphology needs a preliminary ‘inspection’ phase, a collection of
characteristic SEM images was created, in order to provide typical
visually recognizable elements for further reading of the images of
the samples. Figs. 1, 2, 3,
4, 5, 6,
7, 8, 9,
10, 11, 12,
13, 14, 15,
16, 17, 18,
19 Fig. 20 represent a selection of the most
relevant images for the present study, included in the
collection.
On all 5 zones, the following elements were
qualitatively evaluated: The established biofilm, the glycocalyx
matrix, the presence of isolated microorganisms, the relative
presence of microbial morphologies - cocci, rods, filamentous
forms, motile forms (spirochetes, spirilli), areas of nude
cementum, lacunae of cemental resorption, the presence of calculus,
the presence of unstructured (amorphous) material (debris), the
presence of the root canal material (in cases with endodontic
treatment), the presence of red blood cells as result of the
extraction procedures (as they can mask the biofilm). The presence
of these elements varied, depending on the zone. Specific elements
noted in the near-foraminal zone were the periodontal ligament
fibres, in various degrees of decomposition, depending on the
vicinity with the EPL. In the near-foraminal zone, the transition
zone and the periodontal pocket zone, the biofilm was sometimes
found populating the interior of the cemental lacuna.
The SEM images were independently evaluated by one
researcher (SS). Some of the above-mentioned elements were defined
semiquantitatively, as well. Depending on their quantity on the
studied SEM images, the data were recorded by the examiner to the
following categories: (score 0), absence; + (score 1), + ‘low
quantity’; ++ (score 2), ‘significant quantity’; +++ (score 3),
‘abundant’. The scores were used to establish statistical
correlations.
The resorbtions (cemental lacunae) were separately
analyzed, as they are considered zones of special agglomeration of
the biofilm, by offering a particular shelter to the
microorganisms. The analyzed alements in the resorbtion areas were:
The character of the resorbtions (isolated, multiple, generalized);
their near-foraminal presence (as indicating an old apical lesion),
the SEM appearance of the lacunar relief (apparently shallow,
apparently deep), the presence of the established biofilm, the
predominant morphology of the bacteria (cocci, rods, filaments,
motile species), the presence of isolated bacteria. These values
were qualitatively and quantitatively evaluated, as well, as
described before.
Statistical analysis
The qualitative data for biofilm characteristics on
all EPL zones was summarized by computing rates of prevalence. The
relations between the biofilm characteristics assessed
semiquantitatively were investigated using non-parametric
correlational analysis (Spearman rho correlation coefficients and
corresponding significance tests performed using significance level
α=0.05). The data were analysed using the software R version
4.0.0.
Results
Four out of 25 samples were eliminated during the
primary microscopic examination due to following reasons: Apex
fully covered with calculus and no detectable apex, the complete
absence of the biofilm and microorganisms (due possibly to
incorrect manipulation of the sample), the abundant presence of
residual periodontal fibres that prevented the determination of the
target zones on the root surface. Thus, 21 teeth entered the
examination. A total number of 44 images were selected for their
quality and special relevance and were included in a separate
collection (Figs.
S1-S21). In all samples, the cementum presented near the apical
foramen apparently shallow or deep areas of resorption of various
shapes and dimensions, some including clusters of agglomerated,
inserted residual collagen fibres. Within the biofilm in these
cemental lacunae, microorganisms were present, either monomorphic
(cocci, rods, filaments, motile forms), or in association. In some
specimens, small resorbtion areas, containing biofilm, were noted
on the intact cementum. Only 5 (24%) teeth with EPL included in the
study presented a mature biofilm on the inner surface of the
cemental cone, and 2 (9.5%) presented isolated microorganisms, 38%
cocci and 5% rods. In 3 samples (14%), the matrix (glycocalyx) with
few microorganisms was observed on the inner wall of the cemental
cone.
On the near-foraminal zone, mature biofilm was found
in 4 out of 21 samples (19%) and isolated microorganisms only in 1
sample (4.7%). The identified microorganisms were in 28.5% of the
samples cocci, in 9.5% of the samples rods, motile forms (spirilli)
in only 1 sample (4.7%). In 1 sample a matrix poor in
microorganisms was found, and in another 1 sample isolated
microorganisms.
In the ‘transition’ zone, mature biofilm was found
in 8 out of 21 samples (38%) and isolated microorganisms in 5 other
samples (24%). In 57% of the samples cocci were identified and in
further 24% rods, filaments in 24% and no motile forms were found.
In this zone 6 out of 21 samples (28.5%) presented marked
resorbtions; in 3 samples (14%) the resorbtion lacunae were
populated by biofilm. In 24% of the samples, the ‘transition zone’
presented residual periodontal fibers and 24% presented
calculus.
In the periodontal pocket zone, especially on the
calculus deposits, the biofilm percentage increased to 52.3% of the
samples, 62% of these showed cocci, 38% rods, 23.8% filaments but,
surprisingly, no sample presented motile forms. Less resorbtions
were found (4.7%), residual periodontal fibers (38%) and isolated
bacteria (14.2%).
The special separated analysis, of the biofilm in
the cemental resorbtion lacunae, using the same criteria, revealed
in 24% of the samples thick, mature biofilm (5 out of 24 samples),
52% presented cocci, 19% rods and no motile form or filament.
Fig. 20 shows the frequency of the
distributions of all investigated parameters, in all zones of
interest.
The statistical analysis of the data revealed the
presence of mature biofilm on the inner wall of the cemental cone
in 24% of the samples; this drops to 19% in the near-foraminal zone
and then increases to 38% in the ‘transition zone’ and to 52.3% in
the periodontal pocket zone. Abundant quantity of biofilm was found
only in the ‘transition zone’ (9.5%) and in the periodontal pocket
zone (19%).
The extracellular matrix (glycocalyx) poor in
bacteria was found on the inner wall of the cemental cone in 14% of
the samples, on the near-foraminal zone in 4.7% of the samples, in
9.5% in the ‘transition zone’ and more frequently in the
periodontal pocket zone (14.2%). An appreciable amount of
extracellular matrix poor in microorganisms was found only in the
‘transition zone’ in 9.5% of the samples.
Isolated microorganisms were found in 9.5% of the
samples on the inner wall of the cemental cone, 4.7% in the
near-foraminal zone, in 24% of the samples in the ‘transition zone’
and in 14.2% of the samples in the periodontal pocket zone. In the
‘transition zone’, in 9.5% of the samples, the quantity of isolated
microorganisms found was abundant.
The coccoid morphology was found in 38% of the
samples on the inner wall of the cemental cone, in 28.5% in the
juxtaforaminal zone, in 57% of the samples in the ‘transition
zone’, and in 62% in the periodontal pocket samples. Abundant
accumulations of cocci were found in the near-foraminal zone in
4.8% of the samples, in 19% of the samples in the ‘transition zone’
and in 14.3% of the samples in the periodontal pocket zone.
The proportion of the rods increased from 5% of the
samples on the the inner wall of the cemental cone to 9.5% in the
near-foraminal zone, 14% in the ‘transition zone’ and to 38% in the
periodontal pocket zone. An abundant quantity was found only in the
periodontal pocket zone, in 4.8% of the samples. Filamental
morphology of the bacteria was found neither on the inner wall of
the cemental cone zone, nor in the near-foraminal zone, but in 24%
of the samples on the ‘transition zone’ (4.8% in abundant quantity)
and in 23.8% of the samples in the periodontal pocket zone.
Under SEM observation, motile forms
(spirils/spirochetes) were found only in the near-foraminal zone in
4.7% of the samples in reduced quantity (4.8% of the samples). The
cemental resorbtions were found in 14% of the samples on the inner
wall of the cemental cone, in 43% of the samples in the
near-foraminal zone, in 28.5% in the ‘transition zone’ and 4.7% in
the zone of the periodontal pocket. Biofilm was found inside the
resorbtion lacunae in 4.7% of the samples in the near-foraminal
zone, 14% in the ‘transition zone’ and 23.8% in the periodontal
pocket zone.
Calculus was not found on the inner wall of the
cemental cone, but it was found in 4.7% of the samples in the
near-foraminal zone, in 24% of the samples in the ‘transition
zone’, and in 81% of the periodontal pocket zone samples. In 9.5%
of these samples, the quantity found was considered abundand. The
correlational analysis for each parameter on all four investigated
zones is represented in Table
II.
 | Table IISignificant correlations between the
investigated parameters (Spearman's rho and corresponding
P-values).a |
Table II
Significant correlations between the
investigated parameters (Spearman's rho and corresponding
P-values).a
| Variable 1 | Variable 2 | Spearman's rho | P-value |
|---|
| Established biofilm
(1) | Cocci (1) | 0.79 | <0.001 |
| Established biofilm
(1) | Established biofilm
(2) | 0.61 | 0.004 |
| Established biofilm
(1) | Established biofilm
(3) | 0.47 | 0.033 |
| Glycocalyx devoid
of bacteria (1) | Glycocalyx devoid
of bacteria (2) | 0.55 | 0.010 |
| Glycocalyx devoid
of bacteria (1) | Isolated bacteria
(2) | 0.79 | <0.001 |
| Isolated bacteria
(1) | Isolated bacteria
(2) | 0.79 | <0.001 |
| Isolated bacteria
(1) | Isolated bacteria
(3) | 0.50 | 0.022 |
| Cocci (1) | Established biofilm
(2) | 0.66 | 0.001 |
| Cocci (1) | Cocci (2) | 0.92 | <0.001 |
| Rods (1) | Rods (2) | 0.69 | 0.001 |
| Rods (1) | Rods (3) | 0.58 | 0.006 |
| Resorptions
(1) | Resorptions
(2) | 0.62 | 0.003 |
| Resorptions
(1) | Resorptions
(3) | 0.64 | 0.002 |
| Established biofilm
(2) | Cocci (2) | 0.72 | <0.001 |
| Rods (2) | Calculus (2) | 0.69 | 0.001 |
| Rods (2) | Rods (4) | 0.54 | 0.012 |
| Calculus (2) | Biofilm in cemental
resorptions (2) | 1.00 | <0.001 |
| Established biofilm
(3) | Cocci (3) | 0.84 | <0.001 |
| Cocci (3) | Cocci (4) | 0.51 | 0.018 |
| Calculus (3) | Rods (4) | 0.52 | 0.015 |
| Calculus (3) | Calculus (4) | 0.47 | 0.033 |
| Established biofilm
(4) | Cocci (4) | 0.89 | <0.001 |
| Established biofilm
(4) | Filaments (4) | 0.75 | <0.001 |
| Established biofilm
(4) | Naked cementum
(4) | -0.76 | <0.001 |
| Glycocalyx devoid
of bacteria (4) | Isolated bacteria
(4) | 1.00 | <0.001 |
| Rods (4) | Calculus (4) | 0.49 | 0.024 |
Discussion
As far as the authors know, this is the first SEM
study of the biofilm of EPL. This is also the first study
describing a radicular ‘transition’ zone at the intersection of the
endodontic and periodontal microbiota. It is also evident that, the
older the lesion, the fewer the morphological differences in the
biofilms of adjacent regions, which raises the question of common
characteristics for endodontic and periodontal lesions.
The low-vacuum SEM examination of bacterial biofilm
on root surfaces proved useful in the present study because the
method allowed the preservation of samples and avoided high
electrostatic loads during examination. The sample preparation
method for low-vacuum SEM examination differs from the usual SEM
preparation method for biologic samples, in which the dehydration
of the samples is completed by using CO2 in drying
devices at a critical point, followed by fixation of the samples
with adhesive conductive silver on metallic support discs, and the
sputtering of samples with 5-10 nm of pure gold to make them
conductive. This protocol is described minutiously by Leonardo
et al (19), indicating a
thickness of 200 µm of the gold sputtering coating. Another
protocol completes the dehydration of the samples in a
lyophilization device using t-butylic alcohol, and sputters the
samples with osmium oxyde with a 5A thick conductive layer obtained
with a plasma-multicoater device (6). Both described methods are
technique-sensitive and expensive.
In this study, the dehydratation procedere in
increasing alcohol concentrations was completed with a light
air-blow for a few seconds. The dehydratation was maximized by
lowering the pressure inside the microscope vat up to 80-250 Pa
(low-vacuum). This procedure extracts all alcoholic remnants from
the samples, carefully and at slow pace. Despite the careful
induction of the low-vacuum, several samples presented signs of
biofilm disruption, bacterial body damage and root surface cracks,
indicating further need for a better control of the experimental
procedure.
The SEM observations focused on a root surface
including the continuum root surface inside the periodontal pocket
- a so-called ‘transition zone’ (between the apical part of the
root surface inside the periodontal pocket and the former initial
apical lesion, corresponding to the former confluence zone between
them), the near-foraminal zone (the surface of the apex inside the
former apical lesion), the inner wall of the cemental cone. Part of
the continuum described above is the ‘plaque-free-zone’ (PFZ)
described by Brady (20), a
near-apical root zone situated in the periodontal pocket underneath
the most apical extension of the calculus tartar, where bacteria
organized as biofilm (plaque) tend to disappear, as a consequence
of the permanent and sustained cell defence of the host near the
epithelial junction. In our experiment, it was considered that the
conventional zones delimitated as above are potentially containing
biofilm with noteworthy individual characteristics.
The delimitation of the observation zones on the
root surface was conventional and not without difficulties. The
morphology serving to delimitation of both the inner wall of the
cemental cone and the near-foraminal zone was relatively clear. The
‘transition’ zone was apically delimited by the typical landmark of
the former apical lesion (cemental resorbtions with typical texture
and clear limits in EPL with primary endodontic origin and in the
rare case of combined simultaneous EPL) and coronally by the apical
limit of calculus deposits existant in the periodontal pockets. In
the case of primary periodontal EPL, the ‘transition’ zone was
conventionally defined as starting at 2 mm distance from the
periforaminal crest. In this study, the endo-periodontal microbial
population organized as a biofilm, was studied in all 4
conventional zones described: The inner wall of the cemental cone,
the near-foraminal zone, the ‘transition’ zone, the periodontal
pocket zone. The observations followed the variations of
distribution of characteristic morphological elements of the
biofilm in a continuous mode, from the terminal zone of the root
canal to the radicular wall of the periodontal pocket. A first
conventional division of the peri-foraminal region of apices with
pulpal infections and apical lesions of pulpal origin is known in
the literature as ‘extraradicular zone’. This described the apical
external zone, outside the root canal (4,7,19,21).
A study from 2005 explains that the term ‘extraradicular zone’ is
used clinically in contrast to the ‘root canal’, that defines
anatomically the apical foramen (21). Although the ‘extraradicular zone’ is
situated inside the chronic periapical lesion, it is considered
distinct of the lesion. The lesion represents the volume of
resorbed alveolar bone around the apex, which often contains
granulation tissue. The approximately 2 mm area around the apical
foramen (designated in our study as ‘near-foraminal’ or
‘juxta-foraminal’ zone) was separately investigated only in one
study (15).
The morphology of the bacteria and biofilms on
apices associated with refractory and chronic periapical
periodontitis was thoroughly investigated in a classic study
(6). Another SEM study of the
periodontal biofilm investigated seven perio-pathogenic bacteria in
the biofilm of the ‘plaque-free zone’ by scanning immunoelectron
microscopic techniques, using both secondary and back-scattered
imaging, with rabbit antibodies specific for each bacteria
(5). Brady (20) described first the PFZ as an area on
the root of extracted teeth, situated between the apical plaque
limit and the epithelial attachment. The area raised interest for
many researchers in the past (22-24).
Brady observed that this zone displayed a surprisingly low number
of bacteria, hence its name. Today, it is considered that the
apical limit of this zone is not clearly delimited. The PFZ is
situated adjacent to the epithelial attachment, and the bacteria
found seem to resist the antimicrobial host response, whereas most
of the microorganisms found at the apical limit of this region are
destroyed (25). Starting from the
concept of PFZ, in our study the limits of the ‘transition’ zone
were defined between the bottom of the former periodontal pocket
and the former apical lesion.
In our study, the frequency of detection of mature
biofilm was found only in 24% of the samples on the inner wall of
the cemental cone, in 19% of samples in the near-foraminal zone
(zone corresponding to the former endodontic lesion), and is
constantly increasing with distance to the apical foramen: 38% in
the ‘transition’ zone and 52.3% in the periodontal pocket. In the
literature, available data vary greatly. A study on 21 extracted
teeth found apical biofilm and microorganisms in all investigated
samples with pulpal necrosis and radiographically visible lesions
(19). These results match the
results of another study using an optical microscope, which
detected the biofilm in 10 out of 16 extracted teeth with pulpal
necrosis and periapical granuloma (26), but contradict the data described by
later research, that found biofilm only on 3.7% of the teeth with
apical necrosis and periapical lesions (27). More recently, mature extraradicular
biofilm was found in 20 out of 27 patients with refractory apical
periodontitis (21). Finally,
established biofilm was found on 106 roots with apical
periodontitis bacteria, with only one exception. The same study
noted that the presence of biofilm in cysts, abscesses and
granulomas was 95, 83 and 69.5%, respectively (28). A microbiological study reported the
microbial colonization of the external apex surface of teeth with
pulpal necrosis and apical periodontitis with cocci, rods,
coccobacilli, filaments, spirochetes and the presence of biofilm in
the apical 2 mm, in the vicinity of the foramen, in 83.3% of the
cases (15). The literature
includes reports of biofilms with large masses of microorganisms on
teeth with long-term endodontic lesions and periapical lesions,
constituted from various morphotypes-cocci, bacilli and filaments
(29). This demonstrates the
heterogeneity of microbial colonization in long-term apical
lesions, as shown by a series of studies (4,16,30).
In addition to the data found in the current
literature, (e.g., the frequency of presence of mature biofilm),
our study recorded various other relevant data: The matrix poor in
microorganisms, the presence of isolated microorganisms,
semiquantitative data on the composition of the biofilm with
respect to the microbial morphology (cocci, rods, filaments, motile
forms), the presence of resorptions, of calculus, of periodontal
residual fibers, of amorphous debris.
The comparative distribution of the matrix poor in
microorganisms follows surprisingly the same pattern: 14% of
samples on the inner wall of the cemental cone, decreases to 4.7%
in the near-foraminal zone, increases to 9.5% in the ‘transition’
zone and to 14.2% in the periodontal pocket. The scientific
interest for the study of the biofilm matrix poor in microorganisms
and for isolated bacteria, is justified by a study on
experimentally infected teeth in monkeys, that found no periapical
bacterial colonies (31). The
authors concluded that extraction or surgical procedures may lead
to contamination of the periapical tissues and subsequently to
possible false-positive findings in human samples. The presence of
a matrix poor in microorganisms on the apical radicular surface can
be explained, in our opinion, either by a primary colonization
stage (implausible in EPL, because of their remote onset) or to
local defense mechanisms against biofilm accumulation, possible
characteristic for EPL. On the contrary, the total absence of
matrix and biofilm (the so-called ‘naked cementum’) may be due to
the destruction of biofilm during the histological sample
preparation.
The existing literature does not present data
regarding the presence of the different bacterial morphologies in
the apical and periodontal biofilm. A classic study observed a
morphological variety of cocci, rods and filaments, as well as
associations between rods and filaments (19). These results matched the results of
another study, that found bacteria, yeasts and biofilm in the
vicinity of the foramen, in the areas of radicular resorption and
on the external surface of human teeth apices with pulpal necrosis
and chronic apical lesions (16).
Some later studies identified the bacterial species in the biofilm
with PCR immunohistochemical methods, insisting on the role of
P. gingivalis in the primary extraradicular colonization
(21). Our SEM study shows
semiquantitative data on the relative proportion of the coccoid
morphologies (cocci being considered primary colonizers) in the 4
experimental zones. Abundant accumulations of cocci were found in
the near-foraminal zone in 4.8% of the samples, in 19% in the
‘transition’ zone and, in 14.3% of the samples in the periodontal
pocket zone, and very little on the inner wall of the cemental
cone.
Rods were found in our study in 5% of the samples on
the inner wall of the cemental cone, 9.5% in the near-foraminal
zone, 14% in the ‘transition’ zone and to 38% in the periodontal
pocket zone. An abundance of rods was found only in the periodontal
pocket zone in 4.8% of the samples in agreement with literature
data (5). Coccoid microorganisms
were observed in this study mostly on the biofilm surface (probably
during release phases from clusters), and in monomorphic and mixed
agglomerations. In an in vitro study on gutta-percha cones,
it was found that cocci were localized in deeper biofilm layers, as
they play an important role in the biofilm initiation (32).
In contradiction to the data from the literature,
that found rods and filaments on the entire peri-foraminal and
extraradicular area (a real ‘eruption’ of filaments) (5), in our study filaments were not found
on the inner wall of the cemental cone zone, neither in the
juxta-foraminal zone, but in 24% of the samples in the ‘transition’
zone (4.8%, in abundance) and in 23.8% of the samples in the
periodontal pocket zone. The rods and filaments populating the
terminal root canal are described in the literature as in equal
quantity with the cocci (4),
situation which was not found in this study.
Finally, spirils/spirochetes were found only in the
near-foraminal zone in 4.7% of the samples in low quantities. There
is no explanation for their absence, except that they might be
captured in the deeper biofilm layers, which prevented their
visibility under SEM. Spirochetes associated with endodontic and
periodontal infections were described in the ‘plaque-free-zone’
(20), their dimensions were
measured as 140 microns long and 2 microns thick, longer and
thicker as treponema (33). They
were found in the 2 mm near the apex of teeth with pulpal necrosis
and periapical lesions (15).
Another study detected spirochetes longer than 20 microns (5). None of these studies detected motile
forms in EPL, while the only spirilar form detected in our study
was 25 microns long and 1.5 microns thick. The fact that motile
bacteria and filaments were not observed in our study does not mean
they do not exist in the biofilm of EPL, they could be hidden in
deeper layers of the biofilm, inaccessible to free observation from
above.
As a general observation, in our study we found a
common variation of detection frequency of all investigated
characteristics: A slight decrease or no change from the inner wall
of the cemental cone to the near-foraminal zone, followed by a
slight raise towards the transition zone, and a more pronounced
increase towards the periodontal pocket zone.
Of special interest for our research was the
distribution of cemental resorptions and their population with
isolated and aggregated bacteria. This study includes observation
of SEM characteristics of cemental resorptions in EPL, important
for biofilm formation and persistence (e.g., frequency, relative
depth, localization, fiber- and biofilm content). The incidence of
radicular resorptions as potential sites for persistent biofilm on
39 apical thirds of extracted teeth was evaluated in a recent study
(34). All samples presented
irregular resorption areas with different depths, different
configurations and extensions, especially localized around the
apical foramen. It is already shown that cemental resorption of
teeth with apical lesions were deep and included the whole
peri-foraminal surface (19),
whereas external resorptions were found only in few specimens
(5). Our study found the highest
frequency of cemental resorptions in the juxta-foraminal zone (43%
of the samples), zone that presented a high incidence (9.5% of the
samples). This distribution is a logical consequence of the
prolonged presence of the chronic apical lesion in the
near-foraminal zone, which initiates and maintains the
cementoclastic processes that lead to resorptions.
On the contrary, our study found biofilm in the
cemental lacunae. There are data in the literature that show the
retention and colonization of microorganisms in the cemental
resorption sites (4,16). In 1973, Brady (20) found a population of short and long
bacilli, spirochetes and filaments in cemental resorptions in the
‘plaque-free-zone’. All these data are connected by the concept of
bacterial adhesion by Quirynen et al (35), in which the roughness of the surface
and the free superficial energy of the solid substrate play an
important role. On a rough surface, the bacteria are more protected
against the detachment forces, so the change from reversible to
irreversible attachment takes place easier and more often. This is
illustrated in our study by the presence of biofilm in the cemental
lacunae: In 4.7% of the samples in the near-foraminal zone, 14% in
the transition zone and 23.8% in the periodontal pocket zone. The
increased frequency of biofilm detection in the ‘transition’ zone
(in 4.8% of samples considered even as abundant) is interesting,
when compared with the near-foraminal zone, demonstrating the
influence of the periodontal lesion on the apical microbiology. The
findings in this study show a coexistence of resorption sites with
abundant microorganisms and areas of naked radicular cement with
absolutely no bacteria. The finding of a ‘perfect hide-away’ of
cemental resorptions for the biofilm shows once again its
resistance to therapeutic measures.
The images in this study show the existence and
persistence of bacterial colonies organized as biofilm also in
fissures of hard radicular surfaces, in cementum and calculus
deposits. The decisive influence of the surface roughness and free
superficial energy on the bacterial adhesion and biofilm formation
is demonstrated in this study by the relation between the frequency
of calculus distribution (in 4.7% of the samples in the
juxta-foraminal zone, in 24% in the ‘transition’ zone samples, and
in 81% of the periodontal pocket zone samples, as expected) and the
presence of mature biofilm: The comparison of the graphics of the
two distributions reveals the increase from the apical foramen to
the periodontal pocket. Despite this, in our study we found areas
covered by calculus but free of biofilm, even inside the
periodontal pocket, demonstrating possible biofilm disruptions
during the preparation of the samples.
The presence of residual periodontal ligament fibers
and their relation with the biofilm microorganisms in the apical
region was recently studied in a group of 18 teeth (36). In teeth with normal healthy pulp and
with necrotic pulp but without radiographic visible apical lesions,
the apical surfaces were covered with collagen fibers in the total
absence of bacteria, whereas in necrotic teeth with radiographic
visible lesions, the apices did not present collagen fibers, but
areas of resorption with microorganisms were found in all samples.
In contrast with these findings, the present study on EPL found
residual periodontal ligament fibers in 14.2% of the samples in the
juxta-foraminal zone, in 24% of the samples in the ‘transition’
zone and in 38% of the samples from the periodontal pocket zone.
Moreover, periodontal fibers were found inside the cemental
resorptions, with an abundant and various microbial content. These
findings are in contradiction with the findings of Leonardo et
al (19), but in accordance to
the findings of the Nakano-Hasegawa et al (29), which found bacterial biofilm in all
teeth with pulpal necrosis and periapical lesions, where the
necrosis affected the periapical ligaments and the external surface
of the apex (19,29). In accordance with our findings, the
latter study found a superior structure and organization of the
biofilm, with a greater microbial mass of various morphotypes
(cocci, rods, filaments) (29).
This may prove, according to several researchers, the heterogeneity
of microbial colonization in long-term lesions (16,30).
It can also be noted that the periodontal ligament destructions in
EPL appears diminished and fragmented, when compared with
circumscribed apical lesions.
While not specifically studying the bacterial
coaggregation in biofilms (the recognition of genetically distinct
cell types and their adhesion) (37), this study revealed a special
morphologic coaggregation type in endo-periodontal biofilms. Apart
from monomicrobial multiplication without detachment, coaggregation
is one of the two mechanisms in biofilm formation. Special examples
for biofilm inter-microbial coaggregation in the periodontal
biofilm are the ‘corncob’ formation, in which streptococci adhere
to filaments of Bacterionema matruchotii (38) or bacterial filaments on which
Gram-negative rods adhere (39).
The coaggregation type observed in our study has the aspect of a
spheroid bacterial agglomeration covered with a glycoproteic matrix
with circular extensions, situated over the mature biofilm, with a
general aspect of ‘wrinkled flower’.
Rods and filaments were correlated to the presence
of rough calculus surfaces. Generally speaking, the strong
correlation between mature biofilm and the presence of cocci
appears in all investigated zones, while the presence of rods and
filaments appeared to depend on the roughness of the surfaces
(calculus and cemental resorptions). Data in our study indicate a
strong correlation between the microbial flora of the three EPL
zones, regardless of the organization form of the microflora
(biofilms or isolated bacteria), provided rough surfaces due to
calculus or resorptions are available.
The SEM investigation of the radicular surfaces
involved in EPL in our study revealed less surfaces covered by
biofilm than expected. There is no explanation for this
observation, knowing also that the resistance to antimicrobial
therapy of EPL is attributed to the persistence and inaccessibility
of the biofilm.
Limitations of the present study include the
conventional choice of the radicular zones and of the morphological
characteristics of the biofilm. The interpretation of the results
was done with caution, because of the age of the EPL, the frequency
of acute episodes in apical lesions and periodontal pockets and the
risk of biofilm destruction during sample preparation.
Several conclusions can be drawn from the present
study. The SEM investigation of radicular surfaces involved in EPL
revealed less surfaces covered by biofilm than expected. The
microbial morphologies described by the present SEM investigation
were mostly coccoid forms, seldom rods or filaments. Spirochetes
were found only accidentally. These findings are contradictory to
literature data.
Our data showed that the mature biofilm appears to
be associated with the roughness of the support, due especially to
the presence of cemental resorptions and calculus. Despite the
communication between the periapical lesion and periodontal pocket,
the biofilm elements seem to be better represented in the
periodontal pocket than in other zones of the EPL.
The present study found relatively little
correlative data. The strong correlation between the mature biofilm
and the presence of cocci appears on all investigated zones, and
the presence of rods and filaments appear to depend on the rugosity
of the surfaces (calculus, cemental resorptions); the data indicate
a strong correlation between the microbiota of the 3 zones in the
EPL. On the contrary, the correlations found might confirm a
similar organization of the apical and periodontal microbiota,
especially in old EPL. Later on, the data of the present study
should be completed with combined analyses SEM-TEM (for
visualization of the horizontal distribution and composition of the
biofilm); histobacteriological and immunobacteriological analysis
for detection of microbiological species in correlation with
clinical parameters of affected teeth (mobility, pocket depth and
sensitivity to percussion); accurate analysis of the coaggregation
and association in the biofilm; correlations between the biofilm
presence and the success of therapeutic measures, because EPL tend
to resist treatment.
Supplementary Material
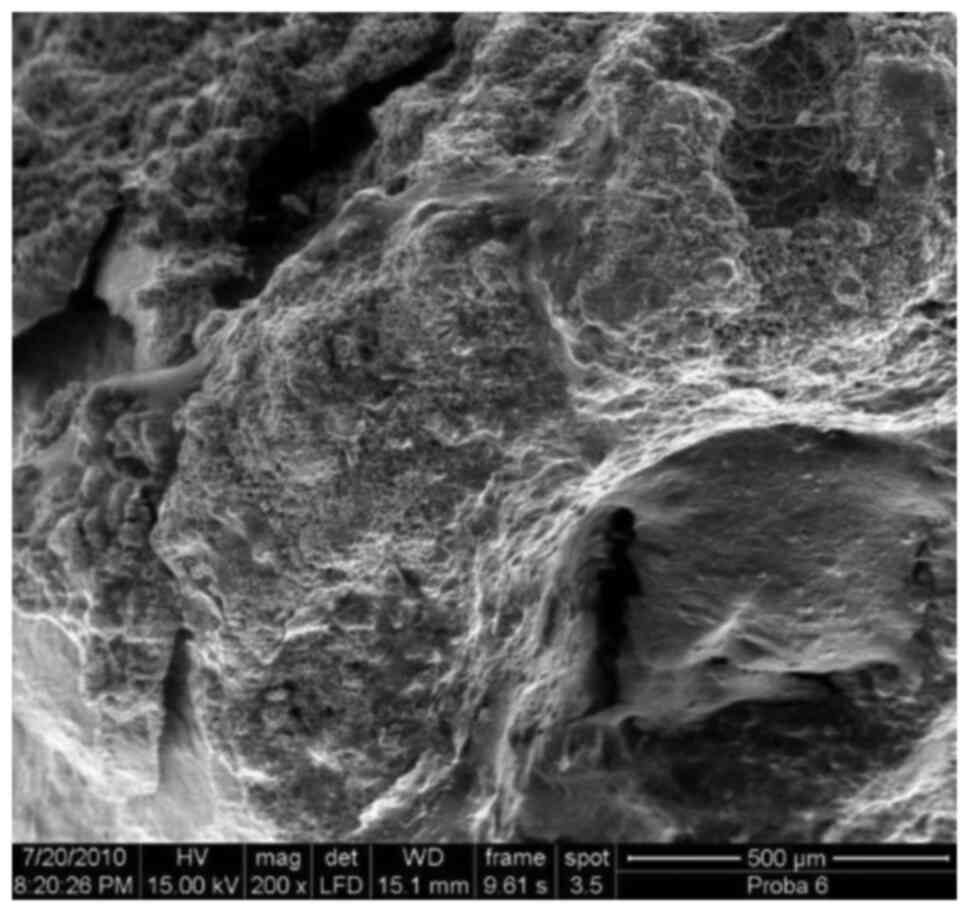
SEM image of an apex, including 3 out
of 4 investigated zones, x50.
Accessory foramen, x100.
Old, multilayered established biofilm
on the root surface in periodontal pocket, x1,200.
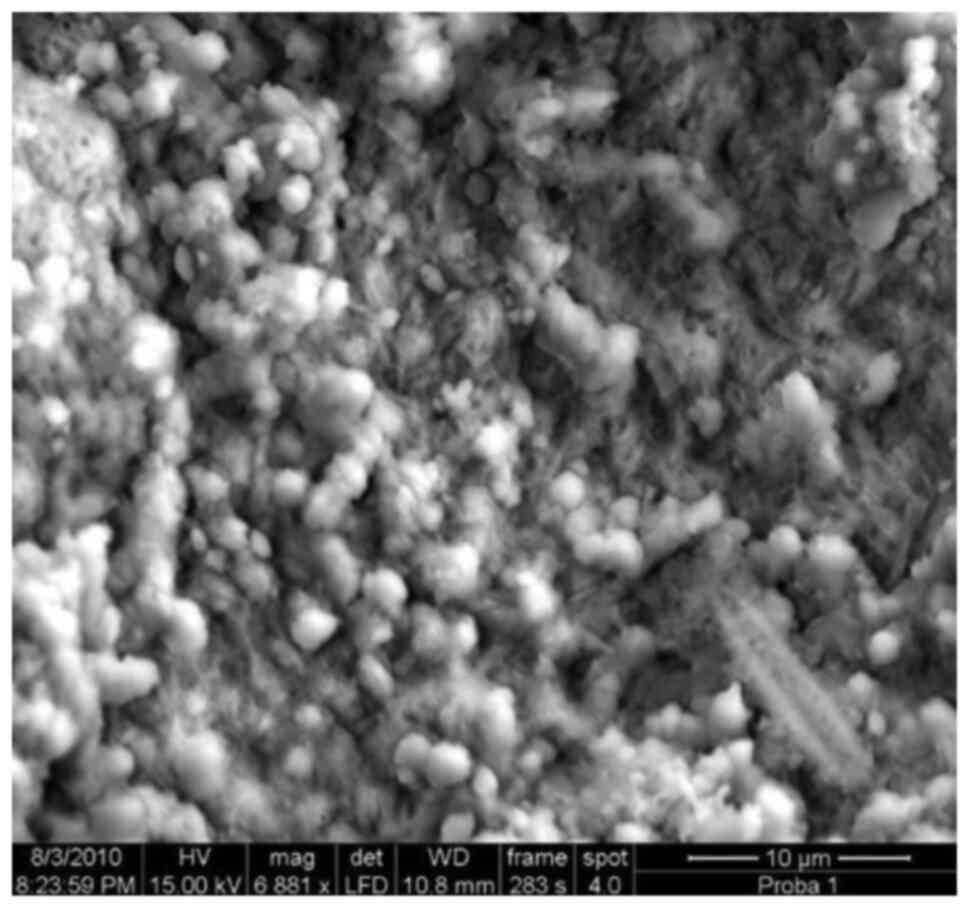
Agglomeration of coccoid bacteria.
Note the fine pellicular strands of glycocalyx partially covering
the layer of cocci, x4,100.
Layer of rods underneath a thick
biofilm layer including mostly coccoid bacteria (9 o'clock),
x8,000.
Residual periodontal fibers of normal
density on the (still) attached root surface, x3,000.
Multilayered aspect of the biofilm
with filamentous microorganisms in the deep layer, x5,900.
Biofilm including mixed microflora.
Large amounts of glycocalyx, abundant mixture of coccoid bacteria
and rods, x7,700.
Nude cement at high magnification,
void of biofilm or isolarted bacteria, x7,900.
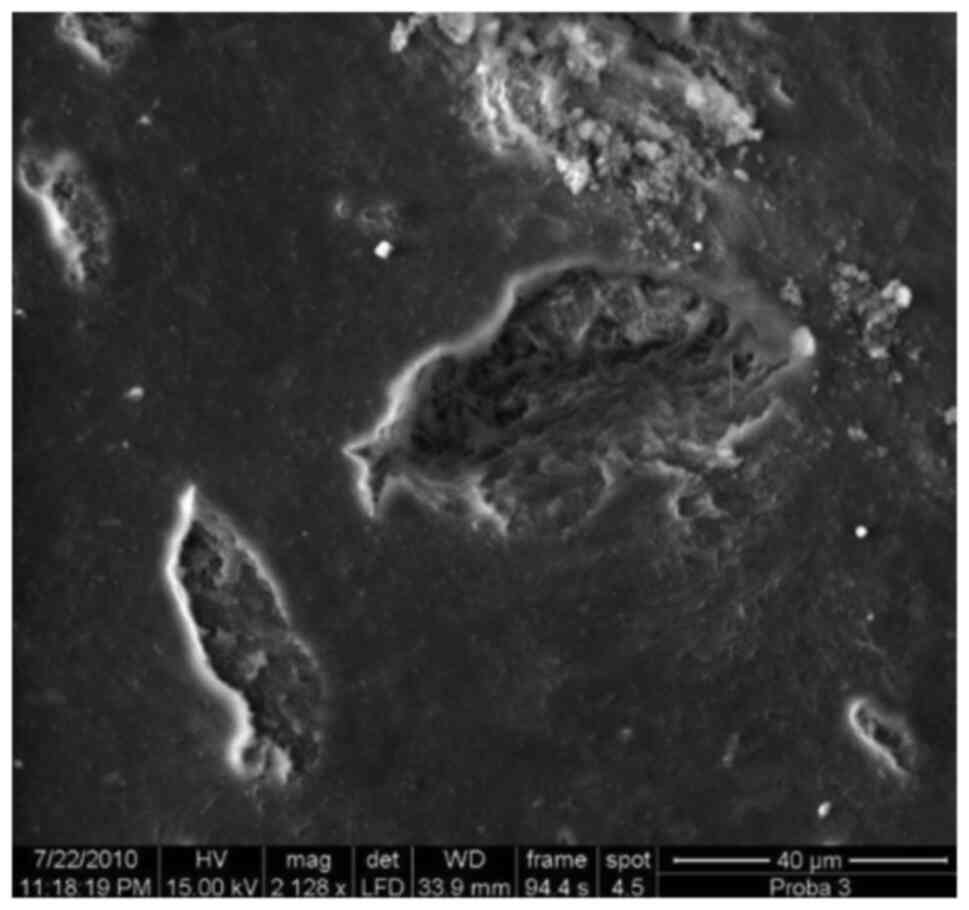
Deep cemental resorbtion with
honeycomb aspect, x1,050.
Laminary coagulum on the root surface.
Note the shrinked erythrocytes included in large fibrin strands,
x2,600.
Microbial agglomeration in a cemental
lacuna. Note amounts of glycocalyx, cocci, rods, residual
periodontal fibers, debris, x7,000.
Abundant mixed flora bordering a
cemental fissure: Note a deep layer of rods, coccoid organisms
(free or included in glycocalyx), isolated superficial
erythrocytes, x3,000.
Laminary, curtain.like (blanket-like)
glycocalyx, x1,500.
‘Fractured’ biofilm layer on cement
surface. (A) Note the filamentous glycocalyx adherences to the
substrate, the intercommunicating voids, the bacteria on the
surface ready to dispersion, the streamers, x2,200. (B) Note the
compact aspect (‘cloud-blanket’-like) of the biofilm, x15,500.
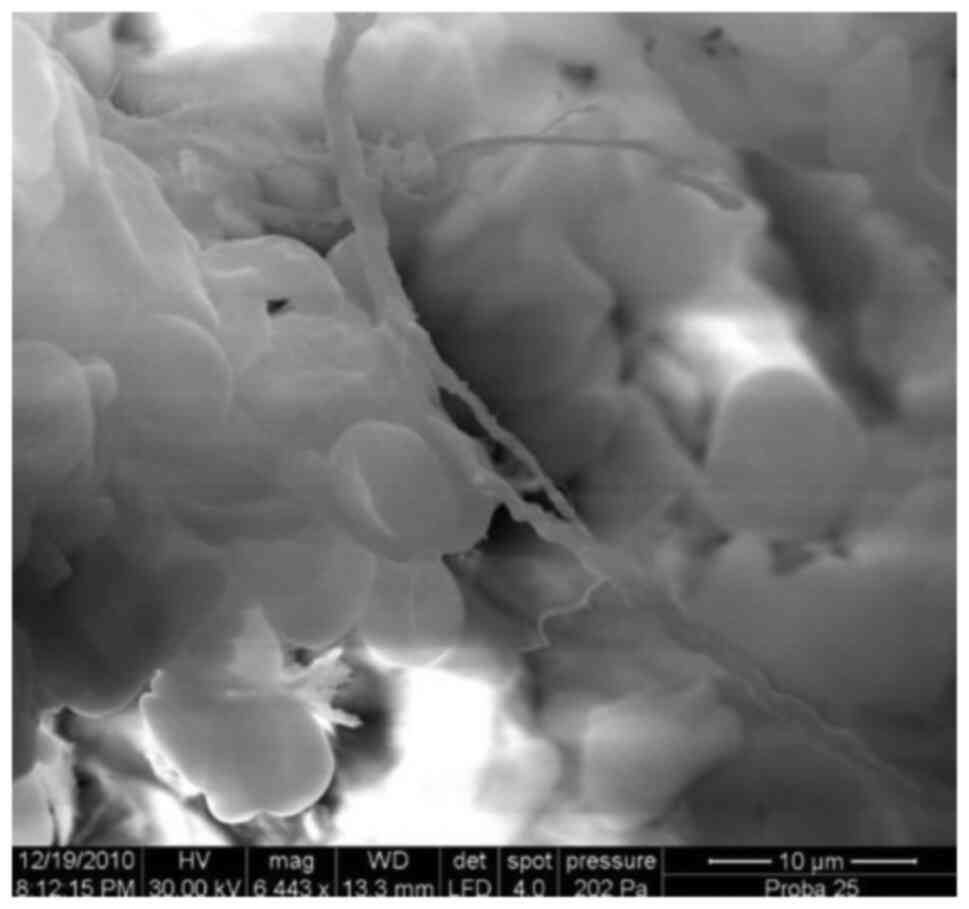
Compact, abundant ‘cloud blanket’-like
biofilm layer, x7,500.
Pellicular glycocalyx stretched over
previous bacterial agglomeration, x4,400.
Root canal filling image. Note the
master cone, the sealer pellicle, pristine and resorbed cemental
areas in the near-foraminal zone, x200.
Mummified pulp, magnifications x150
(A) and x1,000 (B).
Residual pulpal fibers network inside
the apical part of the root canal with included bacteria,
x10,000.
Apical foramen covered in calculus.
Note the circular secondary resorbtions on the calculus surface,
x100.
Descriptive statistics for the
investigated biofilm parameters.
Acknowledgements
The authors would like to thank Eng. Dr Cosmin
Locovei for his invaluable help in the SEM analysis and
documentation, and Mr. Cristian Popescu and Mrs. Claudia Zaharia
for their kind assistance with the statistical analysis of the
data.
Funding
No funding was received.
Availability of data and materials
The datasets used/analyzed in this study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SIS, DR, CL and LN participated in the sample
collection and data acquisition. AR, PS, MC, AO and DR participated
in the study design. SIS and LN drafted and critically revised the
manuscript for important intellectual content. HC, MB and SM
drafted and critically revised the manuscript for important
intellectual content, and were also involved in the conception of
the study. SS and AD performed the statistical analysis. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Research Ethics
Committee of the ‘Victor Babes’ University of Medicine and Pharmacy
in Timisoara, Romania (ethics approval no. 12b/2009). All subjects
were informed about the nature and the purpose of the study, and
each subject signed an informed consent document giving permission
for the dental procedures and sampling of biological material.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fritz B, Stavnsbjerg C, Markvart M,
Damgaard PB, Nielsen SH, Bjørndal L, Qvortrup K and Bjarnsholt T:
Shotgun sequencing of clinical biofilm following scanning electron
microscopy identifies bacterial community composition. Pathog Dis.
77(ftz013)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Carrassi A, Abati S and Santarelli G: The
role of scanning electron microscopy in periodontal research.
Scanning Microsc. 2:1123–1138. 1988.PubMed/NCBI
|
|
3
|
Satheesh K, MacNeill SR, Rapley JW and
Cobb CM: The CEJ: A biofilm and calculus trap. Compend Contin Educ
Dent. 32:30. 32–37; quiz 38, 40. 2011.PubMed/NCBI
|
|
4
|
Tronstad L, Barnett F and Cervone F:
Periapical bacterial plaque in teeth refractory to endodontic
treatment. Endod Dent Traumatol. 6:73–77. 1990.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Noiri Y and Ebisu S: Identification of
periodontal disease-associated bacteria in the ‘plaque-free zone’.
J Periodontol. 71:1319–1326. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Noiri Y, Ehara A, Kawahara T, Takemura N
and Ebisu S: Participation of bacterial biofilms in refractory and
chronic periapical periodontitis. J Endod. 28:679–683.
2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tronstad L, Barnett F, Riso K and Slots J:
Extraradicular endodontic infections. Endod Dent Traumatol.
3:86–90. 1987.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Karcz J, Bernas T, Nowak A, Talik E and
Woznica A: Application of lyophilization to prepare the nitrifying
bacterial biofilm for imaging with scanning electron microscopy.
Scanning. 34:26–36. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jeng JH, Chen KW, Lin CP, Chou HG and Lan
WH: Ultrastructural changes of the tooth root surface by Nd:YAG
laser irradiation followed by citric acid and tetracycline. J
Formos Med Assoc. 98:242–247. 1999.PubMed/NCBI
|
|
10
|
Ko SJ, Kim MK, Bang JK, Seo CH, Luchian T
and Park Y: Macropis fulvipes venom component macropin exerts its
antibacterial and anti-biofilm properties by damaging the plasma
membranes of drug resistant bacteria. Sci Rep.
7(16580)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Speer AG, Cotton PB, Rode J, Seddon AM,
Neal CR, Holton J and Costerton JW: Biliary stent blockage with
bacterial biofilm. A light and electron microscopy study. Ann
Intern Med. 108:546–553. 1988.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ganderton L, Chawla J, Winters C, Wimpenny
J and Stickler D: Scanning electron microscopy of bacterial
biofilms on indwelling bladder catheters. Eur J Clin Microbiol
Infect Dis. 11:789–796. 1992.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zee KY, Samaranayake LP and Attström R:
Scanning electron microscopy of microbial colonization of ‘rapid’
and ‘slow’ dental-plaque formers in vivo. Arch Oral Biol.
42:735–742. 1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Calenic B, Greabu M, Caruntu C, Nicolescu
MI, Moraru L, Surdu-Bob CC, Badulescu M, Anghel A, Logofatu C and
Boda D: Oral keratinocyte stem cells behavior on diamond like
carbon films. Rom Biotechnol Lett. 21:11914–11922. 2016.
|
|
15
|
Molven O, Olsen I and Kerekes K: Scanning
electron microscopy of bacteria in the apical part of root canals
in permanent teeth with periapical lesions. Endod Dent Traumatol.
7:226–229. 1991.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lomçali G, Sen BH and Cankaya H: Scanning
electron microscopic observations of apical root surfaces of teeth
with apical periodontitis. Endod Dent Traumatol. 12:70–76.
1996.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Silness J and Loe H: Periodontal disease
in pregnancy. II. Correlation between oral hygiene and periodontal
condition. Acta Odontol Scand. 22:121–135. 1964.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Glickman I: Bifurcation involvement in
periodontal disease. J Am Dent Assoc. 40:528–538. 1950.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Leonardo MR, Rossi MA, Silva LA, Ito IY
and Bonifácio KC: EM evaluation of bacterial biofilm and
microorganisms on the apical external root surface of human teeth.
J Endod. 28:815–818. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Brady JM: A plaque-free zone on human
teeth-scanning and transmission electron microscopy. J Periodontol.
44:416–428. 1973.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Noguchi N, Noiri Y, Narimatsu M and Ebisu
S: Identification and localization of extraradicular
biofilm-forming bacteria associated with refractory endodontic
pathogens. Appl Environ Microbiol. 71:8738–8743. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Friedman MT, Barber PM, Mordan NJ and
Newman HN: The ‘plaque-free zone’ in health and disease: A scanning
electron microscope study. J Periodontol. 63:890–896.
1992.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bass CC: A demonstrable line on extracted
teeth indicating the location of the outer border of the epithelial
attachment. J Dent Res. 25:401–415. 1946.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Saglie R, Johansen JR and Tollefsen T:
Plaque-free zones on human teeth in periodontitis. J Clin
Periodontol. 2:190–197. 1975.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vrahopoulos TP, Barber PM and Newman HN:
The apical border plaque in chronic adult periodontitis. An
ultrastructural study. I. Morphology, structure, and cell content.
J Periodontol. 63:243–252. 1992.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ribeiro FC: Distribution of bacteria in
the mineralized structures of teeth with pulp necrosis and apical
granuloma. Bauru, Sao Paulo: Faculdade de Odontologia de Bauru,
Universidade de Sao Paulo: 172, 1997.
|
|
27
|
Siqueira JF Jr, Rôças IN, Souto R, de
Uzeda M and Colombo AP: Checkerboard DNA-DNA hybridization analysis
of endodontic infections. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 89:744–748. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ricucci D and Siqueira JF Jr: Fate of the
tissue in lateral canals and apical ramifications in response to
pathologic conditions and treatment procedures. J Endod. 36:1–15.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nakano-Hasegawa M, Yammazaki S, Kaneda Y,
Takizawa H, Maeda N and Nakamura S: The formation of biofilms by
microorganisms isolated from infected root canals. J Endod.
25(299)1999.
|
|
30
|
Sundqvist G: Bacteriological studies of
necrotic dental pulps. Umeå University Odontol Dissertation, No. 7,
University of Umeå, Umeå, Sweden, 1976. https://www.diva-portal.org/smash/get/diva2:719968/FULLTEXT02.pdf.
|
|
31
|
Walton RE and Ardjmand K: Histological
evaluation of the presence of bacteria in induced periapical
lesions in monkeys. J Endod. 18:216–227. 1992.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Takemura N, Noiri Y, Ehara A, Kawahara T,
Noguchi N and Ebisu S: Single species biofilm-forming ability of
rootcanal isolates on gutta-percha points. Eur J Oral Sci.
112:523–529. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dahle UR, Tronstad L and Olsen I:
Characterization of new periodontal and endodontic isolates of
spirochetes. Eur J Oral Sci. 104:41–47. 1996.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Felippe WT, Ruschel MF, Felippe GS,
Pozzobon MH and Felippe MC: SEM evaluation of the apical external
root surface of teeth with chronic periapical lesion. Aust Endod J.
35:153–157. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Quirynen M, Bollen CM, Vandekerckhove BN,
Dekeyser C, Papaioannou W and Eyssen H: Full- vs. partial mouth
disinfection in the treatment of periodontal infections: Short-term
clinical and microbiological observations. J Dent Res.
74:1459–1467. 1995.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rocha CT, Rossi MA, Leonardo MR, Rocha LB,
Nelson-Filho P and Silva LA: Biofilm on the apical region of roots
in primary teeth with vital and necrotic pulps with or without
radiographically evident apical pathosis. Int Endod J. 41:664–669.
2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kolenbrander PE, Ganeshkumar N, Cassels FJ
and Hughes CV: Coaggregation: Specific adherence among human oral
plaque bacteria. FASEB J. 7:406–413. 1993.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mouton C, Reynolds HS and Genco RJ:
Characterization of tufted streptococci isolated from the ‘corn
cob’ configuration of human dental plaque. Infect Immun.
27:235–245. 1980.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Listgarten MA: Structure of the microbial
flora associated with periodontal health and disease in man. A
light and electron microscopic study. J Periodontol. 47:1–18.
1976.PubMed/NCBI View Article : Google Scholar
|