Introduction
Gestational diabetes mellitus (GDM) is defined as
any degree of glucose intolerance with onset or first recognition
during pregnancy, excluding patients who exhibited diabetes prior
to gestation but were first diagnosed during pregnancy (1). GDM has become a major public health
concern due to an increased prevalence, and the associated short-
and long-term complications for the mother and the offspring
(2). GDM is a multifactorial
disease that is induced by environmental factors interacting with
genes, and genetic factors are a major determinant of the disease
(2). Additionally, the prevalence
of GDM varies from country to country and region to region
(3), with epidemiological studies
indicating that the prevalence of GDM is 9% in the United States of
America (3) compared with 3.0-21.2%
in Asian countries (4). Therefore,
the investigation of GDM has become worldwide in recent years
(5); however, at present, the
mechanisms underlying the development and progression of GDM are
not completely understood.
GDM can alter the physiological condition of the
mother; even when aggressively managed, GDM leaves its mark on
offspring (6). In addition, the
origin of the majority of GDM-associated adverse pregnancy outcomes
involve the placenta (7). As a
result, the placenta, which is the only interface that connects the
mother and the fetus, has become an important organ for studying
the pathogenesis of abnormal glucose metabolism, including GDM
(7). Studies have indicated that
the placenta exhibits immature placental villi or alterations in
villus branch morphology during GDM, which lead to alterations in
placental morphology and function, resulting in limited
intrauterine growth and an increased risk of preterm birth
(7). Therefore, chorionic
trophoblast cells, which have invasive and endocrine functions,
serve an important role during GDM (8). The active substances that are secreted
by chorionic trophoblast cells, including hormones and
neuropeptides, serve an important role during energy metabolism and
transfer between mother and baby, maintenance of early pregnancy
and trophic corpus luteum (9). The
human chorionic trophoblast HTR8-/SVneo cell line is an
immortalized cell line that was established and identified by a
previous study (10). In the
present study, the association between GDM and pathophysiology was
assessed by determining the expression of miR-345-3p in the
placenta and peripheral blood using in vitro
experiments.
miRNAs are a class of highly evolutionarily
conserved, single-chain, small-molecule, non-coding RNAs, measuring
18-23 nucleotides in length, that serve a regulatory role at the
epigenetic level (11). Previous
studies investigating miR-345 have focused on cancer, including
prostate (12,13) and colorectal cancer (14), as well as acute lymphocytic leukemia
(15). Studies have identified
miR-345 as a key regulator in a variety of types of cancer,
primarily via its target genes and signaling pathways (12-16).
miR-345 can also act as a tumor suppressor to affect cell
epigenetic regulation, proliferation, apoptosis, differentiation,
metabolism and chemosensitivity (17-19).
Another study reported that miR-345 is differentially expressed
during the differentiation of mouse C2C12 myoblasts into myotube
differentiation port and umbilical cord mesenchymal stem cells
(20). The aforementioned studies
indicated that miR-345-3p serves an important regulatory role
during cell growth, apoptosis, migration and invasion. Based on the
key role of placental trophoblasts during the development and
progression of GMD; it was hypothesized that miR-345-3p may be
involved during the development and progression of GMD by
regulating the biobehavior of placental trophoblast cells.
To explore the proposed hypothesis, the present
study aimed to investigate the role and molecular mechanisms
underlying miR-345-3p during GDM. The expression of miR-345-3p was
measured in placental tissue samples from patients with GDM, and
the regulatory association between miR-345-3p and BAK1 was assessed
at the cellular level. The results of the present study suggested
that miR-345-3p served a protective role during GDM by decreasing
BAK1 expression; therefore, miR-345-3p may serve as a potential
therapeutic target for human GDM. The results of the present study
also highlighted the proapoptotic function of miR-345-3p during
GDM, and supported its potential use as a diagnostic and
therapeutic target for the disease.
Materials and methods
Clinical sample collection
A total of 30 blood samples and placental villous
tissues were collected from 30 pregnant women with GDM (age range:
24-39 years) and 30 healthy pregnant women (age range: 24-39 years)
form February 2017 to June 2018. Exclusion criteria were as
follows: Age <18 years old; hypertensive disorder of pregnancy;
other medical diseases during pregnancy (including pregnancy with
diabetes); exposure to harmful substances during pregnancy;
abnormal placenta or umbilical cord; maternal and child blood type
incompatibility; and multiple pregnancies. Patients with GDM who
did not fulfill any of the exclusion criteria were included in the
present study. Both groups of pregnant women were hospitalized in
Wuhan Children's Hospital. There were no significant differences in
maternal birth, age, geographical origin or occupation between the
two groups. The present study was approved by the Ethics Committee
of Wuhan Children's Hospital (Wuhan Maternal and Child Healthcare
Hospital). Written informed consent was obtained from all patients,
and all patients approved the use of their blood samples and
placental tissues in the present study.
GDM diagnostic criteria
Pregnant women at gestation week 24-28 were selected
for the present study. Patients with GDM were diagnosed according
to the American Diabetes Association standard for clinical
diagnosis and treatment of diabetes (2011 edition) and standard
oral glucose tolerance test (OGTT). The OGTT was performed as
follows: Women fasted for 8-14 h or more, consumed sugar and
subsequently consumed 75 g glucose powder dissolved in 200 ml warm
water within 5 min. The blood glucose levels of the elbow vein were
measured once every hour for 2 h. The normal ranges for these
aforementioned 3 blood glucose levels (including fasting level) are
>5.1, 10.0 and 8.5 mmol/l, respectively. If any reading reached
or exceeded the normal value, GDM was diagnosed.
Cell acquisition and culture
Human chorionic trophoblast cells (HTR8-/SVneo
cells) were purchased from Shanghai Huzheng Industrial Co., Ltd.
(cat no. HZ-CC337655). HTR8/SV-neo cells were routinely cultured in
DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) high sugar
complete medium containing 10% FBS (Invitrogen; Thermo Fisher
Scientific, Inc.) with 5% CO2 at 37̊C.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from blood, tissues and
cells using the TRIzol reagent (Thermo Fisher Scientific, Inc.)
according to manufacturer's protocols. A cDNA Synthesis kit
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to perform RT
according to the manufacturer's protocols. Reverse transcription
reaction condition was as following: 25̊C for 5 min, 42̊C for 60
min and 80̊C for 2 min. qPCR analysis was performed using the
LightCycler 480 SYBR Green I Master kit (Roche Applied Science)
according to manufacturer's protocols and a LightCycler 480 II
instrument (Roche Applied Science). The following thermocycling
conditions were used for qPCR: Initial denaturation at 95̊C for 5
min; followed by 45 cycles of 94̊C for 10 sec, primer pair-specific
annealing at 55̊C for 20 sec and 72̊C for 30 sec, followed by a
final extension step at 72̊C for 10 min. mRNA and miRNA expression
levels were quantified using the 2-ΔΔCq method (21), and normalized to the internal
reference genes β-actin and U6, respectively. Primer sequences were
as follows: miR-345-3p forward, 5'-GGTTTTTGGATTGGGTTGTAGAGTG-3' and
reverse, 5'-AACCAAAACAATCCCTTACCACTAC-3'; BAK1 forward,
5'-GCTCCCAACCCATTCACTAC-3' and reverse, 5'-TCCCTACTCCTTTTCCCTGA-3';
U6 forward, 5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'; GAPDH forward,
5'-CTTTGGTATCGTGGAAGGACTC-3' and reverse,
5'-GTAGAGGCAGGGATGATGTTCT-3'.
Identification of miR-345-3p target
BAK1
TargetScan (version 7.2; www.targetscan.org/vert_72) was used to predict the
target gene of miR-345-3p and to identify target gene
3'-untranslated region (UTR) specific binding sites, in combination
with the National Center for Biotechnology Information database
(https://www.ncbi.nlm.nih.gov/). The
binding sites between miR-345-3p and 3'UTR BAK1 were observed.
Subsequently, a dual-luciferase reporter assay was performed to
investigate whether miR-345-3p directly interacted with BAK1.
Dual-luciferase reporter assay
Wild-type (WT-BAK1) and mutant (MUT-BAK1) 3'UTR BAK1
were cloned into pmiR-RB-Report™ dual luciferase reporter gene
plasmid vectors (Guangzhou RiboBio Co., Ltd.), according to the
manufacturer's protocol. 293T cells (5x104 cells per
well; American Type Culture Collection) were co-transfected with
WT-BAK1 or MUT-BAK1, and miR-345-3p mimic or mimic control using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Following incubation at 37̊C for 48 h, luciferase activities were
detected using a Dual-luciferase assay system (Promega
Corporation), according to the manufacturer's protocol. Firefly
luciferase activity was normalized to Renilla luciferase
activity.
Cell transfection
Cells were seeded into a six well plate
(5x104 cells per well) and allowed to reach 80-90%
confluence. HTRB-/SVneo cells were transfected with 1 µg
BAK1-plasmid (Cat no. sc-400646-ACT; Santa Cruz Biotechnology,
Inc.), 1 µg control-plasmid (cat no. sc-437275; Santa Cruz
Biotechnology, Inc.), 50 nM miR-345-3p mimic (HmiR0210-MR03;
GeneCopoeia), 50 nM mimic control (cat. no. CmiR0001-MR03;
GeneCopoeia), 1 µg control-plasmid + 50 nM miR-345-3p mimic or 1 µg
BAK1-plasmid + 50 nM miR-345-3p mimic using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Following incubation for 48 h, transfection efficiency was
determined by RT-qPCR.
Cell viability
Cells in the logarithmic growth phase were plated
(5x104 cells per well) into a 96-well plate. At ~90%
confluence, HTR8-/SVneo cells were transfected with miR-345-3p
mimic, mimic control, control-plasmid + miR-345-3p mimic or
BAK1-plasmid + miR-345-3p mimic for 48 h at 37̊C. Subsequently, 20
µl MTT solution (5 mg/ml) was added to each well and cultured at
37̊C for 4 h. The solution was discarded and DMSO (150 µl) was
added to each well at 37̊C for 10 min with gentle agitation to
sufficiently dissolve the purple formazan. The optical density (OD)
value of each well was measured at a wavelength of 562 nm using a
microplate reader. Cell viability was calculated as follows:
(Experimental group OD value/control OD value) x100%. A total of 6
wells were used for each group and the experiment was performed in
triplicate.
Detection of apoptosis by flow
cytometry
At 48 h post-transfection, cell apoptosis was
determined using the AnnexinV-FITC/PI kit [cat no 70-AP101-100;
Hangzhou Multi Sciences (Lianke) Biotech Co., Ltd.], according to
the manufacturer's protocol. Early and late apoptosis were detected
using a FACSCalibur flow cytometer (BD Biosciences) with FlowJo
software (version 7.2.4; FlowJo LLC).
Transwell assay for the detection of
cell migration and invasion
Non-Matrigel-coated and Matrigel-coated Boyden
chambers (BD Biosciences) were used to detect the migratory and
invasive abilities of HTRB-/SVneo cells, respectively. HTRB-/SVneo
cells were transfected with miR-345-3p mimic, mimic control,
control-plasmid + miR-345-3p mimic or BAK1-plasmid + miR-345-3p
mimic for 48 h. Following starvation overnight in serum-free DMEM
at 37̊C for 24 h, cells were obtained by 0.25% trypsinization. The
cells (5x106 cells/ml) in serum-free medium were plated
in the upper chambers of the Transwell plates. DMEM containing 2.5%
FBS was plated into the lower chambers of the Transwell plates.
After incubation at 37̊C for 24 h, cells remaining on the upper
chamber surface were wiped off using a cotton swab. Subsequently,
the upper chamber of the Transwell insert was washed with PBS, and
cells on the lower surface of the Transwell chamber were fixed with
4% paraformaldehyde at room temperature for 30 min. Then the cells
were stained using 0.1% crystal violet (Beyotime Institute of
Biotechnology) for 20 min at room temperature. The number of
migratory cells was counted under a light microscope
(magnification, x40) in 5 fields of view, and the total number of
cells was recorded and the mean number of migratory cells was
calculated.
Western blotting
Total protein from HTRB-/SVneo cells was extracted
using RIPA buffer (Beyotime Institute of Biotechnology) at 4̊C for
1 h. The protein concentration was determined with a bicinchoninic
acid assay kit (Beyotime Institute of Biotechnology). Proteins (30
µg/lane) were separated via 10% SDS-PAGE and transferred onto PVDF
membranes. Subsequently, the membranes were blocked with 5% skim
milk in TBS containing Tween 20 (TBST) at room temperature for 1.5
h. The membranes were incubated with primary antibodies targeted
against: Bcl-2 (Cat no. sc-23960; 1:1,000; Santa Cruz
Biotechnology, Inc.), Bax (Cat no. sc-20067; 1:1,000; Santa Cruz
Biotechnology, Inc.), matrix metallopeptidase (MMP)9 (Cat no.
ab38898; 1:1,000; Abcam) and GAPDH (Cat no. Ab181602; 1:1,000;
Abcam). After washing with TBST, the membrane was incubated with
the corresponding horseradish peroxidase-labeled secondary antibody
(1:2,000; cat no. 7074; Cell Signaling Technology, Inc.) at room
temperature for 2 h at room temperature. Protein bands were
visualized using enhanced chemiluminescence kit (Applygen
Technologies, Inc.). Protein expression levels were quantified
using ImageJ software (version 1.46; National Institutes of Health)
with GAPDH as the loading control.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 18.0; SPSS, Inc.). Data are presented as the mean
± standard deviation. One-way ANOVA followed by a Tukey's post hoc
test was used to analyze the differences among multiple groups. The
unpaired Student's t-test was used to analyze the statistical
significance between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miR-345-3p in the
peripheral blood and placental tissues of patients with GDM
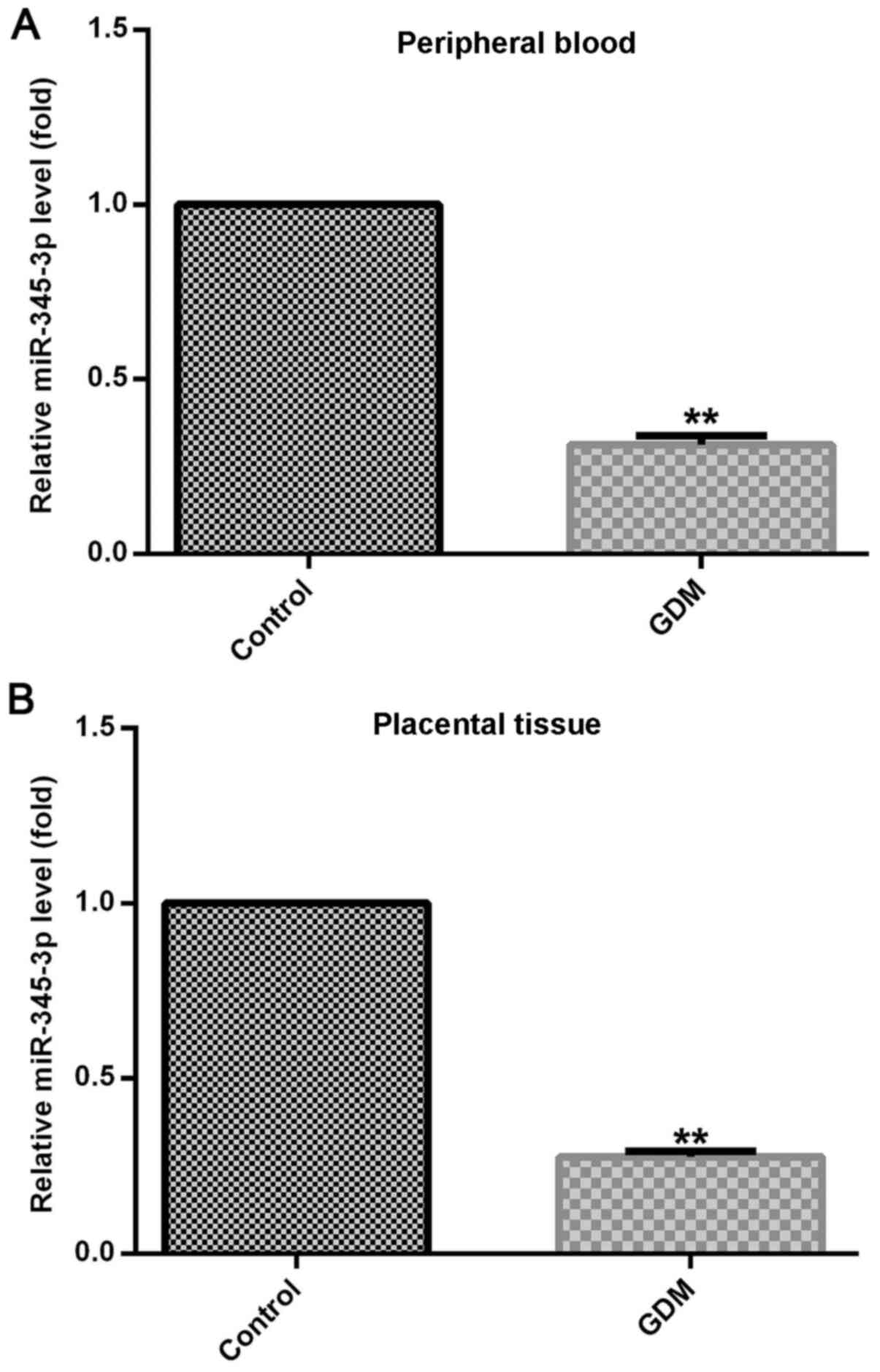
RT-qPCR was used to detect miR-345-3p expression
levels in maternal placental tissues and peripheral blood of
patients with GDM and healthy control subjects. miR-345-3p
expression was significantly decreased in the placental tissues and
peripheral blood of patients with GDM compared with normal pregnant
women (Fig. 1).
BAK1 is the target gene of
miR-345-3p
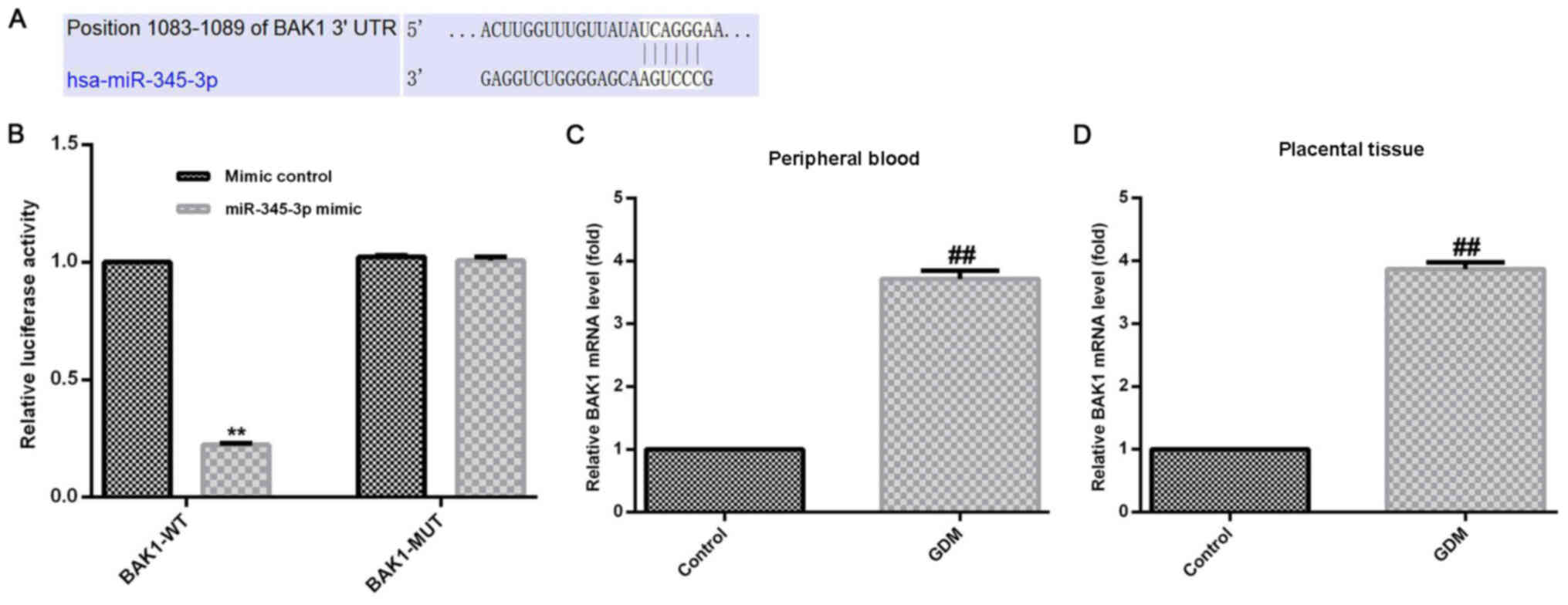
The target gene of miR-345-3p was predicted using
TargetScan. The results indicated that miR-345-3p had hundreds of
potential target genes, including BAK1 (Fig. 2A). The BAK1 protein belongs to the
BCL2 protein family, and localizes to mitochondria to induce
apoptosis (22). As placental
trophoblast growth serves a key role during the development and
progression of GDM (23), it was
hypothesized that BAK1 may serve important functions during the
growth of placental trophoblast cells. Moreover, the role of BAK1
during GDM is not completely understood; therefore, BAK1 was
selected for further investigation in the present study.
A dual-luciferase reporter assay was performed to
verify that BAK1 was a target gene of miR-345-3p. miR-345-3p mimic
significantly decreased the luciferase activity of 293T cells
transfected with BAK1-WT compared with the mimic control group,
while miR-345-3p mimic displayed no significant effect on the
luciferase activity of HTRB-/SVneo cells transfected with BAK1-MUT.
The results indicated that BAK1 was a target of miR-345-3p
(Fig. 2B).
Expression of BAK1 in the peripheral
blood and placental tissues of patients with GDM
RT-qPCR was performed to determined BAK1 mRNA
expression levels in the maternal placental tissues and peripheral
blood of patients with GDM and the normal pregnancy control group.
The BAK1 mRNA expression levels were significantly increased in the
maternal peripheral blood and placental tissues of the GDM group
compared with the control group (Fig.
2C and D).
Negative regulation of BAK1 by
miR-345-3p in HTRB-/SVneo cells
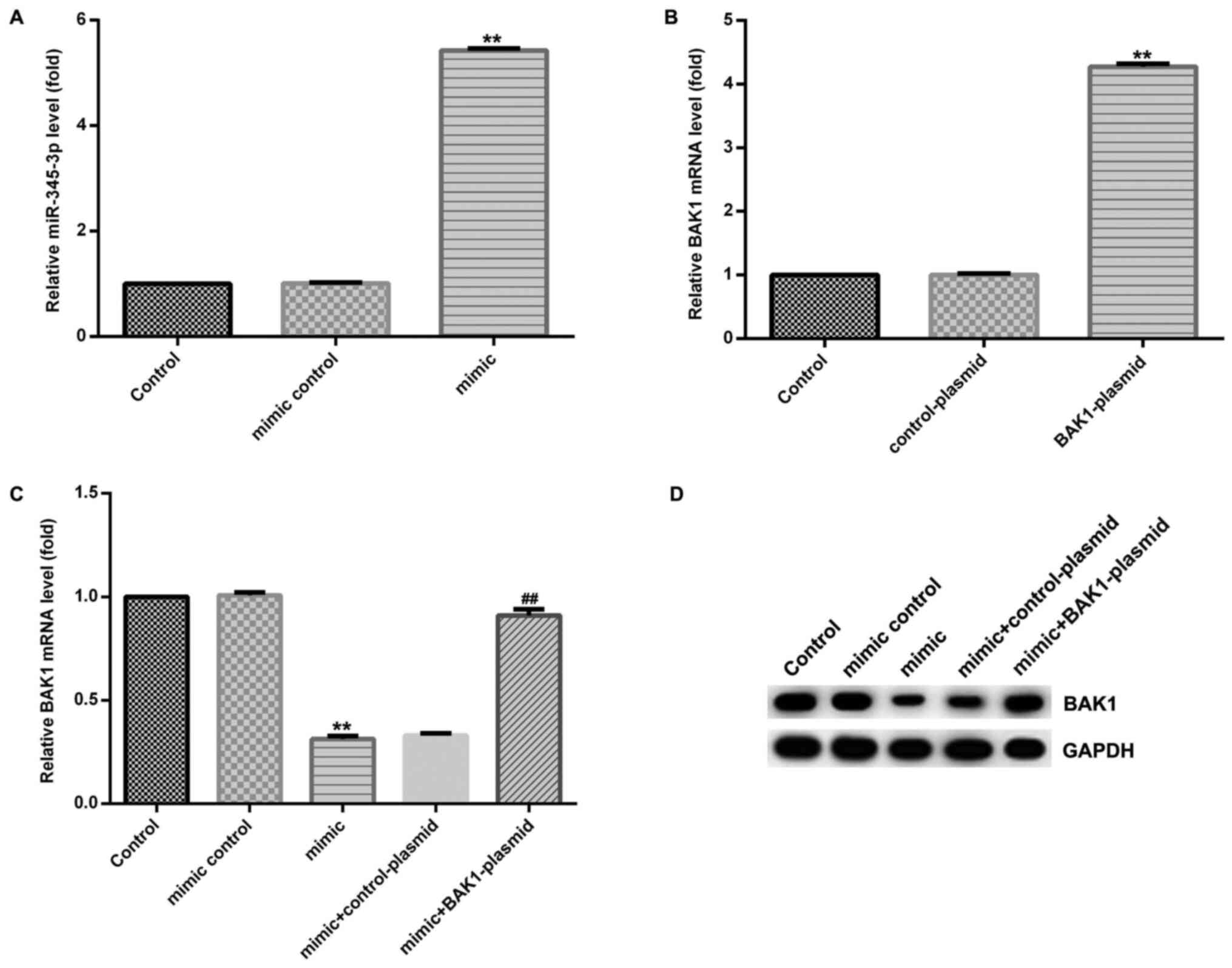
To investigate the effect of miR-345-3p on BAK1
expression, HTRB-/SVneo cells were transfected with BAK1-plasmid,
control-plasmid, miR-345-3p mimic, mimic control, control-plasmid +
miR-345-3p mimic or BAK1-plasmid + miR-345-3p mimic for 48 h. The
expression of miR-345-3p in the miR-345-3p mimic group was
significantly increased compared with the control group (Fig. 3A). BAK1 mRNA expression was
increased in the BAK1-plasmid group compared with the control group
(Fig. 3B). In addition, BAK1
expression was significantly decreased in the miR-345-3p mimic
group compared with the control group; however, co-transfection
with BAK1-plasmid reversed the miR-345-3p mimic-induced decreased
in BAK1 expression (Fig. 3C and
D).
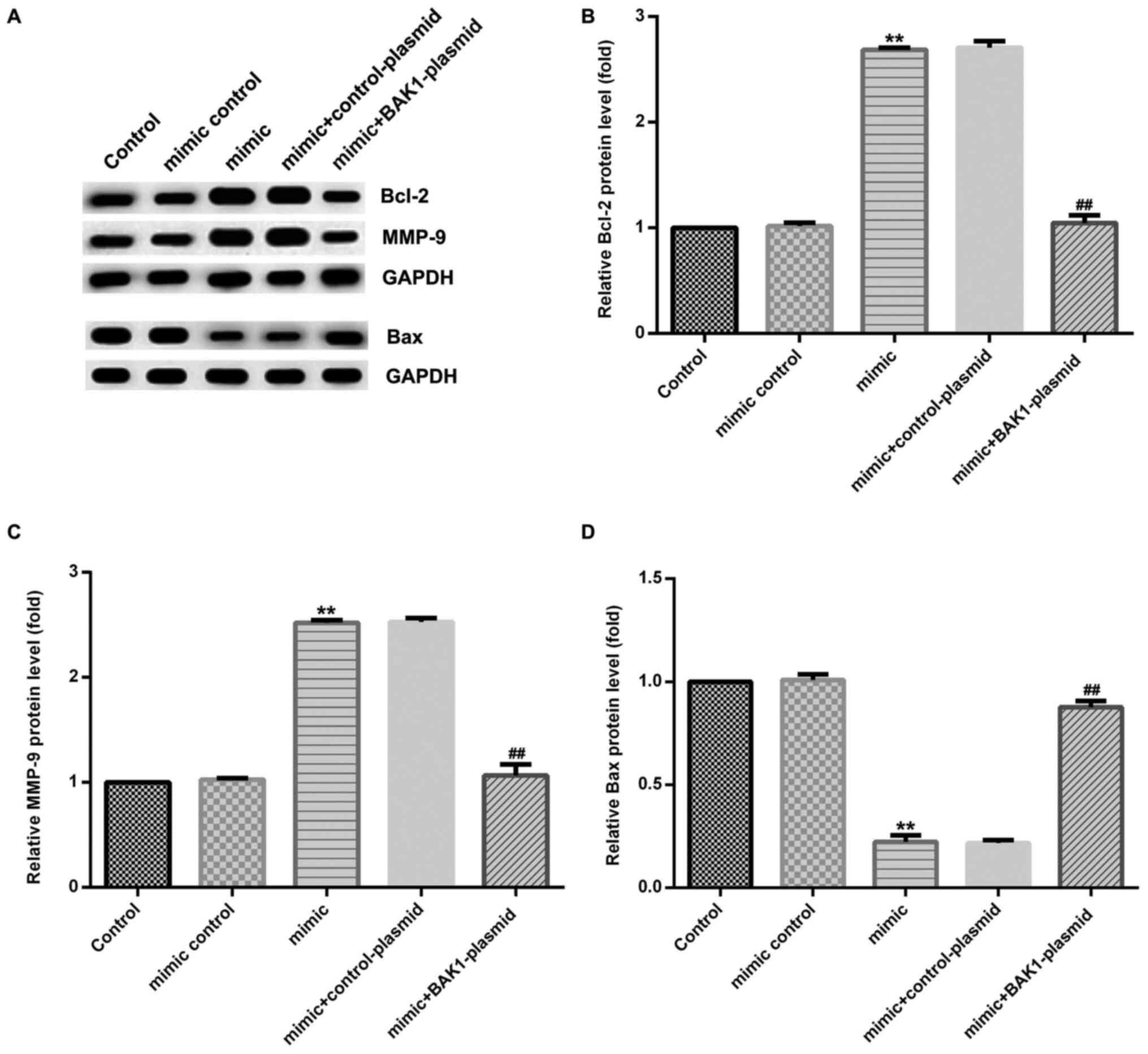
Effect of miR-345-3p on HTRB-/SVneo
cells
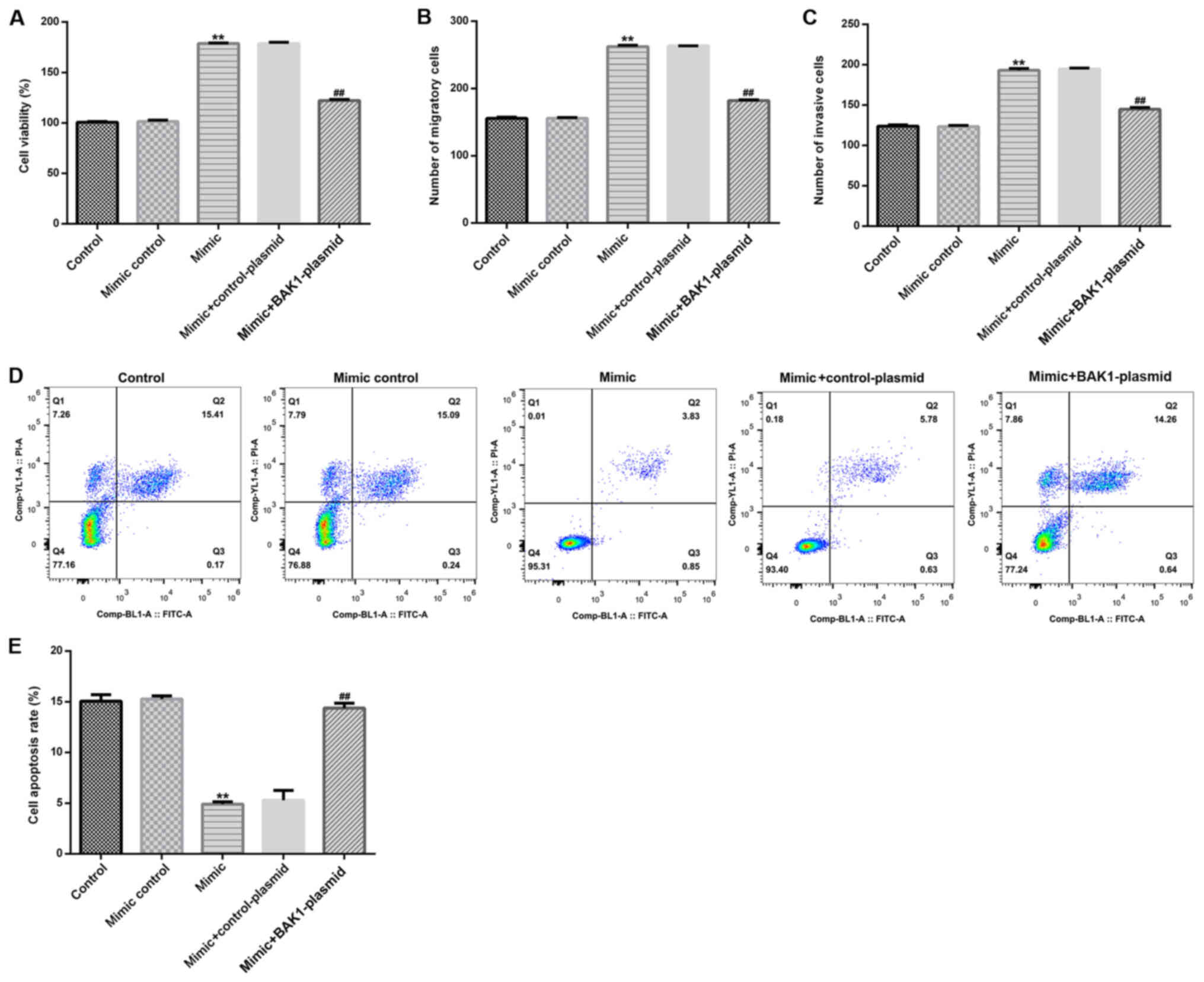
In the present study, the effects of miR-345-3p on
HTRB-/SVneo cells were assessed by detecting cell viability,
apoptosis, migration and invasion, as well as detecting the
expression levels of Bcl-2, Bax and MMP-9. Compared with the
control group, miR-345-3p mimic significantly enhanced HTRB-/SVneo
cell viability (Fig. 4A), migration
(Fig. 4B) and invasion (Fig. 4C), and significantly inhibited cell
apoptosis (Fig. 4D and E). Transfection with the BAK1-plasmid
significantly reversed the miR-345-3p mimic-mediated effects on
HTRB-/SVneo cell activity.
Furthermore, miR-345-3p mimic significantly enhanced
the protein expression levels of Bcl-2 (Fig. 5A and B) and MMP-9 (Fig. 5A and C), and significantly decreased the protein
expression levels of Bax (Fig. 5A
and D) in HTRB-/SVneo cells
compared with the control group. Similarly, miR-345-3p
mimic-induced alterations to protein expression levels were
significantly reversed by BAK1-plasmid.
Discussion
GDM is one of the most common complications that
occurs during pregnancy. The disease has a multi-sourced etiology
(3), which may be the result of a
combination of genetic and environmental factors; however, the
pathogenesis of GDM is not completely understood. A number of
studies have demonstrated that miRNAs are ubiquitously expressed in
various organisms and exhibit biological characteristics, including
highly conserved and time-dependent expression, as well as tissue
expression specificity (11,24,25).
miRNAs serve a post-transcriptional regulatory role by binding to
the 3'UTR of mRNAs. Li et al (26) conducted an miRNA microarray analysis
that indicated that 9 miRNAs were significantly altered in GDM
samples: miR-508-3p was upregulated, and miR-27a, miR-9, miR-30d,
miR-362-5p, miR-137, miR-92a, miR-33a and miR-502-5p were
downregulated, suggesting that miRNA may serve a regulatory role
during the development of GDM. Therefore, investigating the
expression and regulatory mechanisms underlying miRNAs in patients
with GDM is required to provide novel insight into the pathogenesis
of GDM.
To investigate the effect and mechanisms underlying
miR-345-3p during GDM, the expression of miR-345-3p was measured in
the peripheral blood and placental tissues of patients with GDM and
healthy pregnant women using RT-qPCR. The results indicated that
the expression of miR-345-3p in the peripheral blood and placental
tissues of patients with GDM was significantly decreased compared
with healthy pregnant women, indicating that low-level miR-345-3p
expression may be associated with GDM pathophysiology. In addition,
miRNAs have been reported to serve an important role during
diabetes (11,27,28).
Monfared et al (11)
demonstrated the extensive role of miR-135a as a gene regulator
following GDM transplantation, which suggested that miR-135a may
serve as a potential indicator for the prevention, treatment and
management of GDM. The serum miRNA pattern of type 1 diabetes and
the signaling pathways that may be associated with its pathogenesis
have been previously identified (27). Erener et al (27) reported that miR-29b is highly
expressed in retinal ganglion cell neurons in a rat model of
diabetes, and indicated that miR-29b may be associated with the
pathogenesis of diabetic retinopathy. Additionally, miR-29b has
also been associated with the progression of renal fibrosis,
including diabetic nephropathy (29). The aforementioned studies indicated
that miRNAs may be associated with the pathogenesis of
diabetes.
miRNAs are involved in a number of cellular
biological processes by regulating post-transcriptional expression
levels, either by degrading target genes or inhibiting the
translation of target genes. To identify the biological functions
of miRNAs, screening and validation of miRNA target genes has
become a research focus. Therefore, the present study used
HTRB-/SVneo cells to investigate the target genes of miR-345-3p,
predicting potential target genes via TargetScan and verifying
these target genes using a dual-luciferase reporter assay. The
results indicated that BAK1 was a target gene of miR-345-3p.
Subsequently, whether BAK1 was expressed in the peripheral blood
and placental tissues of patients with GDM was assessed. The
expression of BAK1 in the peripheral blood and placental tissues of
patients with GDM was detected using RT-qPCR. The results indicated
that BAK1 expression was significantly increased in the peripheral
blood and placental tissues of patients with GDM compared with
healthy pregnant women.
BAK1 was expressed in patients with GDM; therefore,
the association between BAK1 and miR-345-3p was assessed in
HTRB-/SVneo cells. Transfection of HTRB-/SVneo cells with
BAK1-plasmid, control-plasmid, miR-345-3p mimic, mimic control or
BAK1-plasmid + miR-345-3p mimic for 48 h indicated that miR-345-3p
mimic significantly decreased BAK1 expression compared with the
control group. miR-345-3p mimic-mediated effects on BAK1 expression
were reversed by co-transfection with BAK1-plasmid. Therefore, the
results suggested that miR-345-3p exhibited a negative regulatory
effect on BAK1. MTT, flow cytometry, Transwell assay and western
blot analyses were subsequently performed to investigate the
effects of BAK1 expression on miR-345-3p-mediated cellular
functions in HTRB-/SVneo cells, including cell viability,
apoptosis, migration and invasion. miR-345-3p mimic significantly
increased HTRB-/SVneo cell viability, migration and invasion, and
inhibited cell apoptosis compared with the control group. The
miR-345-3p-mediated effects on cellular functions were reversed by
BAK1 overexpression. In addition, transfection with the miR-345-3p
mimic significantly increased the expression of Bcl-2 and MMP9 in
HTRB-/SVneo cells, and decreased the expression of Bax, which was
also reversed by BAK1 overexpression. Cell functions, including
cell viability, migration, invasion and apoptosis are closely
associated with cell differentiation, tissue and organ development
(30), indicating that miR-345-3p
may serve an important regulatory role during the development and
function of the placenta during embryonic development. In the
present study, miR-345-3p expression levels were significantly
decreased in the placental tissues of patients with GDM compared
with normal pregnant women, which suggested that GDM may be
associated with dysfunction of trophoblast cell migration and
invasion. Therefore, during GDM, miR-345-3p downregulation in
placental trophoblast cells may increase the expression of
downstream target genes, thereby affecting trophoblast cell
proliferation, viability and apoptosis. Alterations to cellular
function lead to abnormalities in placental structure and function,
particularly placental invasion and secretion, during embryonic
development (31,32).
The results of the present study indicated that
HTRB-/SVneo cell functions were sensitive to high levels of
miR-345-3p. Among the HTRB-/SVneo cell functions evaluated in the
present study, migration, invasion and cell viability were enhanced
by miR-345-3p over-expression, which is important for the molecular
pathogenesis of GDM (23). Invasion
is an important function of trophoblast cells (33,34),
with adhesion and invasion of the maternal uterine epithelium being
the premise of placenta formation (35). Insufficient extravillous trophoblast
invasion of the endometrium has been reported to cause
pregnancy-associated complications, including miscarriage,
pregnancy-induced hypertension and fetal growth restriction
(36). Moreover, the migration and
invasion of trophoblast cells has been suggested to be associated
with insulin resistance (37). The
results of the present study suggested that miR-345-3p expression
decreased in GDM maternal placenta tissue and blood. miR-345-3p
upregulation increased the migration and invasion abilities of
placental trophoblast cells, which may be one of the causes of GDM.
Previous study has indicated that the high glucose environment in
pregnant women with GDM affects trophoblast cell proliferation,
apoptosis and cell cycle regulation (38). The expression of the target gene
BAK1 was increased in patients with GDM compared with normal
pregnant women, suggesting that BAK1 promoted the migration and
invasion of trophoblast cells, and potentially induced the high
glucose environment. The present study provided novel insight for
targeted treatment strategies for patients with GDM.
In conclusion, the present study assessed the
function of miR-345-3p during GDM. The results revealed that
miR-345-3p was abnormally expressed in patients with GDM, and high
expression of miR-345-3p during GDM served a protective role by
inhibiting placental trophoblast apoptosis, and promoting cell
proliferation and migration via targeting BAK1. As the present
study was only a preliminary study investigating the role of
miR-345-3p during GDM, the possibility of using miR-345-3p to
diagnose GDM requires further investigation. For example, the role
of miR-345-3p/BAK1 during glucose metabolism and insulin signaling
should be explored. In addition, the expression of miR-345-3p/BAK1
in patients with GDM prior and subsequent to insulin therapy
requires further investigation. Furthermore, the effect of
miR-345-3p/BAK1 during GDM should be investigated in
vivo.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL contributed to study design, data collection,
statistical analysis, data interpretation and manuscript
preparation. JZ contributed to data collection, statistical
analysis and manuscript preparation. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Wuhan Children's Hospital (Wuhan Maternal and Child
Healthcare Hospital). Written informed consent was obtained from
all patients included in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Metzger BE and Coustan DR: Summary and
recommendations of the Fourth International Workshop-Conference on
Gestational Diabetes Mellitus. The Organizing Committee. Diabetes
Care. 21 (Suppl 2):B161–B167. 1998.PubMed/NCBI
|
|
2
|
Chiefari E, Arcidiacono B, Foti D and
Brunetti A: Gestational diabetes mellitus: An updated overview. J
Endocrinol Invest. 40:899–909. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhu Y and Zhang C: Prevalence of
gestational diabetes and risk of progression to type 2 diabetes: A
global perspective. Curr Diab Rep. 16(7)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yuen L and Wong VW: Gestational diabetes
mellitus: Challenges for different ethnic groups. World J Diabetes.
6:1024–1032. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Egan AM, Bogdanet D, Griffin TP,
Kgosidialwa O, Cervar-Zivkovic M, Dempsey E, Allotey J, Alvarado F,
Clarson C, Cooray SD, et al: A core outcome set for studies of
gestational diabetes mellitus prevention and treatment.
Diabetologia: Mar 20, 2020 (Epub ahead of print).
|
|
6
|
Tryggestad JB, Vishwanath A, Jiang S,
Mallappa A, Teague AM, Takahashi Y, Thompson DM and Chernausek SD:
Influence of gestational diabetes mellitus on human umbilical vein
endothelial cell miRNA. Clin Sci (Lond). 21:1955–1967.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gabbay-Benziv R and Baschat AA:
Gestational diabetes as one of the ‘great obstetrical
syndromes’-The maternal, placental, and fetal dialog. Best Pract
Res Clin Obstet Gynaecol. 29:150–155. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Winship A, Sorby K, Correia J, Rainczuk A,
Yap J and Dimitriadis E: Interleukin-11 up-regulates endoplasmic
reticulum stress induced target, PDIA4 in human first trimester
placenta and in vivo in mice. Placenta. 53:92–100. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Antoniotti GS, Coughlan M, Salamonsen LA
and Evans J: Obesity associated advanced glycation end products
within the human uterine cavity adversely impact endometrial
function and embryo implantation competence. Hum Reprod.
33:654–665. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Graham CH, Hawley TS, Hawley RG,
MacDougall JR, Kerbel RS, Khoo N and Lala PK: Establishment and
characterization of first trimester human trophoblast cells with
extended lifespan. Exp Cell Res. 206:204–211. 1993.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Monfared YK, Ghadimi F, Foroughi F,
Honardoost M, Hashemipour S, Sefidi F and Sarookhani MR:
Determination and comparison miR135a in the serum between women
with GDM, non-pregnant type 2 diabetes, healthy pregnant and
control group. Middle East J Fam Med. 2:193–197. 2018.
|
|
12
|
Eminaga O, Fries J, Woetzel F, Alakus H,
Warnecke-Eberz U and Heidenreich A: MP66-12 the expression profiles
of miR-210, miR-375, miR-378, miR-345, miR-143 miR-183 and miR-98
in the progression of prostate cancer from high-grade prostatic
intraepithelial neoplasia to metastatic diseases. J Urology.
4(e877)2016.
|
|
13
|
Mou T, Xie F, Zhong P, Hua H, Lai L, Yang
Q and Wang J: MiR-345-5p functions as a tumor suppressor in
pancreatic cancer by directly targeting CCL8. Biomed Pharmacother.
111:891–900. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Danese E, Minicozzi AM, Benati M, Paviati
E, Lima-Oliveira G, Gusella M, Pasini F, Salvagno GL, Montagnana M
and Lippi G: Reference miRNAs for colorectal cancer: Analysis and
verification of current data. Sci Rep. 7(8413)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ying X, Zhang W, Fang M, Zhang W, Wang C
and Han L: miR-345-5p regulates proliferation, cell cycle, and
apoptosis of acute myeloid leukemia cells by targeting AKT2. J Cell
Biochem. 2:1620–1629. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang SY, Shiboski S, Belair CD, Cooperberg
MR, Simko JP, Stoppler H, Cowan J, Carroll PR and Blelloch R:
MiR-19, miR-345, miR-519c-5p serum levels predict adverse pathology
in prostate cancer patients eligible for active surveillance. PLoS
One. 6(e98597)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tinay I, Tan M, Gui B, Werner L, Kibel AS
and Jia L: Functional roles and potential clinical application of
miRNA-345-5p in prostate cancer. Prostate. 78:927–937.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Srivastava SK, Bhardwaj A, Arora S, Tyagi
N, Singh S, Andrews J, McClellan S, Wang B and Singh AP:
MicroRNA-345 induces apoptosis in pancreatic cancer cells through
potentiation of caspase-dependent and -independent pathways. Brit J
Cancer. 113:660–668. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tinay I, Gui B, Werner L, Rafiei S,
Gelpi-Hammerschmidt F, Kibel A and Jia L: 24-Upregulation of
microRNAs miR-9, miR-330-3p and miR-345 in prostate cancer. Eur
Urol Suppl. 15:e1286–e1287. 2016.
|
|
20
|
Siengdee P, Trakooljul N, Murani E,
Schwerin M, Wimmers K and Ponsuksili S: MicroRNAs regulate cellular
ATP levels by targeting mitochondrial energy metabolism genes
during C2C12 myoblast differentiation. PLoS One.
10(e0127850)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using realtime quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Uren RT, O'Hely M, Iyer S, Bartolo R, Shi
MX, Brouwer JM, Alsop AE, Dewson G and Kluck RM: Disordered
clusters of Bak dimers rupture mitochondria during apoptosis.
Elife. 6(pii: 19944)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Loegl J, Nussbaumer E, Cvitic S, Huppertz
B, Desoye G and Hiden U: GDM alters paracrine regulation of
feto-placental angiogenesis via the trophoblast. Lab Invest.
97:409–418. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li J, Song L, Zhou L, Wu J, Sheng C, Chen
H, Liu Y, Gao S and Huang W: A MicroRNA signature in gestational
diabetes mellitus associated with risk of macrosomia. Cell Physiol
Bioche. 1:243–252. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Erener S, Marwaha A, Tan R,
Panagiotopoulos C and Kieffer TJ: Profiling of circulating
microRNAs in children with recent onset of type 1 diabetes. JCI
Insight. 4(e89656)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Silva VA, Polesskaya A, Sousa TA, Corrêa
VM, André ND, Reis RI, Kettelhut IC, Harel-Bellan A and De Lucca
FL: Expression and cellular localization of microRNA-29b and RAX,
an activator of the RNA-dependent protein kinase (PKR), in the
retina of streptozotocin-induced diabetic rats. Mol Vis.
17:2228–2240. 2011.PubMed/NCBI
|
|
29
|
Chen HY, Zhong X, Huang XR, Meng XM, You
Y, Chung AC and Lan HY: MicroRNA-29b inhibits diabetic nephropathy
in db/db mice. Mol Ther. 22:842–853. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Knöfler M, Haider S, Saleh L, Pollheimer
J, Gamage TKJB and James J: Human placenta and trophoblast
development: Key molecular mechanisms and model systems. Cell Mol
Life Sci. 76:3479–3496. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pollheimer J, Vondra S, Baltayeva J,
Beristain AG and Knöfler M: Regulation of placental extravillous
trophoblasts by the maternal uterine environment. Front Immunol.
9(2597)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Haider S, Meinhardt G, Saleh L, Kunihs V,
Gamperl M, Kaindl U, Ellinger A, Burkard TR, Fiala C, Pollheimer J,
et al: Self-renewing trophoblast organoids recapitulate the
developmental program of the early human placenta. Stem Cell
Reports. 11:537–551. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang X, Peng S, Cui K, Hou F, Ding J, Li
A, Wang M and Geng L: MicroRNA-576-5p enhances the invasion ability
of trophoblast cells in preeclampsia by targeting TFAP2A. Mol Genet
Genomic Med. 8(e1025)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Baines KJ and Renaud SJ: Transcription
factors that regulate trophoblast development and function. Prog
Mol Biol Transl Sci. 145:39–88. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Aplin JD: Developmental cell biology of
human villous trophoblast: Current research problems. Int J Dev
Biol. 54:323–329. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Meekins JW, Pijnenborg R, Hanssens M,
McFadyen IR and van Asshe A: A study of placental bed spiral
arteries and trophoblast invasion in normal and severe
pre-eclamptic pregnancies. Brit J Obstet Gynaec. 8:669–674.
1994.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mayama R, Izawa T, Sakai K, Suciu N and
Iwashita M: Improvement of insulin sensitivity promotes
extravillous trophoblast cell migration stimulated by insulin-like
growth factor-I. Endocr J. 60:359–368. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Aires MB and Anne Carolline Veríssimo dos
Santos: Effects of maternal diabetes on trophoblast cells. World J
Diabetes. 6:338–344. 2015.PubMed/NCBI View Article : Google Scholar
|