Introduction
Osteoarthritis is a non-inflammatory and
degenerative joint disease that is mainly characterized by
arthralgia and ankylosis (1). At
present, the disease mostly occurs in women, and its prevalence
increases with age (2). Statistics
have revealed that it affected approximately 240 million people
around the world in 2016(3), with
its incidence having increased from 13.8 to 21.6% in 2018(4). Although the cause of the disease
remains unclear, studies at home and abroad suggest that the cause
is closely related to age and obesity, and possibly correlated with
excessive joint activity, arthrotrauma, heredity, and osteoporosis
(5,6). Osteoarthritis mainly occurs in
cartilage. The original and normal cartilage tissue undergoes
morphological changes and destruction, and then forms new spurs at
the articular margin (7). Moreover,
the disease may also involve ligaments, joint capsules, synovium,
and surrounding muscles during its course. Patients with slight
conditions suffer from arthralgia and limited daily activities,
while those with serious conditions suffer from joint dysfunction
and paralysis (8,9). Currently, osteoarthritis is diagnosed
by complex joint examinations, which include erythrocyte
sedimentation rate, mucin, rheumatoid factors, X-ray, CT, and MRI;
however, the disease has generally progressed to the advanced stage
when detected by bone scanning and arthroscopy (10).
The challenges produced by osteoarthritis have
become increasingly serious, thus researchers at home and abroad
are striving to find an early screening program that is more
effective, in order to improve the rehabilitation effect on
patients. With the advancement of research, it has been revealed
that as non-coding short-stranded RNAs with a length of
approximately 22 nt, microRNAs (miRs) inhibit the translation and
transcription of target genes by binding to the 3' untranslated
region (3'UTR) of downstream target gene mRNAs, thus altering the
expression of the target gene (11). microRNAs play an important role in
numerous diseases (12). It has
been confirmed that microRNA-141 (miR-141) inhibits bone resorption
and has aberrant expression in osteoporosis rats, which is closely
related to changes in normal bone morphology (13,14).
However, its role in osteoarthritis remains unclear. It is
theorized that this miR may be closely correlated with
osteoarthritis, and thus may be particularly significant to the
future diagnosis and treatment of the disease.
Neutrophil-lymphocyte ratio (NLR) has a great predictive value for
coronary artery disease (15), and
a significant monitoring value for inflammation development
(16). Therefore, miR-141 and NLR
in patients with osteoarthritis were analyzed in the present study,
to explore their significance and provide new insights for the
clinical diagnosis and treatment of the disease.
Materials and methods
General information
One hundred and forty-two patients with
osteoarthritis (the study group) admitted to Shanghai
TCM-Integrated Hospital, Shanghai University of TCM from January
2017 to January 2019 and 150 healthy controls (the control group)
were enrolled in this study for a prospective analysis. The present
study was approved by the Ethics Committee of Shanghai
TCM-Integrated Hospital, Shanghai University of TCM. All research
subjects or their immediate families signed the informed consent
form.
Inclusion and exclusion criteria
Inclusion criteria were as follows: i) Patients who
met clinical manifestations of knee osteoarthritis (17); ii) patients confirmed with knee
osteoarthritis by a series of examinations at Shanghai
TCM-Integrated Hospital, Shanghai University of TCM; iii) patients
with complete medical records; iv) patients who had not received
any antibiotic treatment within 3 months before admission; iv)
patients willing to cooperate and participate in the investigation
of medical staff at Shanghai TCM-Integrated Hospital, Shanghai
University of TCM. Exclusion criteria were as follows: i) Patients
complicated with tumors, cardiovascular and cerebrovascular
diseases, autoimmune diseases, organ dysfunction, infectious
diseases, or neurological disorders; ii) patients allergic to drugs
used in the present study; iii) patients with physical disabilities
and who could not take care of themselves; iv) patients who
transferred to other hospitals.
Methods
After admission, the patients were orally
administered loxoprofen sodium tablets (Daiichi Sankyo
Pharmaceutical (Shanghai) Co., Ltd.; State Food and Drug
Administration (China) Approval no. H20030769), 60 mg x3 times/day,
and Gukang capsules (Guizhou Weikang Zifan Pharmaceutical Co.,
Ltd.; SFDA Approval no. Z20025657), 0.4 g/capsule, 3 capsules x3
times/day. The course of treatment was one month in total. Before
treatment, the fasting venous blood (5 ml) of patients was
extracted and placed into EDTA anticoagulant tubes (2 ml) and blood
collection tubes (3 ml) containing inert separation gel and
coagulants. NLR expression in the venous blood of the EDTA
anticoagulant tubes was detected by a flow cytometer, whereas the
venous blood in the blood collection tubes was centrifuged at 1,505
x g for 10 min at 24˚C, to collect the serum. Part of the serum was
used for subsequent experiments, while the rest was placed in an
RNase-free EP tube and stored at -80˚C for later use.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) detection
An EasyPure miRNA kit (cat. no. ER601-01; Beijing
TransGen Biotech Co., Ltd.) was used to extract total RNA from the
collected serum. An ultraviolet spectrophotometer and agarose gel
electrophoresis were used to detect its purity, concentration, and
integrity. TransScript® miRNA RT Enzyme Mix and 2xTS
miRNA Reaction Mix (cat. no. AT351-01; Beijing TransGen Biotech
Co., Ltd.) were used to reversely transcribe the total RNA, with
the steps in strict accordance with the manufacturer's kit. Then,
PCR amplification was carried out. The system consisted of 1 µl of
cDNA, each 0.4 µl of upstream and downstream primers, 10 µl of
TranStart® Tip Green qPCR SuperMix (cat. no. AQ141-01;
Beijing TransGen Biotech Co., Ltd.), 0.4 µl of Passive Reference
Dye (50X) (cat. no. 600536; Beijing Biomars-Technology Co., Ltd.),
and ddH2O finally added to make up to 20 µl. The
conditions were as follows: Pre-denaturation at 94˚C for 30 sec,
denaturation at 94˚C for 5 sec, annealing and extension at 60˚C for
30 sec, for a total of 40 cycles. Each sample was provided with 3
repeated wells, and the experiment was conducted in triplicate. In
the present study, U6 was used as an internal reference and
2-ΔΔCq was used to analyze the data (18). Primer sequences are presented in
Table I.
 | Table IPrimer sequences of miR-141 and
internal reference. |
Table I
Primer sequences of miR-141 and
internal reference.
| Gene | Forward | Reverse |
|---|
| miR-141 |
5'-CTCAAGGCAACCTACCGAAAAG-3' |
5'-TATCGGACCCATCACGGAGTGG-3' |
| U6 |
5'-GATTAGAACCGTCGGTAACGGAA-3' |
5'-AGCGATCTCGTTGGCCTTTCTACC-3' |
Outcome measures
Assessments included: i) Differences in miR-141 and
NLR expression; ii) diagnostic values of miR-141 and NLR for
osteoarthritis; iii) correlations of miR-141 and NLR with the
clinical pathology of osteoarthritis; iv) correlations of miR-141
and NLR with Lysholm Knee Scoring Scale (LKSS) (19), Western Ontario and McMaster
Universities Osteoarthritis Index (WOMAC) score (20), and visual analogue scale (VAS) score
(21) in the study group; v)
correlation of miR-141 with NLR in the study group; and iv) risk
factors for osteoarthritis.
Statistical methods
SPSS 24.0 (Shanghai Yuchuang Network Technology Co.,
Ltd.) was used for statistical calculation. Graphpad 8 (SOFTHEAD,
Inc.) was used to plot figures and verify the results. Count data
including sex and dwelling environment were expressed by rate, and
chi-square test was used for their comparison between groups.
Measurement data for miR-141 and NLR were expressed by the mean ±
standard deviation. Independent sample t-test was used for
comparison between groups, and paired t-test was used for
comparison before and after treatment. One-way ANOVA and LSD post
hoc test were used for the comparison among multiple groups.
Receiver operating characteristic (ROC) curves were plotted to
analyze the diagnostic and predictive values. Binary Logistic
regression analysis was used for the combined detection, to obtain
the constants and coefficients of the regression equation, and then
the ROC curves were analyzed. Pearson correlation coefficient was
used for correlation analysis. Multivariate Logistic regression was
used to analyze risk factors. A P<0.05, was considered to
indicate a statistically significant difference.
Results
Comparison of general information
There were significant differences between the study
and control groups in past medical history and exercise habits
(P<0.001), not in age, body mass index (BMI), total cholesterol,
triglyceride, low density lipoprotein, creatinine, urine nitrogen,
sex, smoking, dwelling environment, and nationality (P>0.050;
Table II).
 | Table IIComparison of general information [n
(%)]. |
Table II
Comparison of general information [n
(%)].
|
Characteristics | Study group
(n=142) | Control group
(n=150) | t or
χ2 | P-value |
|---|
| Age (years) | 59.4±9.1 | 60.1±10.2 | 0.618 | 0.537 |
| BMI
(kg/cm2) | 23.15±1.89 | 23.06±1.98 | 0.397 | 0.692 |
| Total cholesterol
(mmol/l) | 4.42±1.20 | 4.52±1.09 | 0.746 | 0.456 |
| Triglyceride
(mmol/l) | 1.85±0.87 | 1.90±0.77 | 0.521 | 0.603 |
| Low density
lipoprotein (mmol/l) | 2.67±0.62 | 2.58±0.72 | 1.142 | 0.255 |
| Creatinine
(µmol/l) | 72.85±12.52 | 71.19±11.96 | 1.159 | 0.248 |
| Urea nitrogen
(mmol/l) | 6.24±1.63 | 6.42±1.82 | 0.889 | 0.375 |
| Course of disease
(years) | 3.54±1.54 | | | |
| Sex | | | 0.070 | 0.791 |
|
Male | 35 (24.65) | 39 (26.00) | | |
|
Female | 107 (75.35) | 111 (74.00) | | |
| Past medical
history | | | 49.172 | <0.001 |
|
Diabetes | 56 (39.44) | 24 (16.00) | | |
|
Hypertension | 42 (29.58) | 18 (12.00) | | |
|
None | 44 (30.99) | 108 (72.00) | | |
| Smoking | | | 0.024 | 0.876 |
|
Yes | 48 (33.80) | 52 (34.67) | | |
|
No | 94 (66.20) | 98 (65.33) | | |
| Dwelling
environment | | | 1.417 | 0.233 |
|
City | 92 (64.79) | 87 (58.00) | | |
|
Countryside | 50 (35.21) | 63 (42.00) | | |
| Nationality | | | 0.372 | 0.798 |
|
Han | 138 (97.18) | 148 (98.67) | | |
|
Ethnic
minorities | 4 (2.82) | 2 (1.33) | | |
| Exercise
habits | | | 42.642 | <0.001 |
|
Yes | 16 (11.27) | 69 (46.00) | | |
|
No | 126 (88.73) | 81 (54.00) | | |
| Severity | | | | |
|
Early
stage | 38 (26.76) | | | |
|
Middle and
advanced stages | 104 (86.62) | | | |
| Classification | | | | |
|
Primary | 104 (73.24) | | | |
|
Secondary | 38 (26.76) | | | |
Comparison of miR-141 and NLR
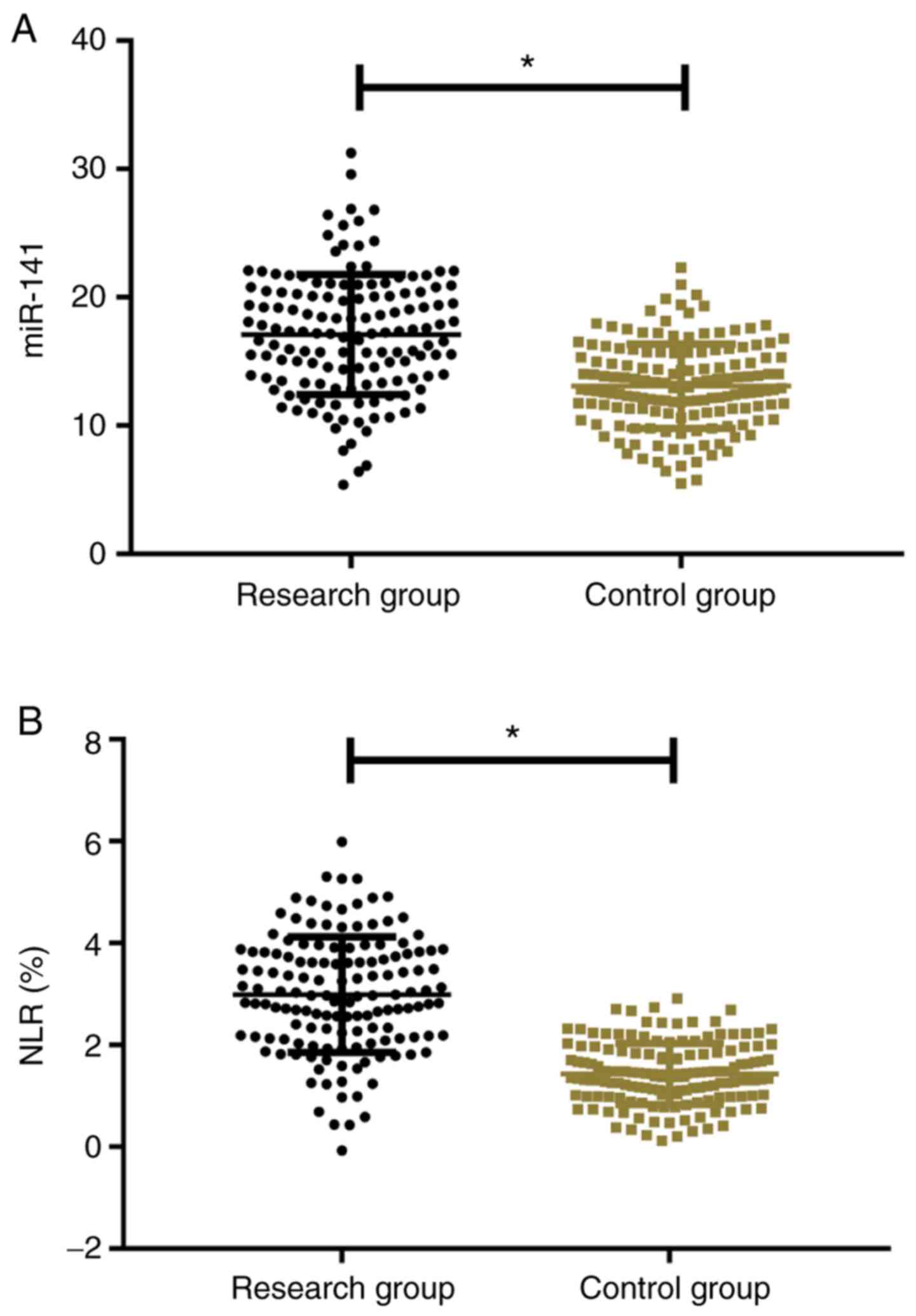
The expression level of miR-141 in the study group
was significantly higher than that in the control group (17.52±4.58
vs. 13.24±3.09) (P<0.001). NLR in the study group (2.81±1.14%)
was significantly higher than in the control group (1.42±0.62%)
(P<0.001; Fig. 1).
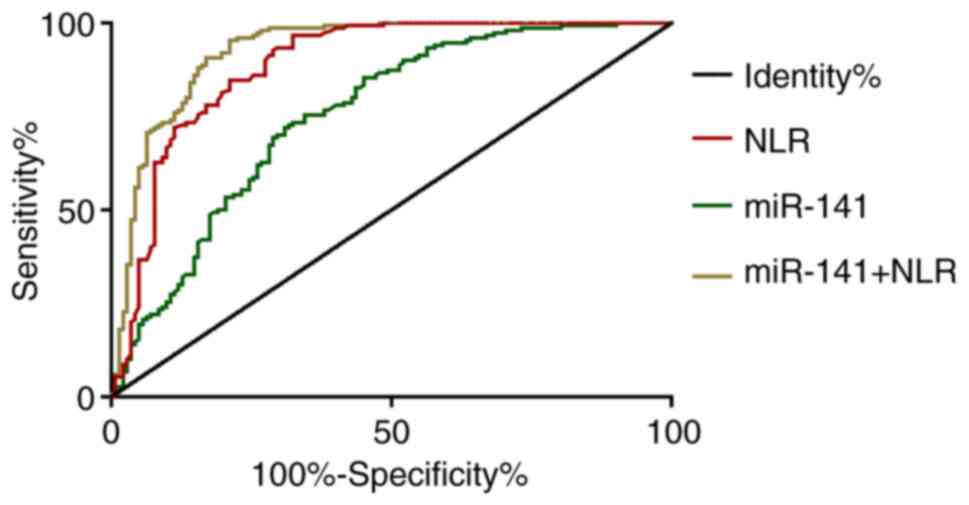
Diagnostic values of miR-141 and
NLR
According to the ROC curves, when the cut-off value
was 14.85, the sensitivity and specificity of miR-141 for
osteoarthritis diagnosis were 72.00 and 69.01%, respectively. When
the cut-off value was 2.51, the sensitivity and specificity of NLR
were 96.67 and 67.61%, respectively. With miR-141 and NLR as
independent variables, binary logistic regression analysis was
performed to obtain the logistic regression model: Logit
(P)=-8.688+0.288 miR-141+2.088 NLR. When the cut-off value was
0.63, the sensitivity and specificity of the model were 95.33 and
78.87%, respectively (Tables III
and IV and Fig. 2).
 | Table IIIAnalysis of ROC curves. |
Table III
Analysis of ROC curves.
| Parameters | miR-141 | NLR | miR-141+NLR |
|---|
| Cut-off value | 14.85 | 2.51 | 0.63 |
| AUC | 0.753 | 0.887 | 0.927 |
| SE | 0.029 | 0.020 | 0.016 |
| 95% CI | 0.697-0.809 | 0.847-0.927 | 0.895-0.958 |
| P-value | <0.001 | <0.001 | <0.001 |
| Sensitivity
(%) | 72.00 | 96.67 | 95.33 |
| Specificity
(%) | 69.01 | 67.61 | 78.87 |
 | Table IVBinary logistic regression
analysis. |
Table IV
Binary logistic regression
analysis.
| Factors | B | SE | Wald | Df | Sig | Exp (B) |
|---|
| miR-141 | 0.288 | 0.051 | 32.195 | 1 | P<0.001 | 1.334 |
| NLR | 2.088 | 0.262 | 63.571 | 1 | P<0.001 | 8.070 |
Clinicopathological associations
between miR-141, NLR and osteoarthritis
miR-141 was closely associated to the course of
disease, severity, and classification of diseases of patients with
osteoarthritis (P<0.001), not significantly associated with
their age, BMI, total cholesterol, triglyceride, low density
lipoprotein, creatinine, urine nitrogen, sex, past medical history,
smoking, dwelling environment, nationality, and exercise habits
(P>0.05). NLR was closely associated to the age, course of
disease, and severity of patients with osteoarthritis (P<0.001),
not significantly associated with their BMI, total cholesterol,
triglyceride, low density lipoprotein, creatinine, urine nitrogen,
sex, past medical history, smoking, dwelling environment,
nationality, exercise habits, and classification of diseases
(P>0.05; Table V).
 | Table VClinicopathological associations
between miR-141, NLR and osteoarthritis. |
Table V
Clinicopathological associations
between miR-141, NLR and osteoarthritis.
|
Characteristics | n | miR-141 | t or F-value | P-value | NLR | t or F-value | P-value |
|---|
| Age (years) | | | 0.239 | 0.811 | | 4.881 | <0.001 |
|
<59.4 | 42 | 17.34±4.14 | | | 2.12±0.98 | | |
|
≥59.4 | 100 | 17.55±5.01 | | | 3.09±1.12 | | |
| BMI
(kg/cm2) | | | 0.408 | 0.684 | | 0.245 | 0.807 |
|
<23.15 | 38 | 17.73±4.66 | | | 2.92±0.84 | | |
|
≥23.15 | 104 | 18.14±5.52 | | | 2.97±1.15 | | |
| Total cholesterol
(mmol/l) | | | 0.666 | 0.506 | | 1.034 | 0.303 |
|
<4.42 | 80 | 17.45±3.63 | | | 2.71±0.92 | | |
|
≥4.42 | 62 | 17.88±4.04 | | | 2.90±1.27 | | |
| Triglyceride
(mmol/l) | | | 0.194 | 0.846 | | 0.167 | 0.868 |
|
<1.85 | 83 | 17.85±3.75 | | | 2.71±0.98 | | |
|
≥1.85 | 59 | 17.72±4.17 | | | 2.68±1.16 | | |
| Low density
lipoprotein (mmol/l) | | | 0.186 | 0.852 | | 1.719 | 0.088 |
|
<2.67 | 79 | 17.57±4.87 | | | 2.99±1.12 | | |
|
≥2.67 | 63 | 17.72±4.63 | | | 2.58±1.71 | | |
| Creatinine
(µmol/l) | | | 0.519 | 0.605 | | 1.112 | 0.268 |
|
<72.85 | 68 | 17.14±4.59 | | | 3.13±1.43 | | |
|
≥72.85 | 74 | 17.58±5.43 | | | 2.86±1.46 | | |
| Urea nitrogen
(mmol/l) | | | 0.180 | 0.857 | | 1.131 | 0.260 |
|
<6.24 | 70 | 17.59±4.72 | | | 2.75±1.10 | | |
|
≥6.24 | 72 | 17.73±4.54 | | | 2.52±1.31 | | |
| Course of disease
(years) | | | 7.216 | <0.001 | | 5.227 | <0.001 |
|
<3.54 | 49 | 14.57±5.63 | | | 2.50±1.24 | | |
|
≥3.54 | 93 | 20.24±3.69 | | | 3.62±1.20 | | |
| Sex | | | 0.164 | 0.870 | | 0.612 | 0.542 |
|
Male | 35 | 17.85±3.75 | | | 2.80±1.24 | | |
|
Female | 107 | 17.72±4.17 | | | 2.92±0.92 | | |
| Past medical
history | | | 0.756 | 0.472 | | 10.332 | <0.001 |
|
Diabetes | 56 | 17.73±4.54 | | | 2.85±1.11 | | |
|
Hypertension | 42 | 17.59±4.58 | | | 2.72±0.92 | | |
|
None | 44 | 18.62±3.64 | | | 2.04±0.62 | | |
| Smoking | | | 0.281 | 0.779 | | 0.559 | 0.577 |
|
Yes | 48 | 18.08±3.95 | | | 2.98±1.14 | | |
|
No | 94 | 17.86±4.62 | | | 3.12±1.53 | | |
| Dwelling
environment | | | 0.024 | 0.981 | | 0.779 | 0.437 |
|
City | 92 | 17.16±4.59 | | | 3.12±1.20 | | |
|
Countryside | 50 | 17.14±4.86 | | | 2.96±1.11 | | |
| Nationality | | | 0.080 | 0.936 | | 0.138 | 0.890 |
|
Han | 138 | 16.93±4.68 | | | 2.98±1.14 | | |
|
Ethnic
minorities | 4 | 17.12±4.25 | | | 3.06±1.13 | | |
| Exercise
habits | | | 0.188 | 0.851 | | 0.408 | 0.684 |
|
Yes | 16 | 17.63±4.58 | | | 2.85±1.24 | | |
|
No | 126 | 17.86±4.62 | | | 2.71±1.30 | | |
| Severity | | | 4.152 | <0.001 | | 3.628 | <0.001 |
|
Early
stage | 19 | 14.62±5.86 | | | 2.12±1.63 | | |
|
Middle and
advanced stages | 123 | 21.21±6.52 | | | 3.25±1.20 | | |
| Classification | | | 12.321 | <0.001 | | 0.419 | 0.676 |
|
Primary | 104 | 15.12±2.85 | | | 2.75±1.31 | | |
|
Secondary | 38 | 22.62±4.05 | | | 2.85±1.10 | | |
Correlations of miR-141 and NLR with
LKSS, WOMAC, and VAS scores
Before treatment, LKSS, WOMAC, and VAS scores in the
study group were (52.62±6.21), (63.35±7.21), and (8.26±2.62)
points, respectively. According to the analysis with Pearson
correlation coefficient, miR-141 was negatively correlated with
LKSS score (r=-0.671, P<0.001), positively correlated with WOMAC
score (r=0.778, P<0.001), and positively correlated with VAS
score (r=0.846, P<0.001). NLR was negatively correlated with
LKSS score (r=-0.649, P<0.001), positively correlated with WOMAC
score (r=0.722, P<0.001), and positively correlated with VAS
score (r=0.825, P<0.001). In the study group, miR-141 was
positively correlated with NLR (r=0.889, P<0.001) (Fig. 3).
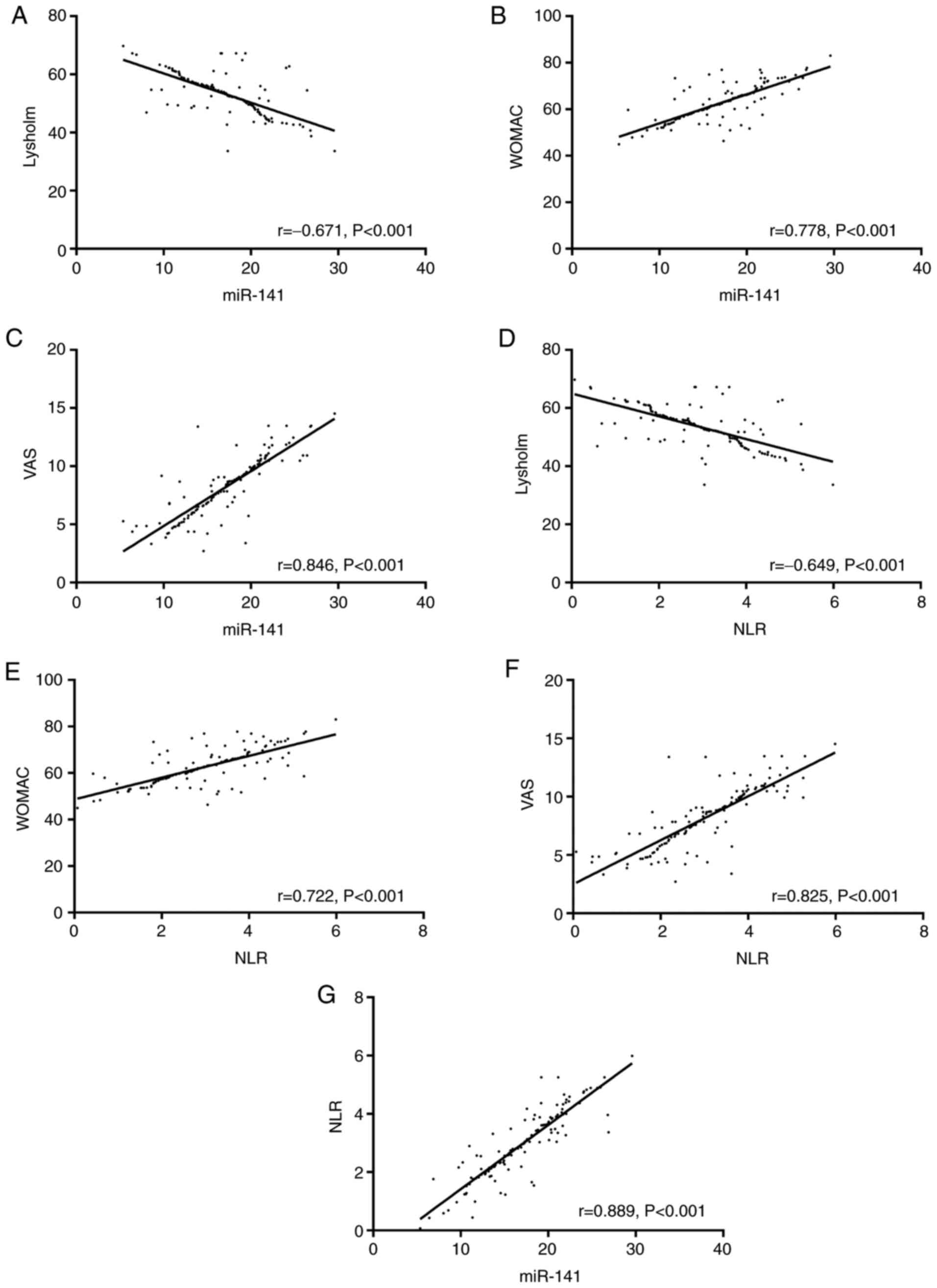 | Figure 3Correlations of miR-141 and NLR with
LKSS, WOMAC, and VAS scores. (A) miR-141 was negatively correlated
with LKSS score (r=-0.671, P<0.001). (B) miR-141 was positively
correlated with WOMAC score (r=0.778, P<0.001). (C) miR-141 was
positively correlated with VAS score (r=0.846, P<0.001). (D) NLR
was negatively correlated with LKSS score (r=-0.649, P<0.001).
(E) NLR was positively correlated with WOMAC score (r=0.722,
P<0.001). (F) NLR was positively correlated with VAS score
(r=0.825, P<0.001). (G) miR-141 was positively correlated with
NLR (r=0.889, P<0.001). miR, microRNA; NLR,
neutrophil-lymphocyte ratio; LKSS, Lysholm Knee Scoring Scale;
WOMAC, Western Ontario and McMaster Universities Osteoarthritis
Index; VAS, visual analogue scale. |
Analysis of related risk factors
With the comparison of general information between
the two groups as the results of univariate analysis, the
indicators with differences were assigned values (Table VI). Then, forward logistic
regression (LR) was selected for multivariate logistic regression
analysis. The results showed that the course of disease, severity,
and the classification of diseases were not independent risk
factors for osteoarthritis. However, past medical history (OR:
12.245; 95% CI: 2.652-54.262), miR-141 (OR: 1.256; 95% CI:
1.154-1.452), and NLR (OR: 25.627; 95% CI: 1.953-86.621) were the
independent risk factors. Exercise habits (OR: 0.497; 95% CI:
0.085-3.215) was the independent protective factor for the disease
(Table VII).
 | Table VIAssignment. |
Table VI
Assignment.
| Factors | Assignment |
|---|
| Past medical
history | None=0; diabetes=1;
hypertension=2 |
| Exercise
habits | Yes=0; no=1 |
| Course of
disease | A continuous
variable analyzed by original data |
| Severity | Early stage=0;
middle and advanced stages=1 |
| Classification of
diseases | Primary=0;
secondary=1 |
| miR-141 | A continuous
variable analyzed by original data |
| NLR | A continuous
variable analyzed by original data |
 | Table VIIMultivariate logistic analysis. |
Table VII
Multivariate logistic analysis.
| | 95% CI |
|---|
| Factors | B | SE | Wald | Sig | OR | Lower | Upper |
|---|
| Past medical
history | 2.524 | 0.862 | 9.412 | 0.002 | 12.245 | 2.652 | 54.262 |
| Exercise
habits | 1.168 | 0.983 | 1.512 | 0.026 | 0.497 | 0.085 | 3.215 |
| miR-141 | 0.225 | 0.035 | 54.216 | P<0.001 | 1.256 | 1.154 | 1.452 |
| NLR | 3.542 | 1.236 | 5.921 | 0.012 | 25.627 | 1.953 | 86.621 |
Discussion
As a chronic inflammation with articular cartilage
degeneration as the core, osteoarthritis is mainly manifested as
multi-directional and multi-level bone structural lesions (22). It causes arthralgia, joint
dysfunction, and joint deformity, affecting the normal life and
actions of patients (23).
Therefore, a new clinical index for its early screening is being
explored to effectively diagnose and treat this disease. For
example, a study by Li et al suggested that ADAMTS-4 may be
a potential marker for osteoarthritis (24). Park et al revealed that the
detection of CTX-II in serum and urine by BeadChip had a markedly
high diagnostic value for the disease (25). Previous studies have revealed that
differences in miR expression are closely associated with cartilage
destruction, chondrocyte apoptosis, and synovitis during the
development and progression of certain osteoarticular diseases
(26,27). miR-141, which belongs to the miR-200
family and is located at 12p 13.31 in the human body, was revealed
to be closely related to gastric and non-small cell lung cancer
(28,29), however, its role in osteoarthritis
is still unclear. In the present study, the roles of miR-141 and
NLR in osteoarthritis were explored, and the findings indicated
that they are essential for the diagnosis and treatment of the
disease.
In the present study, miR-141 and NLR were
significantly increased in the peripheral blood of patients with
osteoarthritis, suggesting that they may be involved in the
development and progression of the disease. This is consistent with
the findings of Dong et al (miR-141 in endometrial cancer)
and Gundogdu et al (NLR in osteoarthritis) (30,31),
which support the results of the present study. The mechanism of
action of miR-141 in osteoarthritis remains unclear. As an
osteoblastic negative regulatory factor, miR-141 inhibits the
cascade action of Dlx5/Runx2/Msx2 axis after transcription, thus
reducing osteogenic activities (32). Karlsson et al revealed that
the Msx2 gene is involved in the bone formation of patients with
osteoarthritis (33). Therefore, it
is speculated that miR-141 may inhibit Msx2 and then inhibit the
activation of osteoblasts, thereby deteriorating osteoarthritis.
NLR, a blood inflammatory marker obtained through the ratio of
neutrophils to lymphocytes, has a better predictive value for
inflammation than traditional and single inflammatory markers
(34). In our study, NLR in
patients with osteoarthritis was significantly increased,
suggesting that adverse conditions caused by inflammatory
stimulation significantly reduce the lymphocytes of patients. The
mechanism of NLR affecting the disease may be achieved through
IL-6, IL-8, and TNF-α (35). A
study by Goldring and Otero et al revealed the significant
increase of the inflammatory cytokines in patients with
osteoarthritis (36), which also
confirms our theory. Inflammatory cytokines can enhance the
cellular activity of the synovial tissue, thus neutrophils,
platelets, or lymphocytes in peripheral blood significantly
increase, while other indices are stable in patients with tumors
and immune diseases (37).
Therefore, NLR is commonly used as an indicator to evaluate the
health of patients, however its diagnostic specificity for a
certain disease is poor (38). In
the present study, the combined detection of miR-141 and NLR had
better sensitivity and specificity for predicting osteoarthritis.
This reveals that the combined detection can be used for the early
screening of osteoarthritis in clinical practice, and can improve
its early diagnostic rate, which is important for the early
treatment of the patients. At present, the diagnosis of
osteoarthritis depends on imaging technology. The detection of
miR-141 combined with NLR has several advantages. Firstly, the
evaluation is objective. The assessment of results is not affected
by the previous experience and subjective consciousness of doctors.
Secondly, detection methods are simple. The peripheral blood is
only needed for detection. Thirdly, the preservation time is long.
The preservation time of peripheral blood in the low-temperature
environment is long and the detection is less affected by
environmental factors (39).
According to the analysis of the correlations of
miR-141 and NLR with clinical pathology, miR-141 was closely
associated to the course of disease, severity, and the
classification of diseases. NLR was closely associated to age
(40), past medical history, the
course of disease, and severity. These results indicated that
miR-141 and NLR are closely associated with osteoarthritis
progression. There were differences in NLR between patients of
different ages. This may be because the immune and metabolic
functions of older patients decline, which makes them more
vulnerable to inflammatory responses and diseases. Similarly, the
findings of Soysal et al (41), support this theory. There were
significant differences in the two indicators between patients with
different classification of diseases, which may be due to the fact
that patients with primary osteoarthritis usually have no trauma,
infection, congenital malformation, genetic defects, and
abnormalities in systemic metabolism and endocrine, with lighter
conditions and lower treatment difficulty compared with those of
patients with secondary arthritis (osteoarthritis occurring based
on local original joint lesions). This is similar to the
experimental results of Lunebourg et al (42). According to the correlations of
miR-141 and NLR with LKSS, WOMAC, and VAS scores, miR-141 and NLR
were negatively correlated with LKSS score, but positively
correlated with WOMAC and VAS scores. This suggests that the two
indicators are closely related to the conditions of patients with
osteoarthritis. The two were also significantly correlated, which
indicates that they have consistent synergy and may be closely
related to the inflammatory responses of patients. According to the
multivariate logistic regression analysis, past medical history,
miR-141, and NLR were independent risk factors for osteoarthritis,
demonstrating that miR-141 and NLR may be potential therapeutic
targets for the disease. Exercise habits were the independent
protective factor for the disease, suggesting that proper exercise
can prevent its development, thus the importance of regular
exercise should be emphasized for middle-aged people.
The present study aimed to explore the roles of
miR-141 and NLR in osteoarthritis, but it has limitations due to
the limited experimental conditions. For example, the underlying
mechanisms of the effects of miR-141 and NLR in osteoarthritis are
still conjecture and lack the support of basic experiments. We hope
in future that relevant experimental analysis can be conducted
according to our conjecture. In addition, the age range of the
subjects included in the present study was small and the
differences in regions and ethnicities was limited, therefore the
results may be biased. Moreover, sequences of the miR family are
numerous, thus there may be other miRs especially expressed in
osteoarthritis, which require further analysis and discussion. In
future, this study will be improved in order to achieve optimal
experimental results.
In summary, miR-141 and NLR were significantly
increased in the peripheral blood of patients with osteoarthritis.
Their combined detection has a good diagnostic value for the
disease and may become a potential therapeutic target for it in the
future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China: The research of Bi syndrome
and body constitution as risk factors in predicting progression of
osteoarthritis (funding project no. 81373665) and the Shanghai
Science and Technology Commission Project: Optimization of TCM
comprehensive diagnosis and treatment plan for senile Cervical
Radiculopathy (funding project no. 18401901700).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RZ conceived the study and wrote the manuscript. CC
performed the PCR experiments. XX analyzed and interpreted the data
of patients. GL and HG helped with the statistical analysis. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shanghai TCM-Integrated Hospital, Shanghai University
of TCM. Patients who participated in the present research, signed
the informed consent and had complete clinical data. Signed written
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Malfait AM: Osteoarthritis year in review
2015: Biology. Osteoarthritis Cartilage. 24:21–26. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Loeser RF, Collins JA and Diekman BO:
Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol.
12:412–420. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nelson AE: Osteoarthritis year in review
2017: Clinical. Osteoarthritis Cartilage. 26:319–325.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Palazzo C, Nguyen C, Lefevre-Colau MM,
Rannou F and Poiraudeau S: Risk factors and burden of
osteoarthritis. Ann Phys Rehabil Med. 59:134–138. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yu D, Jordan KP, Bedson J, Englund M,
Blyth F, Turkiewicz A, Prieto-Alhambra D and Peat G: Population
trends in the incidence and initial management of osteoarthritis:
Age-period-cohort analysis of the clinical practice research
Datalink, 1992-2013. Rheumatology (Oxford). 56:1902–1917.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mathiessen A and Conaghan PG: Synovitis in
osteoarthritis: Current understanding with therapeutic
implications. Arthritis Res Ther. 19(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sharma L: Osteoarthritis year in review
2015: Clinical. Osteoarthritis Cartilage. 24:36–48. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Collins NJ, Prinsen CA, Christensen R,
Bartels EM, Terwee CB and Roos EM: Knee injury and osteoarthritis
outcome score (KOOS): Systematic review and meta-analysis of
measurement properties. Osteoarthritis Cartilage. 24:1317–1329.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wallace IJ, Worthington S, Felson DT,
urmain RD, Wren KT, Maijanen H, Woods RJ and Lieberman DE: Knee
osteoarthritis has doubled in prevalence since the mid-20th
century. Proc Natl Acad Sci USA. 114:9332–9336. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang Y, Teichtahl AJ and Cicuttini FM:
Osteoarthritis year in review 2015: Imaging. Osteoarthritis
Cartilage. 24:49–57. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Feinberg MW and Moore KJ: MicroRNA
regulation of atherosclerosis. Circ Res. 118:703–720.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang S, Zhang W, Cai M, Zhang Y, Jin F,
Yan S, Baloch Z, Fang Z, Xue S, Tang R, et al: Suppression of bone
resorption by miR-141 in aged rhesus monkeys. J Bone Miner Res.
33:1799–1812. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hu H, Zhang G, Tian G, Lv G and Jin Y:
miRNA profiling reveals the upregulation of Osteogenesis-associated
miRNAs in ovariectomy osteoporosis mice. Clin Exp Obstet Gynecol.
45:817–822. 2018.
|
|
15
|
Balta S, Celik T, Mikhailidis DP, Ozturk
C, Demirkol S, Aparci M and Iyisoy A: The relation between
atherosclerosis and the neutrophil-lymphocyte ratio. Clin Appl
Thromb Hemost. 22:405–411. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fu X, Li T, Dai Y and Li J: Preoperative
systemic inflammation score (SIS) is superior to neutrophil to
lymphocyte ratio (NLR) as a predicting indicator in patients with
esophageal squamous cell carcinoma. BMC Cancer.
19(721)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tichopad A, Kitchen R, Riedmaier I, Becker
C, Ståhlberg A and Kubista M: Design and optimization of
reverse-transcription quantitative PCR experiments. Clin Chem.
55:1816–1823. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lysholm J and Gillquist J: Evaluation of
knee ligament surgery results with special emphasis on use of a
scoring scale. Am J Sports Med. 10:150–154. 1982.PubMed/NCBI View Article : Google Scholar
|
|
20
|
McConnell S, Kolopack P and Davis AM: The
western ontario and mcmaster universities osteoarthritis index
(WOMAC): A review of its utility and measurement Properties.
Arthritis Rheum. 45:453–461. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wewers ME and Lowe NK: A critical review
of visual analogue scales in the measurement of clinical phenomena.
Res Nurs Health. 13:227–236. 1990.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Johnson VL and Hunter DJ: The epidemiology
of osteoarthritis. Best Pract Res Clin Rheumatol. 28:5–15.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Thakur M, Dickenson AH and Baron R:
Osteoarthritis pain: Nociceptive or neuropathic? Nat Rev Rheumatol.
10(374)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li W, Du C, Wang H and Zhang C: Increased
serum ADAMTS-4 in knee osteoarthritis: A potential indicator for
the diagnosis of osteoarthritis in early stages. Genet Mol Res.
13:9642–9649. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Park YM, Kim SJ, Lee KJ, Yang SS, Min BH
and Yoon HC: Detection of CTX-II in serum and urine to diagnose
osteoarthritis by using a Fluoro-microbeads guiding chip. Biosens
Bioelectron. 67:192–199. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li D, Liu J, Guo B, Liang C, Dang L, Lu C,
He X, Cheung HY, Xu L, Lu C, et al: Osteoclast-derived exosomal
miR-214-3p inhibits osteoblastic bone formation. Nat Commun.
7(10872)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu Y, Zhang Y, Zhang Y and Wang JJ:
CircRNA hsa_circ_0005105 upregulates NAMPT expression and promotes
chondrocyte extracellular matrix degradation by sponging miR-26a.
Cell Biol Int. 41:1283–1289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhou X, Ye F, Yin C, Zhuang Y, Yue G and
Zhang G: The interaction between MiR-141 and lncRNA-H19 in
regulating cell proliferation and migration in gastric cancer. Cell
Physiol Biochem. 36:1440–1452. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tejero R, Navarro A, Campayo M, Viñolas N,
Marrades RM, Cordeiro A, Ruíz-Martínez M, Santasusagna S, Molins L,
Ramirez J, et al: miR-141 and miR-200c as markers of overall
survival in early stage non-small cell lung cancer adenocarcinoma.
PLoS One. 9(e101899)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dong Y, Si JW, Li WT, Liang L, Zhao J,
Zhou M, Li D and Li T: miR-200a/miR-141 and miR-205 upregulation
might be associated with hormone receptor status and prognosis in
endometrial carcinomas. Int J Clin Exp Pathol. 8:2864–2875.
2015.PubMed/NCBI
|
|
31
|
Gundogdu G and Gundogdu K: A novel
biomarker in patients with knee osteoarthritis: Adropin. Clin
Rheumatol. 37:2179–2186. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sangani R, Periyasamy-Thandavan S, Kolhe
R, Bhattacharyya MH, Chutkan N, Hunter M, Isales C, Hamrick M, Hill
WD and Fulzele S: MicroRNAs-141 and 200a regulate the SVCT2
transporter in bone marrow stromal cells. Mol Cell Endocrinol.
410:19–26. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Karlsson C, Dehne T, Lindahl A, Brittberg
M, Pruss A, Sittinger M and Ringe J: Genome-wide expression
profiling reveals new candidate genes associated with
osteoarthritis. Osteoarthritis Cartilage. 18:581–592.
2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sunbul M, Gerin F, Durmus E, Kivrak T,
Sari I, Tigen K and Cincin A: Neutrophil to lymphocyte and platelet
to lymphocyte ratio in patients with dipper versus non-dipper
hypertension. Clin Exp Hypertens. 36:217–221. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Han R, Wu D, Deng S, Liu T, Zhang T and Xu
Y: NLRP3 inflammasome induces pyroptosis in lung tissues of
radiation-induced lung injury in mice. Xi Bao Yu Fen Zi Mian Yi Xue
Za Zhi. 33:1206–1211. 2017.PubMed/NCBI(In Chinese).
|
|
36
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Adamski JK, Kelsey A and Brennan B:
Inflammatory myofibroblastic tumors following the treatment of
malignancy in Childhood: Case reports. J Pediatr Hematol Oncol.
36:159–162. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Diaz-Martinez J, Campa A, Delgado-Enciso
I, Hain D, George F, Huffman F and Baum M: The relationship of
blood neutrophil-to-lymphocyte ratio with nutrition markers and
health outcomes in hemodialysis patients. Int Urol Nephrol.
51:1239–1247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Koolwal D: Anticoagulant EDTA induced
storage effect (Artifacts) on peripheral blood cells. Clin Lab
Haematol. 27:336–342. 2018.
|
|
40
|
Niazi S, Krogh Nielsen M, Sørensen TL and
Subhi Y: Neutrophil-to-lymphocyte ratio in age-related macular
degeneration: A systematic review and meta-analysis. Acta
Ophthalmol. 97:558–566. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Soysal P, Stubbs B, Lucato P, Luchini C,
Solmi M, Peluso R, Sergi G, Isik AT, Manzato E, Maggi S, et al:
Inflammation and frailty in the elderly: A systematic review and
meta-analysis. Ageing Res Rev. 31:1–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lunebourg A, Parratte S, Gay A, Ollivier
M, Garcia-Parra K and Argenson JN: Lower function, quality of life,
and survival rate after total knee arthroplasty for posttraumatic
arthritis than for primary arthritis. Acta Orthop. 86:189–194.
2015.PubMed/NCBI View Article : Google Scholar
|