Introduction
Bronchiolitis is common in infants between 1 and 6
months. The characteristic lesion is the bronchioles of the lungs,
which is a common acute lower respiratory tract infectious disease
in children. The major clinical symptoms of the disease are
wheezing, shortness of breath, and tri-concavity signs. The disease
often occurs in winter, and the most common pathogen is respiratory
syncytial virus (1). Researchers
have shown that respiratory syncytial virus (RSV) bronchiolitis is
one of the high risk factors for Bronchiolitis in infants and young
children to develop bronchial asthma (2). RSV is an enveloped, non-segmented
antisense RNA virus of the paramyxoviridae family. It is the most
common respiratory pathogen in Bronchiolitis in infants and young
children worldwide (3). Prospective
epidemiological research suggests that RSV-associated bronchiolitis
is significantly correlated with subsequent recurrent wheezing and
asthma in childhood (4). This
phenomenon has also been confirmed in animal models. RSV can be
vertically transmitted from mother to the fetal lung respiratory
tract, and the persistence of the virus after birth has a
significant correlation with persistent airway hyperresponsiveness
(5,6). After years of research, there is still
a lack of reliable biomarkers, effective vaccines and treatment
strategies for RSV bronchiolitis in infants and young children.
High mobility group protein box 1 (HMGB1) is a
highly conserved nuclear DNA binding protein and a molecule of
damage associated molecular pattern (DAMP). HMGB1 is the key
mediator molecule that initiates the innate and adaptive immune
response in inflammation (7). HMGB1
exists mainly in the nucleus and is involved in nucleosome
stabilization, cell differentiation, DNA repair, and gene
transcription. When cells are stimulated by ‘danger signals’,
HMGB1, as the molecule of the alarmins family, can be rapidly
activated and released to the outside of cells. It acts directly as
inflammatory cytokine to initiate and maintain the immune response
caused by infectious or non-infectious diseases, and maintain the
homeostasis balance in the damaged tissue (8). HMGB1 not only participates in the
inflammatory response caused by a variety of cytokines and
chemotactic proinflammatory cells, but also participates in
asthmatic airway sensitization and induction of airway inflammation
by directly affecting immune cells (9). In recent years, it is suggested that
HMGB1 can be used as a biological marker. It plays an important
role in the development of mouse RSV infection and the development
of chronic airway dysfunction (6).
However, there are still few reports on the expression of HMGB1 in
clinical patients with bronchiolitis and its role in activating
immune cells during the development of disease. Therefore, this
experiment probes into the expression of HMGB1 in the clinical
sample and in vitro cell model. The effect of HMGB1 on the
function of macrophage inflammation activation was explored, aiming
at providing a new train of thought for the diagnosis and treatment
of RSV bronchiolitis.
Patients and methods
Research objects
The study was approved by the Ethics Committee of
Xuzhou Children's Hospital, Xuzhou Medical University (Xuzhou,
China). All the families of the child patients were informed of the
experimental purpose and research plan before blood drawing, and
signed written informed consent. Thirty patients with respiratory
syncytial virus bronchiolitis were hospitalized in Department of
Pediatrics, Xuzhou Children's Hospital, Xuzhou Medical University
from January 2017 to September 2019. Their average age was
(10.23±0.62) months in a ratio of male to female 18:12. Inclusion
criteria: i) children aged up to 2.5 years with the disease for
less than 3 days; ii) the serum-specific RSV-specific IgM was
detected to be positive after admission, iii) children who met the
diagnostic criteria for bronchiolitis in the 7th edition of
Zhufutang Practical Pediatrics; iv) children diagnosed with
bronchiolitis for the first time. Exclusion criteria: i) children
with a family history of asthma-related diseases; ii) children with
congenital heart and pulmonary insufficiency; iii) children with
suspected immune deficiency and allergic diseases; iv) children on
antiviral drugs for 4 weeks before treatment. The healthy control
group comprised of 30 infants and young children who underwent a
physical examination in the hospital during the same period. All of
the infants were full-term babies, without recent suspicious
infection status, and without previous history of diseases such as
immunodeficiency, allergic diseases, and long-term medication
history. Their age and gender match the patient group,
respectively. The age was (10.31±0.63) months, which was comparable
(P=0.077). According to the 2014 version of Bronchiolitis Diagnosis
and Prevention, the severity of the included patients was graded
into mild, moderate and severe according to the feeding amount
(normal/normal to drop by half/drop by more than half), respiratory
rate (normal/>60 times/min/>70 times/min), inspiratory
trident sign on chest wall (mild/moderate/severe), nasal agitating
(none/yes), blood oxygen saturation (>92%/88-92%/<88%) and
mental condition (normal/minor irritability/irritable coma). All
patients included in the research group were followed up for an
18-month outpatient visit to assess whether the children had
symptoms such as chest tightness, dry cough, wheezing, and
expiratory dyspnea, bronchodilation. Bronchodilation test was
positive if FEV1 increased by ≥12% 15 min after inhaling available
β2 receptor agonists. There was reversible airflow restriction if
FEV1/FVC >0.8. The infants were checked to see if they had
asthma in hospital during the follow-up period.
Analysis of peripheral blood
Two milliliters of peripheral venous blood (EDTA)
was extracted from all the subjects and placed at 4˚C. The blood
samples were sent to the laboratory for detection within 4 h. Then
the blood cell analysis and CRP protein expression level of
patients upon admission were collected according to the report
results.
Enzyme linked immunosorbent assay
(ELISA)
HMGB1 kit (E-EL-H1554c; Ellerat) was used according
to the instructions to detect the concentration of HMGB1 in the
peripheral blood plasma of patients. A microplate reader (iMark;
Bio-Rad) was used to read the absorbance at 450 nm.
Cytology experiments Cell culture
Keratinocyte Complete Medium (zq-1303; Zhong Qiao
Xinzhou) was used in this experiment to culture human bronchial
epithelial cells 16HBE (ZQ0001; Zhong Qiao Xinzhou). The monocyte
THP-1 cell line was purchased from (ZQ0086) Zhong Qiao Xinzhou. 90%
1640 (HyClone, Sv30087.01), 10% FBS, 100 µ/ml penicillin and 100
µ/ml streptomycin (Biyuntian, C0222) were used for culture. The
cells were cultured in a constant temperature incubator containing
5% CO2 at 37˚C, and it was used when its density
increased to 90%.
Preparation of RSV and infection
Human recombinant RSV-A2 strain was purchased from
ATCC in the United States. The RSV was purified by discontinuous
sucrose gradient centrifugation at 5,000 x g for 10 min at 4˚C, as
described (7). The titer of the
virus was confirmed to be 109 PFU/ml by methyl cellulose
empty spot method. The virus was repacked and stored in a freezer
at -80˚C for use. Six hours before RSV virus infected 16HBE cells,
the cell culture medium was replaced with basic culture medium.
When the cell density reached 90%, the infection was carried out
for 6 h according to the complex number of multiplication of
infection (MOI)=1.0. The uninfected 16HBE cells were treated with
the same amount of 30% sucrose solution as the infection control
group. The cell culture supernatant and protein were extracted and
stored at -80˚C.
Cell treatment grouping
After the cells in the infected RSV group and the
infected control group grew to 30-50% after passage, the cells were
divided into HMGB1 inhibition group and observation group. DMSO was
added to the observation group, and 0.16 µM (glycyrrhizin, S2302;
Selleck) was added to the HMGB1 inhibition group, until the cells
grew to the appropriate concentration.
Co-culture of macrophages
Transwell plate (3452; Corning) was used for
co-culture of monocytes and 16HBE. THP-1 monocytes with a density
of 1x106/ml (ZQ0086; Zhongqiao Xinzhou) was added to the
lower wells of Transwell at 5 ml/well, and 1x106 HMGB1
inhibition group infected with RSV virus and 16HBE cells in the
observation group were added to the upper wells, respectively.
After co-culture of 16HBE cells in the uninfected HMGB1 inhibition
group and the observation group for 48 h, the total protein in the
lower layer of the cells was extracted for use.
Quantitative real-time polymerase
chain reaction (qRT-PCR)
RNeasy Mini Kit (74104; Qiagen) was used to extract
the total RNA of 16HBE cells infected with RSV and infected control
cells. The RNA samples were quantified using a Nanodrop
spectrophotometer. Reverse transcription was performed according to
the specification of Taqman reagent. The forward and reverse
primers of RSV-A2 virus infection are:
5'-ACTGCAATCAYACAAGATGCAACRA-3' and 5'-CAGATTGRAGAAGCTGATTCCA-3'.
The expression level of gene change was ∆Ct value-ACTB reference
value, the expression was calculated based on the results of the
2-∆∆Ct method.
Western blot protein detection
Cells were added with RIPA lysate (R0020; Solarbio)
and protease inhibitor PMSF (P0100; Solarbio) with a final
concentration of 1 mM, lysed and centrifuged at 15,000 x g for 10
min at 4˚C to extract the cell protein. The protein concentration
was quantified by BCA method, and the final protein concentration
was adjusted. The sample protein concentration was 5-10 µg/µl with
10 µl applied to each well, and the gel was electrophoresed using
an 8% SDS-PAGE separation gel. After electrophoresis, the protein
was transferred to the PVDF membrane by a semi-wet transfer method.
5% Skim milk powder was blocked for 1 h, and then Toll-like
receptor (TLR)4 (1:2,000, 66350-1-Ig; Proteintech), TLR7 (1:500,
17232-1-AP; Proteintech), GAPDH (1:5,000, 60004-1-Ig; Proteintech)
were added. The primary antibody dilution solution was left at 4˚C
overnight, then it was washed three times with 0.1% PBST. The
corresponding HRP-labeled goat anti-rabbit (mouse) immune secondary
antibody (1:3,000, SA00001-1/2; Proteintech) was added, then
incubated at room temperature for 1 h. Then washed 3 times with
0.1% PBST. ECL chemiluminescence solution (PE0010; Solarbio) was
added, after that, it was developed, fixed and images were taken in
a dark room (LAS 4000; ImageQuant) for recording. ImageJ software
was used for image analysis.
Data statistics and analysis
All data were counted and analyzed by SPSS 23.0. The
results of ELISA and qRT-PCR experiments were expressed as mean ±
standard deviation (mean ± SD). The comparison between groups was
performed using t-test of paired samples. The correlation between
the number and the expression of HMGB1 was analyzed using the
Pearson test. The comparison of cells between different treatment
groups was performed using one-way ANOVA, Tamhane test was employed
as the post hoc test. Unconditional logistic regression was used to
analyze the independent risk factors of asthma symptoms in children
with bronchiolitis infected with RSV. P<0.05 indicates that the
difference was statistically significant.
Results
HMGB1 expression and immune cell
levels in peripheral blood of children with RSV bronchiolitis are
significantly increased, and correlate with the severity of the
disease
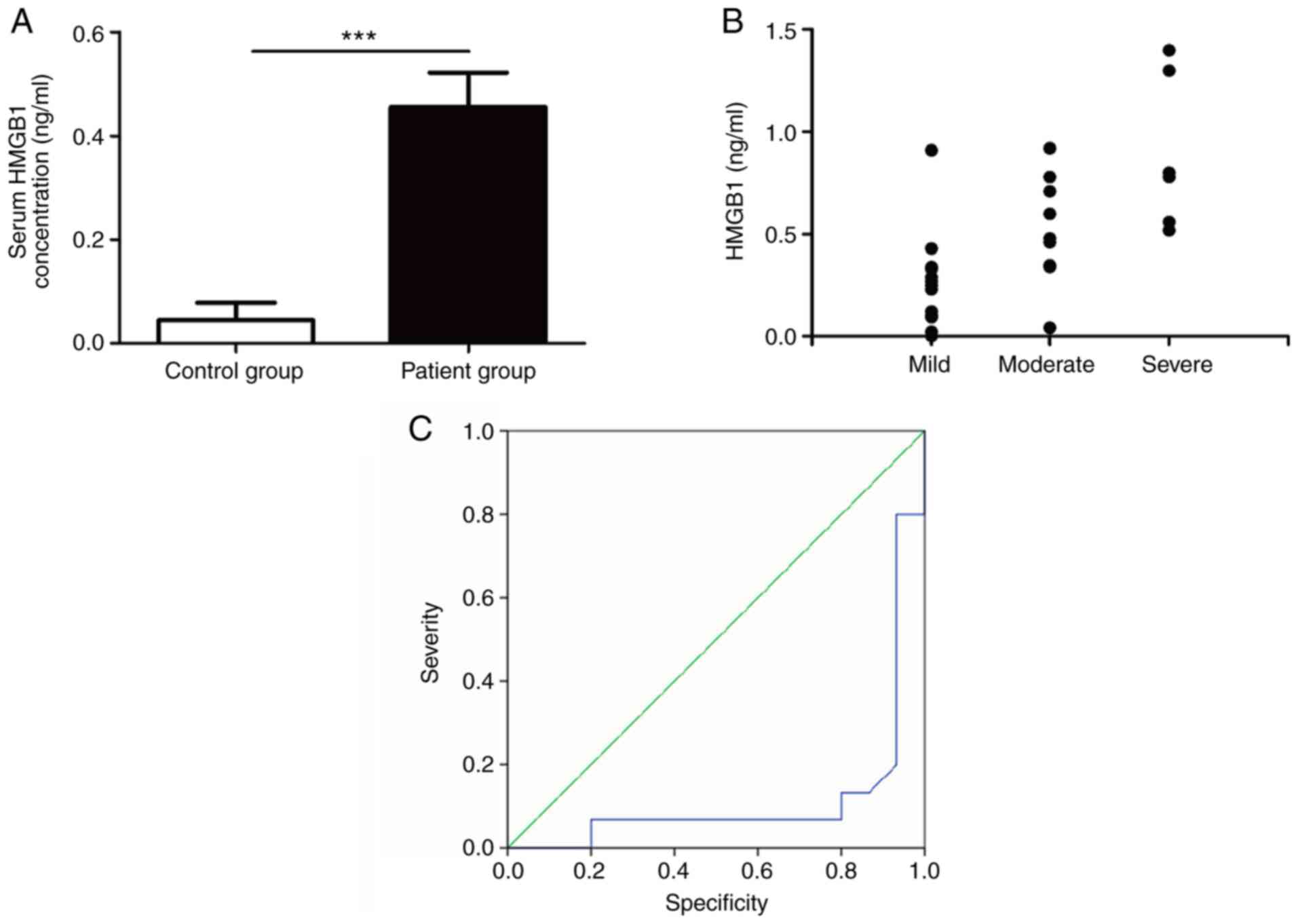
ELISA was used to detect HMGB1 expression level in
the peripheral blood plasma of the two groups of infants. The
results showed that HMGB1 expression level in children with RSV
bronchiolitis was significantly higher than that in the control
group (0.45±0.06 vs. 0.04±0.03, t=5.835, P<0.0001 (Fig. 1A). Spearman Analysis was used for
further analysis of HMGB1 expression level in peripheral blood and
the severity of the disease. The results showed that the HMGB1
expression level was significantly correlated with the severity of
the disease (P<0.01, Fig. 1B).
The ROC curves showed that HMGB1 can be used as a marker to judge
the condition of children with bronchiolitis (95% CI: 0-0.23,
P<0.001) (Fig. 1C).
The significantly increased expression
of HMGB1 in peripheral blood of children with RSV bronchiolitis is
significantly correlated with immune indicators
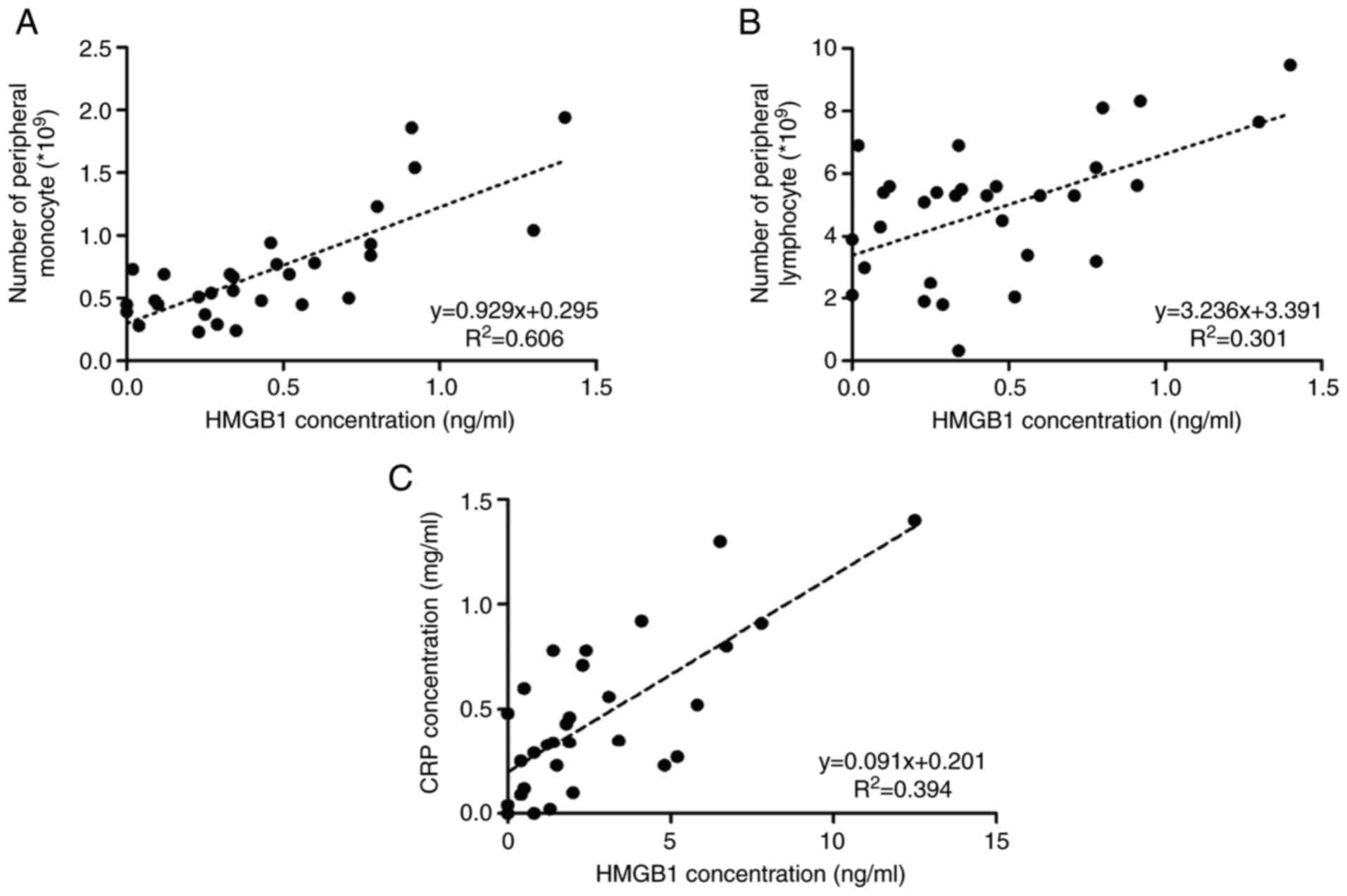
The correlation of HMGB1 expression in peripheral
blood and clinical indicators was analyzed. It was found that the
expression of HMGB1 was correlated with monocytes and lymphocytes
in the peripheral blood, as well as the CRP expression level upon
admission. It suggested that the high expression of HMGB1 in
children with RSV bronchiolitis may be correlated with the
activation of inflammatory immunity in vivo (Fig. 2A-C).
Human bronchial epithelial cell lines
can up-regulate HMGB1 after RSV infection
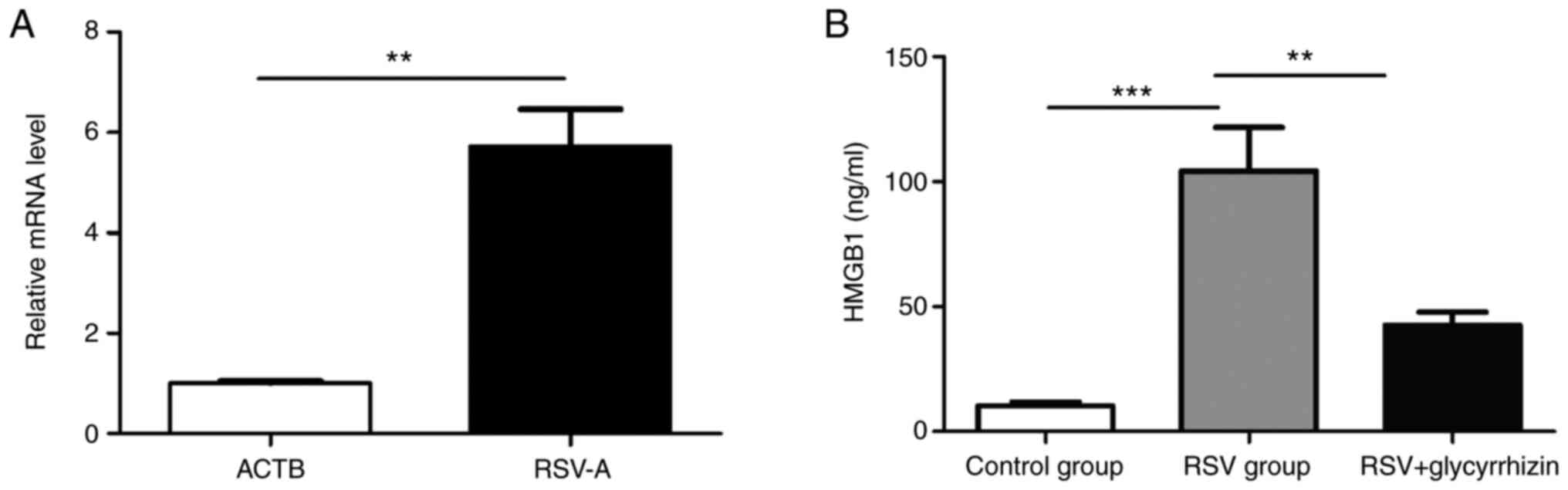
After the 16HBE cells infected with human
recombinant RSV-A2 virus (Fig. 3A)
were verified by qRT-PCR (1.00±0.05 vs. 5.73±0.73, t=6.455,
P<0.01, respectively), it was found that the expression of HMGB1
in the culture medium of 16HBE cells increased significantly after
infection (10.22±1.65 vs. 104.31±17.31, t=5.413, P<0.0001),
which suggested that in vitro cell experiment simulates the
RSV process of bronchial epithelial cells (Fig. 3B). In addition, the results of ELISA
experiments also found that the addition of glycyrrhizic acid to
bronchial epithelial cells after RSV virus infection can
significantly reduce the expression level of HMGB1 (Fig. 3B).
RSV-infected human bronchial
epithelial cell line activates TLR4 expression in monocytes by
up-regulating HMGB1
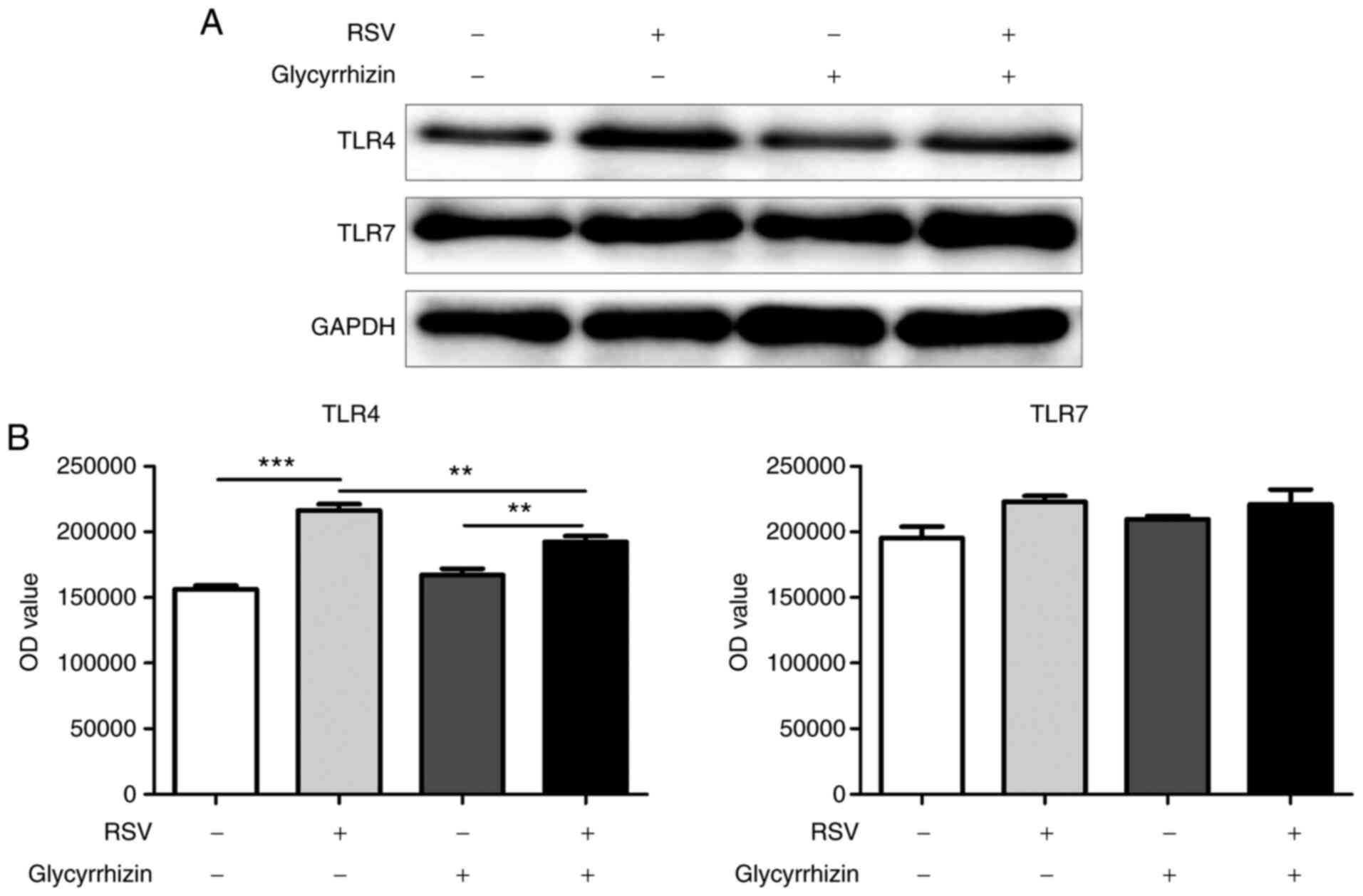
16HBE cells infected with RSV virus were co-cultured
with monocytes, which showed that the expression of TLR4 in
monocytes significantly increased, while TLR7 had no significant
change (Fig. 4A). The expression of
TLR4 in the mononuclear cells can be significantly inhibited after
the addition of glycyrrhizic acid to the culture medium to inhibit
the activity of HMGB1 in the infected RSV cell lines (Fig. 4B). This result suggested that RSV
may activate monocyte-related immune functions through HMGB1 after
16HBE infection.
Relationship between HMGB1 expression
and prognosis in children with RSV infection
The patients with RSV bronchiolitis were followed up
for chest distress, dry cough, wheezing, exhale dyspnea and other
symptoms for 18 months. The bronchodilation test, the reversible
airflow restriction (FEV1/FVC >0.8) test and asthma test were
carried out during follow-up. The results showed that the rate of
reversible airflow was higher in the patients with RSV (1/30, 6/30,
P<0.05) (Table I). The related
factors of asthma in children with bronchiolitis were analyzed by
unconditional logistic regression. The variables of asthma-related
factors included age (<12 months), gender (men), duration of
disease (≤5 days), the number of monocytes in blood, and the
expression of HMGB1 in peripheral blood plasma on admission for the
first time. The results showed that the independent risk factors
for asthma symptoms in children were age (OR=0.84, 95% CI:
3.64-12.58, P<0.05), number of monocytes (OR=2.13, 95% CI:
4.88-12.55, P<0.01) and HMGB1 levels upon admission (OR=1.47,
95% CI: 2.56-5.72, P<0.01).
 | Table IPrognosis of children with RSV in
infection group and control group. |
Table I
Prognosis of children with RSV in
infection group and control group.
| Follow-up
prognosis | Control group
(n=30) | Children with RSV
bronchiolitis (n=30) | P-value |
|---|
| Chest tightness, dry
cough, wheezing, dyspnea and other symptoms | 3 | 2 | 0.640 |
| Bronchodilator test
is positive | 2 | 7 | 0.071 |
| FEV1/FVC
>0.8 | 1 | 6 | 0.044b |
| Asthmaa | 2 | 6 | 0.128 |
Discussion
Bronchiolitis in infants and young children is also
known as asthmatic pneumonia, which usually occurs in winter.
Generally, the disease is relatively severe, which may cause local
epidemic. Acute RSV infection may be complicated by secondary
bacterial infections and respiratory failure. Mechanical
ventilation is needed to prolong hospitalization time (10). In developed countries, most RSV
infections are self-limiting. However, RSV is globally an important
cause of death in children under 5 years of age. As a result, the
extensive disease burden caused by the disease and the mortality
rate indicate the urgent need for markers and related therapeutic
targets in the active phase of RSV infection. This study collected
peripheral blood serum from children with RSV capillary bronchitis
and healthy children. The results showed that the expression of
HMGB1 in children with bronchiolitis infected with RSV was
significantly increased. It is consistent with previous results in
the RSV capillary bronchitis animal model (7), which suggests that HMGB1 may be used
as a biomarker of disease. In addition, the children with RSV
bronchiolitis were followed up for 1-2 years. Combined with the
prognosis of the children and HMGB1 expression level in peripheral
blood at the first admission, the results of Logistic regression
analysis showed that the possibility of asthma and allergic related
diseases increased with the increase of HMGB1 expression level in
the children. Due to the limitation of time and follow-up, this
research could not reach the 3-year follow-up (2), and the sample size was relatively
small. The expression level and prognosis of HMGB1 in the disease
still need to be verified by larger data and high-quality clinical
controlled trials.
In this study, human bronchial epithelial cell line
16HBE was used to infect human recombinant RSV-A2 strain virus. The
results showed that HMGB1 expression level in epithelial cell
culture supernatant could also significantly increase, suggesting
that the model could mimic human bronchial epithelial cells
infected with RSV. Previous research has shown that after cell
infection or apoptosis, HMGB1 can be passively released by necrotic
cells or actively produced by macrophages, dendritic cells and
natural killer cells (11). In
combination with the receptors of TLR2, 4 and RAGE (12), HMGB1 can promote the synthesis of
inflammatory cytokines, induce the chemotaxis of inflammatory
cells, and support the proliferation of interstitial fibroblasts,
the chemotaxis and the synthesis of matrix metalloproteinases
(13), leading to the occurrence of
acute and chronic diseases (14).
Research has shown that both RSV infection and advanced glycation
end product receptor (RAGE) mutations are a risk factor for asthma.
It has recently been reported that HMGB1 as a cytokine can play an
important role in allergic airway sensitivity and airway
inflammation in patients with asthma (6). In our study, co-culture of bronchial
epithelial cell lines and monocytes after RSV infection was found
to significantly increase the expression of TLR4 in monocytes, and
there was no significant change in TLR7 in monocytes after RSV
infection. However, the results of Qi et al (15) and Kim et al (16) were different from our research.
There may be two reasons. One may be that previous reports have
been performed using mouse in vivo models and cell lines.
There may be some differences with human cells. The other reason
may be that cytology experiments directly use RSV to infect immune
cells without considering the potential impact of epithelial cells
on monocytes.
Although HMGB1 has been reported to play a key role
in multiple stages of several DNA (herpes simplex virus type 2) and
RNA (western nile virus, dengue) virus infections, literature
reports on its role in RSV infection remain limited (7). Hou et al (17) reported an increase in HMGB1 levels
in lung tissue of RSV-infected mice. The results of this animal
model are consistent with the results of previous research
(17). Some other research
(18) showed that purified HMGB1
was co-cultured with human airway epithelial cell line (AEC) and
peripheral mononuclear cells (PBMC) and various immune cells
isolated and induced from PBMC, including monocytes. The results
showed that although HMGB1 treatment can significantly increase the
phosphorylation of NF-κB and P38 MAPK in AECs, it failed to promote
the release of cytokines or chemokines. The addition of HMGB1 to
immune cells can simultaneously promote the significant induction
of cytokine/chemokine release and activate the NF-κB and P38 MAPK
pathways. These results suggest that HGMB1 can promote the
inflammatory response involved in proinflammatory mediators
secreted by immune cells and pathogenesis of RSV by promoting the
mechanism. Thus, the results of this experiment further explored
the effects of human bronchial epithelial cells on monocytes. It
can be found that 16HBE cells infected with RSV can significantly
activate the TLR4 signaling pathway in monocytes, thus inducing the
generation of innate immune system activation in vivo,
leading to the activation of inflammatory cytokines and chemokines
in downstream pathways. Therefore, blocking the pro-inflammatory
function of HMGB1 may be an effective way to develop new
treatments. This research was further deepened on the basis of
previous studies and focused on the immunomodulatory effects of
HMGB1 on monocytes from the 16 HBE cell-derived sources.
Monocyte-macrophages are the key to coordinating the
immune system response to infection and injury. We observed that
HMGB1 can mediate a series of downstream cytokine/chemokine
cascades by activating TLR4 in monocytes. It has important
significance in RSV infection. Previous research has shown that
HMGB1 cannot stimulate human bronchial epithelial cells, and its
effect only shows a certain effect in the presence of RSV (7). Therefore, four groups of bronchial
epithelial cells were set up (the control group, the infection
group, the non-infection and inhibition group, and the infection
and inhibition group) and the cells were co-cultured with
monocytes. We found that there was activation induction of the
HMGB1-dependent activation of monocytes in the presence of RSV.
HMGB1 not only activates monocytes. Research has shown that HMGB1
may trigger more inflammatory reactions by activating other immune
cells in the airway to release more pro-inflammatory cytokines,
such as mediating neutrophils, proliferation and activation of acid
granulocytes (19). Combined with
the results of this study, it is suggested that HMGB1 itself or in
combination with other proinflammatory cytokines may also cause
chronic inflammation of airway epithelial cells. These observations
support the idea that increased secretion of HMGB1 can trigger and
maintain the pathological state of the disease. Next, the
appropriate animal model should be used to further confirm the
pathological role of HMGB1 in bronchiolitis infected with RSV and
the physiological process of HMGB1 in the process of RSV
infection.
The transfer of HMGB1 from nucleus to cytoplasm or
extracellular cells not only indicates that it plays an important
role in apoptosis, necrosis and other cell processes, but also
indicates that it can affect the host immunity to respiratory tract
virus infection by regulating autophagy (20,21).
HMGB1 promotes nutrient deficiency and oxidative stress-induced
autophagia in mouse embryonic fibroblasts and cancer cells by
directly interacting with the key regulatory factor Beclin1 of
autophage and apoptosis. Research on the effect of RSV infection on
autophagy has shown that RSV can enter the cytoplasm of host cells
directly through membrane fusion (22). While DC activation is dependent on
the production and immune response of autophagy-mediated
TLR-dependent cytokines, autophagy can also promote the
identification of intracellular pathogens, the maturation of
dendritic cells, and the production of pro-inflammatory cytokines
(23-25).
HMGB1 mediated autophagy can also control the differentiation of
acute promyelocytic leukemia cells by regulating the protein
degradation of promyelocytic leukemia and the retinal acid receptor
α (26). These studies can lay a
solid foundation for further exploration of the role of HMGB1 in
airway inflammation caused by RSV infection, and provide new
insights into its occurrence mechanism, which is helpful to develop
new treatment strategies to treat and prevent bronchiolitis in
children caused by a virus.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MN and ZJ designed the study and drafted the
manuscript. XX, JZ and JY were responsible for the collection and
analysis of the experimental data. MD, GQ and BQ revised the
manuscript critically for important intellectual content and were
also involved in the conception of the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Xuzhou Children's Hospital, Xuzhou Medical University (Xuzhou,
China). Signed informed consents were obtained from the guardians
of the child patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arruvito L, Raiden S and Geffner J: Host
response to respiratory syncytial virus infection. Curr Opin Infect
Dis. 28:259–266. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bacharier LB, Cohen R, Schweiger T,
Yin-Declue H, Christie C, Zheng J, Schechtman KB, Strunk RC and
Castro M: Determinants of asthma after severe respiratory syncytial
virus bronchiolitis. J Allergy Clin Immunol. 130:91–100.e3.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Melvin W and Giovanni P: Respiratory
syncytial virus prevention and therapy: Past, present, and future.
Pediatr Pulmonol. 46:324–347. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sigurs N, Bjarnason R, Sigurbergsson F and
Kjellman B: Respiratory syncytial virus bronchiolitis in infancy is
an important risk factor for asthma and allergy at age 7. Am J
Respir Crit Care Med. 161:1501–1507. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ullah MA, Loh Z, Gan WJ, Zhang V, Yang H,
Li JH, Yamamoto Y, Schmidt AM, Armour CL, Hughes JM, et al:
Receptor for advanced glycation end products and its ligand
high-mobility group box-1 mediate allergic airway sensitization and
airway inflammation. J Allergy Clin Immunol. 134:440–450.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou Y, Jiang YQ, Wang WX, Zhou ZX, Wang
YG, Yang L and Ji YL: HMGB1 and RAGE levels in induced sputum
correlate with asthma severity and neutrophil percentage. Hum
Immunol. 73:1171–1174. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hosakote YM, Brasier AR, Casola A,
Garofalo RP and Kurosky A: RSV infection triggers epithelial HMGB1
release as a damage-associated molecular pattern promoting a
monocytic inflammatory response. J Virol. 90:9618–9631.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rendon-Mitchell B, Ochani M, Li J, Han J,
Wang H, Yang H, Susarla S, Czura C, Mitchell RA, Chen G, et al:
IFN-gamma induces high mobility group box 1 protein release partly
through a TNF-dependent mechanism. J Immunol. 170:3890–3897.
2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang H, Antoine DJ, Andersson U and Tracey
KJ: The many faces of HMGB1: molecular structure-functional
activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol.
93:865–873. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Russell CD, Unger SA, Walton M and
Schwarze J: The human immune response to respiratory syncytial
virus infection. Clin Microbiol Rev. 30:481–502. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gallucci S and Matzinger P: Danger
signals: SOS to the immune system. Curr Opin Immunol. 13:114–119.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu M, Wang H, Ding A, Golenbock DT, Latz
E, Czura CJ, Fenton MJ, Tracey KJ and Yang H: HMGB1 signals through
toll-like receptor (TLR) 4 and TLR2. Shock. 26:174–179.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ojo OO, Ryu MH, Jha A, Unruh H and Halayko
AJ: High-mobility group box 1 promotes extracellular matrix
synthesis and wound repair in human bronchial epithelial cells. Am
J Physiol Lung Cell Mol Physiol. 309:L1354–L1366. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao
L, Huang J, Yu Y, Fan XG, Yan Z, et al: HMGB1 in health and
disease. Mol Aspects Med. 40:1–116. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qi F, Wang D, Liu J, Zeng S, Xu L, Hu H
and Liu B: Respiratory macrophages and dendritic cells mediate
respiratory syncytial virus-induced IL-33 production in TLR3- or
TLR7-dependent manner. Int Immunopharmacol. 29:408–415.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim TH, Oh DS, Jung HE, Chang J and Lee
HK: Plasmacytoid dendritic cells contribute to the production of
IFN-β via TLR7-MyD88-dependent pathway and CTL priming during
respiratory syncytial virus infection. Viruses.
11(730)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hou CC, Zhao HJ, Cai SX, Li WJ, Tong WC
and Liu LY: Respiratory syncytial virus increases the expression
and release of high mobility group Box-1 protein in the lung tissue
of mice. Nan Fang Yi Ke Da Xue Xue Bao. 30:700–703. 2010.PubMed/NCBI(In Chinese).
|
|
18
|
Rayavara K, Kurosky A, Stafford SJ, Garg
NJ, Brasier AR, Garofalo RP and Hosakote YM: Proinflammatory
effects of respiratory syncytial virus-induced epithelial HMGB1 on
human innate immune cell activation. J Immunol. 201:2753–2766.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gremion C and Cerny A: Hepatitis C virus
and the immune system: A concise review. Rev Med Virol. 15:235–268.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Tang D, Kang R, Cheh CW, Livesey KM, Liang
X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, et
al: HMGB1 release and redox regulates autophagy and apoptosis in
cancer cells. Oncogene. 29:5299–5310. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tang D, Kang R, Livesey KM, Cheh CW,
Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ III
and Lotze MT: Endogenous HMGB1 regulates autophagy. J Cell Biol.
190:881–892. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Srinivasakumar N, Ogra PL and Flanagan TD:
Characteristics of fusion of respiratory syncytial virus with HEp-2
cells as measured by R18 fluorescence dequenching assay. J Virol.
65:4063–4069. 1991.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee FE, Walsh EE, Falsey AR, Lumb ME, Okam
NV, Liu N, Divekar AA, Hall CB and Mosmann TR: Human infant
respiratory syncytial virus (RSV)-specific type 1 and 2 cytokine
responses ex vivo during primary RSV infection. J Infect Dis.
195:1779–1788. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Morris S, Swanson MS, Lieberman A, Reed M,
Yue Z, Lindell DM and Lukacs NW: Autophagy-mediated dendritic cell
activation is essential for innate cytokine production and APC
function with respiratory syncytial virus responses. J Immunol.
187:3953–3961. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Reed M, Morris SH, Jang S, Mukherjee S,
Yue Z and Lukacs NW: Autophagy-inducing protein beclin-1 in
dendritic cells regulates CD4 T cell responses and disease severity
during respiratory syncytial virus infection. J Immunol.
191:2526–2537. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang L, Chai W, Wang Y, Cao L, Xie M, Yang
M, Kang R and Yu Y: Reactive oxygen species regulate the
differentiation of acute promyelocytic leukemia cells through
HMGB1-mediated autophagy. Am J Cancer Res. 5:714–725.
2015.PubMed/NCBI
|