Introduction
Acute cerebral infarction (ACI) is an acute ischemic
stroke. It refers to the sudden decrease or interruption of blood
supply to part of the brain cells, resulting in hypoxia of local
tissue and depletion of energy (1).
Eventually, cells in this area lose their activity and undergo
tissue necrosis, further resulting in the loss of nerve function
(2,3). ACI has high morbidity and lethality in
cerebrovascular disease, which has a serious impact on the
psychological and physiological aspects of patients. It reduces the
quality of life of patients and brings a serious economic burden to
families and society (4). The
pathogenesis of ACI is markedly complex. Hypertension, diabetes
mellitus and other diseases have a certain impact on the occurrence
of the disease (5). At present, the
diagnosis of ACI mainly depends on clinical manifestations
(6), signs, and imaging
examination. However, some patients may not exhibit significant
imaging changes 24 h later (7). Due
to the influence of time, imaging application is limited (8). Especially some diseases, including
migraine and epileptic seizures, have similar symptoms as a stroke
(9), which has a certain influence
on the diagnosis of diseases.
It has been reported (10) that S100 protein β (S100β)plays an
important role in cell division, differentiation and apoptosis. It
is mainly expressed in glial cells in the central nervous system
and participates in the production process of cerebrovascular
disease (11). Specifically, the
release of S-100β by glial cell necrosis will lead to the increase
of peripheral blood concentration (12). According to a previous study
(13), there is a close
relationship between the change of S100β level and the size of
cerebral infarction, and the degree of nervous functional defects.
Cystatin C (CysC) has been used as a serum factor index for
assessing glomerular filtration rate. In recent years, it has
received increased attention due to its effect in human vascular
diseases and has been revealed to be directly involved in the
atherosclerosis process (14). The
most common factor of occurrence of ACI is atherosclerosis
(10). Nuclear factor κB (NF-κB)
(15) is a nuclear transcription
activating factor that has been widely studied in recent years. It
is closely related to immune response, inflammatory response as
well as other processes. Research has confirmed that (16) NF-κB can induce the activation of
inflammatory factors in acute cerebral hemorrhage, which can lead
to further damage of brain tissue.
Therefore, the present study aimed to research the
expression and significance of S-100β, CysC and NF-κB in ACI. It is
of great significance to explore the diagnostic biomarkers related
to cerebral infarction in the treatment of disease and
prognosis.
Materials and methods
Baseline data
ACI patients (n=120) that were hospitalized at the
Department of Neurology of Xuzhou Central Hospital (Xuzhou, China)
from August 2016 to August 2018 were selected as the experimental
group. There were 68 males and 52 females, 52-76 years old, with a
mean age of (62.25±2.18). Healthy (n=90)subjects who underwent a
physical examination at Xuzhou Central Hospital during the same
period were selected as the control group. There were 53 males and
37 females, 51-76 years old, with a mean age of (62.36±2.07). There
were no significant differences between the two groups with regard
to age and sex.
Inclusion and exclusion criteria
The inclusion criteria were the following: i)
patients who met the diagnostic criteria for acute ischemic stroke
in China (17) and diagnosed by CT
and MRI; ii) patients who had the disease for the first time; iii)
patients admitted to the hospital within 24 h from onset; and iv)
patients who received no thrombolytic therapy.
The exclusion criteria were the following: i)
patients complicated with cerebral hemorrhage, malignant tumor,
head trauma, severe infection, and autoimmune disease; ii) patients
who received thrombolytic and surgical therapy; iii) patients with
hepatic renal dysfunction; iv) patients with communication and
cognitive dysfunction; and v) patients who did not cooperate with
the experiment.
All patients agreed to participate in the experiment
and signed the informed consent form. The present study was
approved by the Ethics Committee of Xuzhou Central Hospital
(approval no. XZXY-LJ-20190305-007).
Methods. All patients were treated according
to the ‘single disease type quality control indexes for cerebral
infarction’ (18). They were
administered antiplatelet aggregation and anti-arteriosclerosis
therapeutic agents, such as aspirin, clopidogrel and statins. The
blood pressure and blood glucose levels were controlled, brain
edema was relieved, and a protective agent of brain cells and
symptomatic supportive treatment was applied. The degree of nervous
functional defects was assessed by the National Institutes of
Health Stroke Scale (NIHSS) (19).
According to the NIHSS, the 120 ACI patients were divided into the
severe type infarction group (NIHSS score was >15 points, 27
cases), the medium type infarction group (NIHSS score was 4-15
points, 56 cases) and the mild type infarction group (NIHSS score
was <4 points, 37 cases). According to the focal volume of
cerebral infarction (20), patients
were divided into three groups: the small size infarction group
(<5 cm3, 39 cases), the medium size infarction group
(5-10 cm3, 58 cases), and the large size infarction
group (>10 cm3, 23 cases). The prognosis information
was obtained by phone 6 months after patients were discharged, and
the patients were divided into a worsening group (36 cases) and a
non-worsening group (84 cases) according to whether their
conditions were aggravated.
Experimental materials
Peripheral venous blood (2 ml) was collected from
patients in both groups in the early morning on an empty stomach
and centrifuged at 2,000 x g for 5 min. Serum was collected and
stored at -80˚C (AU5800 automatic biochemistry analyzer; Beckman
Coulter). Enzyme-linked immunosorbent assay (ELISA) was used to
detect the expression levels of S-100β, CysC and NF-κB in the
samples of the experimental and control groups. The kit was
purchased from Wuhan Boster Biological Technology Co., Ltd.
Specific experimental methods were carried out in accordance with
the manufacturer's instructions.
Observation indexes
Comparison of baseline data between the two groups
was as follows: i) Comparison of the expression levels of S-100β,
CysC and NF-κB between the two groups. ii) Comparison of the
different degree of nervous functional defects of groups and
S-100β, CysC and NF-κB levels. iii) Comparison of serum S-100β,
CysC and NF-κB levels in patients with different infarct size. iv)
Comparison of S-100β, CysC and NF-κB levels in ACI patients with
different prognosis. v) ROC curve analysis of serum S-100β, CysC
and NF-κB levels for diagnosis of ACI.
Statistical methods
In the present study, SPSS 20.0 software (Beijing
Bizinsight Information Technology Co., Ltd.) was used for
statistical analysis. The enumeration data were assessed by
chi-square test. The measurement data were expressed as the mean
number ± standard deviation. A Student's t-test was used to compare
the two groups. One-way analysis of variance was used for
comparison of multiple groups, such as groups with different levels
of neurological deficits and groups with different infarct size.
Bonferroni test was the post hoc test used with ANOVA. The S-100β,
CysC and NF-κB levels, and the value of prognosis in the diagnosis
of ACI were assessed by the receiver operating characteristic (ROC)
curve. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of baseline data between
the two groups
There was no significant difference in baseline data
such as age and BMI between the two groups (all P>0.05; Table I).
 | Table IComparison of baseline data between
the two groups. |
Table I
Comparison of baseline data between
the two groups.
| Factors | Experimental group
(n=120) | Control group
(n=90) | t/χ2 | P-value |
|---|
| Age, years | 62.25±2.18 | 62.36±2.07 | 0.370 | 0.712 |
|
BMI/kgm2 | 26.58±2.36 | 26.14±2.72 | 1.359 | 0.176 |
| Sex |
|
Male | 68 (56.67) | 53 (58.89) | | |
|
Female | 52 (43.33) | 37 (41.11) | 0.104 | 0.747 |
| Hypertension |
|
Yes | 88 (73.33) | 68 (75.56) | | |
|
No | 32 (26.67) | 22 (24.44) | 0.133 | 0.715 |
| Diabetes
mellitus |
|
Yes | 65 (54.17) | 48 (53.33) | | |
|
No | 55 (45.83) | 42 (46.67) | 0.014 | 0.905 |
| Hyperlipidemia |
|
Yes | 77 (64.17) | 58 (64.44) | | |
|
No | 43 (35.83) | 32 (35.56) | 0.002 | 0.967 |
| Smoking |
|
Yes | 62 (51.67) | 50 (55.56) | | |
|
No | 58 (48.33) | 40 (44.44) | 0.313 | 0.576 |
| Drinking |
|
Yes | 69 (57.50) | 53 (58.89) | | |
|
No | 51 (42.50) | 37 (41.11) | 0.041 | 0.840 |
Comparison of serum S-100β, CysC and
NF-κB levels between the two groups
The serum S-100β, CysC and NF-κB levels of patients
in the experimental group were significantly higher than those in
the control group. The difference was statistically significant
(all P<0.05; Table II).
 | Table IIComparison of serum S-100β, CysC and
NF-κB levels between the two groups. |
Table II
Comparison of serum S-100β, CysC and
NF-κB levels between the two groups.
| Factors | Experimental group
(n=120) | Control group
(n=90) | t | P-value |
|---|
| S-100β
(ρ/µg·l-1) | 1.185±0.15 | 0.923±0.24 | 9.70 | <0.05 |
| CysC (mg/l) | 1.36±0.24 | 1.05±0.14 | 10.93 | <0.05 |
| NF-κB (pg/ml) | 326.4±25.4 | 305.2±16.6 | 6.899 | <0.05 |
Comparison of the groups with
different degree of nervous functional defects and S-100β, CysC and
NF-κB levels
The serum S-100β, CysC and NF-κB levels of patients
with different degree of nervous functional defects were compared,
and the differences were statistically significant (all P<0.05).
The levels of serum S-100β, CysC and NF-κB in the severe and medium
type infarction groups were significantly higher than those in the
mild type infarction group (all P<0.05). The serum S-100β, CysC
and NF-κB levels of patients in the severe type infarction group
were higher than those in the medium type infarction group
(P<0.05; Table III).
 | Table IIIComparison of different degree of
nervous functional defects group and S-100β, CysC and NF-κB
levels. |
Table III
Comparison of different degree of
nervous functional defects group and S-100β, CysC and NF-κB
levels.
| Grouping | Severe type
infarction group (n=27) | Medium type
infarction group (n=56) | Mild type infarction
group (n=37) | F | P-value |
|---|
| S-100β
(ρ/µg·l-1) |
1.432±0.341a |
0.747±0.243b | 0.421±0.123 | 139.53 | <0.05 |
| CysC (mg/l) |
1.73±0.21a |
1.25±0.18b | 1.17±0.16 | 85.455 | <0.05 |
| NF-κB (pg/ml) |
435.3±32.8a |
408.6±21.6b | 376.3±18.6 | 49.550 | <0.05 |
Comparison of serum S-100β, CysC and
NF-κB levels in patients with different infarct size
The serum S-100β, CysC and NF-κB levels of patients
with different infarct size were compared, and the differences were
statistically significant (all P<0.05). The serum S-100β, CysC
and NF-κB levels in patients with large and medium size infarction
were significantly higher than those in the small size infarction
group (all P<0.05). The serum S-100β, CysC and NF-κB levels of
patients in the large size infarction group were higher than those
in the medium size infarction group (P<0.05; Table IV).
 | Table IVComparison of serum S-100β, CysC and
NF-κB levels in patients with different infarct size. |
Table IV
Comparison of serum S-100β, CysC and
NF-κB levels in patients with different infarct size.
| Grouping | Large size
infarction group (n=23) | Medium size
infarction group (n=58) | Small size
infarction group (n=39) | F | P-value |
|---|
| S-100β
(ρ/µg·l-1) |
1.278±0.256a |
0.821±0.223b | 0.435±0.103 | 130.5 | <0.05 |
| CysC (mg/l) |
1.56±0.21a |
1.35±0.17b | 1.07±0.15 | 63.39 | <0.05 |
| NF-κB (pg/ml) |
424.3±30.3a |
403.2±21.4b | 368.3±16.4 | 53.15 | <0.05 |
Comparison of S-100β, CysC and NF-κB
levels in ACI patients with different prognosis
Serum S-100β, CysC and NF-κB levels in patients of
the worsening group were significantly higher than those in the
non-worsening group. The difference was statistically significant
(all P<0.05; Table V).
 | Table VComparison of S-100β, CysC and NF-κB
levels in ACI patients with different prognosis. |
Table V
Comparison of S-100β, CysC and NF-κB
levels in ACI patients with different prognosis.
| Grouping | Worsening group
(n=36) | Non-worsening group
(n=84) | t | P-value |
|---|
| S-100β
(ρ/µg·l-1) | 1.473±0.347 | 0.445±0.142 | 23.10 | <0.001 |
| CysC (mg/l) | 1.36±0.24 | 1.21±0.37 | 2.236 | 0.027 |
| NF-κB (pg/ml) | 432.7±23.5 | 385.4±26.1 | 9.364 | <0.001 |
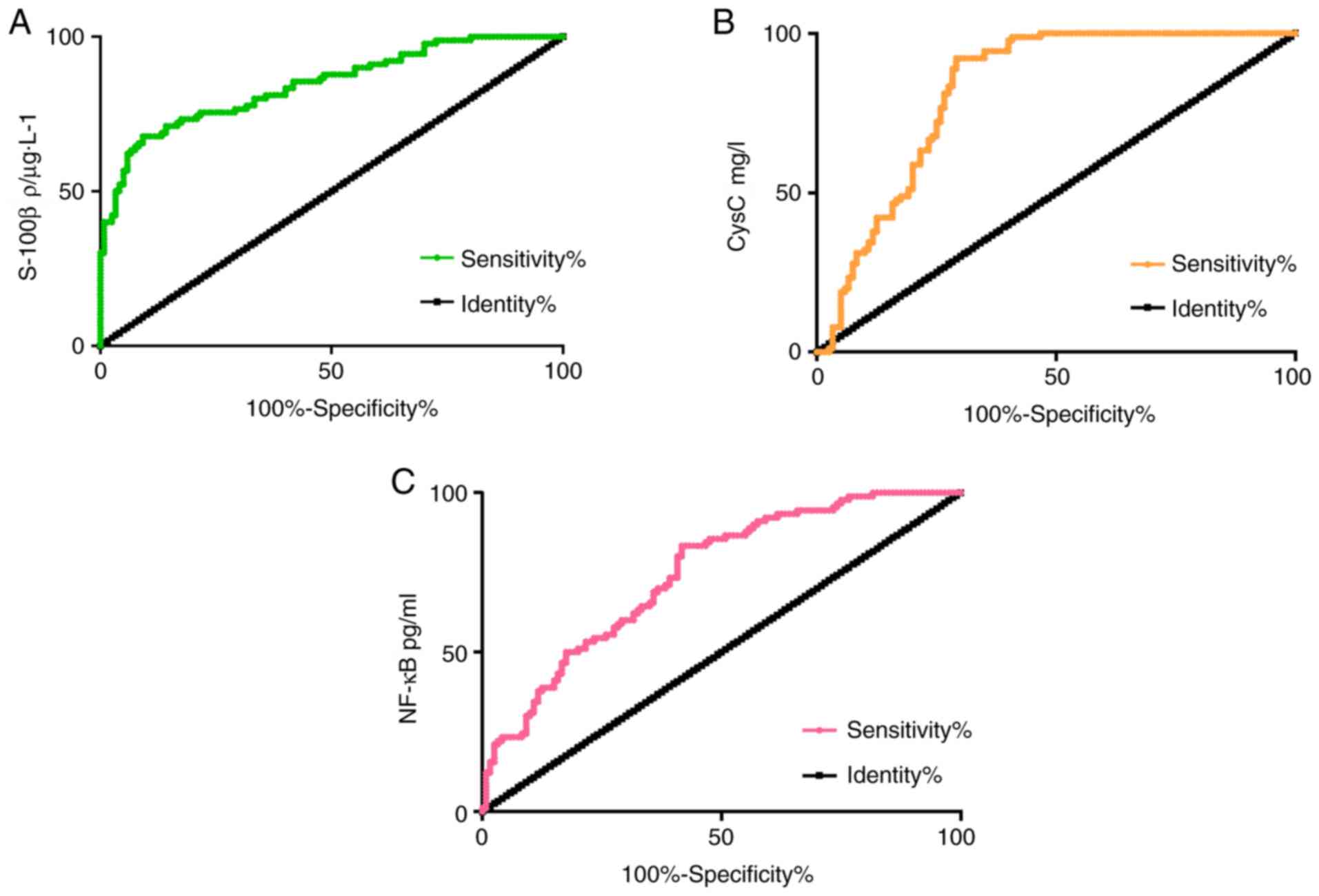
ROC curve analysis of serum S-100β,
CysC and NF-κB levels for diagnosis value of ACI
The diagnostic sensitivity, specificity and AUC of
S-100β to ACI were 67.78, 90.08 and 0.845%, respectively. The 95%
CI was 0.791-0.899, and the critical value was 0.985
ρ/µg·l-1. The diagnostic sensitivity, specificity and
AUC of CysC to ACI were 92.22, 70.00 and 0.823%, respectively. The
95% CI was 0.765-0.879, and the critical value was 1.193 mg/l. The
diagnostic sensitivity, specificity and AUC of NF-κB to ACI was
83.33, 57.50 and 0.745%, respectively. The 95% CI was 0.679-0.810,
and the critical value was 317.7 pg/ml (Fig. 1)
Discussion
ACI, a common cerebrovascular disease, has a great
impact on the health and quality of life of patients (21). There are nuermous clinical methods
and standards for the diagnosis of ACI, but there are some
shortcomings. According to the level value of some related markers,
the doctor can assess the condition of the patient and carry out
treatment (22). Therefore, the
search for serum markers for diagnosis of ACI has a certain
influence on the treatment and prognosis of patients (23).
The expression of S-100β, CysC and NF-κB factors in
ACI patients was investigated in the present study. First, the
serum S-100β, CysC and NF-κB levels in the experimental group were
higher than those in the control group. It was revealed that the
serum S-100β, CysC and NF-κB levels in ACI patients were higher
than those in healthy subjects. The level of detection factors is
conducive to the diagnosis of diseases. Studies (24,25)
have revealed that after the occurrence of atherosclerosis in
cerebral infarction, inflammation and nociceptive stimuli lead to
an increased secretion of protease and serum S-100β levels. CysC
plays an important role in the progression and occurrence of
cardiovascular disease and systemic inflammatory response and
atherosclerosis. In the case of ischemia, CysC is released in large
quantities (26). It has been
reported that CysC can protect the brain against ischemic brain
injury, and exogenous CysC exerts neuroprotective effects by
reducing infarct volume (27). The
high expression of NF-κB can induce the aggravation of inflammatory
response and disease (28). This is
consistent with the present research results.
Then serum S-100β, CysC and NF-κB levels of patients
with different degree of nervous functional defects, different
infarct size, and different prognosis were compared. The results
revealed that the levels of serum S-100β, CysC and NF-κB in severe
and medium type infarction groups were significantly higher than
those in the mild type infarction group. The serum S-100β, CysC and
NF-κB levels of patients in the severe type infarction group were
higher than those in the medium type infarction group in different
degree of nervous functional defects. The serum S-100β, CysC and
NF-κB levels in patients with large and medium size infarction were
significantly higher than those in the small size infarction group.
The serum S-100β, CysC and NF-κB levels in the large size
infarction group were higher than those in the medium size
infarction group in different infarct size. Serum S-100β, CysC and
NF-κB levels in the worsening group were significantly higher than
those in the non-worsening group in different prognosis patients.
According to the results, it is speculated that the increased
levels of S-100β, CysC and NF-κB can be used as ideal indices for
the diagnosis of cerebral infarction and clinical indices to
diagnose the disease. In other words, the higher the S-100β, CysC
and NF-κB levels, the larger the size of cerebral infarction, the
more serious the clinical degree of nervous functional defects.
Studies have shown that (29,30)
serum S-100β levels in patients of acute cerebral infarction are
closely related to the size of focal volume and the severity of the
clinical condition. High levels of S100-β protein can lead to
further damage of nervous functional of patients. Related research
(31) have shown that the
difference in NF-κB response in ACI patients at different periods
is closely related to the progression of the disease. Abnormal
increase of the CysC level is closely related to the occurrence and
progression of cerebral infarction and other cerebrovascular
diseases (32,33), consistent with our research results.
Then, in order to further clarify the diagnostic value of serum
S-100β, CysC and NF-κB levels for ACI, a prediction analysis was
conducted. ROC is a common method for evaluating medical diagnostic
efficacy. The research results revealed that the diagnostic AUC of
CysC to ACI was 0.823, and the diagnostic AUC of NF-κB to ACI was
0.745. S-100β, CysC and NF-κB have high diagnostic values for ACI.
Therefore, it is considered that the detection of serum S-100β,
CysC, NF-κB levels has a positive effect on the diagnosis and
treatment of ACI diseases.
In summary, higher levels of S-100β, CysC and NF-κB
expression value can be detected in ACI patients. With the
progression of the disease degree and the enlargement of the lesion
area, the level of the three markers are increased. This reveals
that the three biomarkers are important for the treatment and
prognosis. However, the present study still has some limitations.
The diagnostic significance of the three combined indices for ACI
disease was not detected, thus, this will be addressed in follow-up
experiments. The present study focuses on the effect of markers on
ACI, therefore, only ROC curves of the three factors were produced
for the diagnostic value of the disease. Other effects of markers
will be further studied in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL conceived and designed the study and drafted the
manuscript. ZL and ZX collected, analyzed, interpreted the
experimental data, and revised the manuscript critically for
important intellectual content. Both authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Xuzhou Central Hospital (Xuzhou, China). Signed written informed
consents were obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun Y, He W and Geng L: Neuroprotective
mechanism of HIF-1α overexpression in the early stage of acute
cerebral infarction in rats. Exp Ther Med. 12:391–395.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shen HJ, He HY and Dai MX: Observation on
the therapeutic effect of aspirin in combined with ozagrel sodium
in the treatment of acute cerebral infarction. J Hainan Med Univ.
23:141–144. 2017.(In Chinese).
|
|
3
|
Qing-Qing R, Wei-Dong Q and Neurology DO:
The role of Th17 in stroke in patients with acute atherosclerotic
cerebral infarction. Chin J Stroke, 2017.
|
|
4
|
Podolecki T, Pudlo R, Mazurek M, Koziel M,
Jedrzejczyk-Patej E, Boidol J, Przybylska K, Sokal A, Kowalski O,
Kowalczyk J, et al: The incidence, clinical significance, and
treatment effects of depression in cardiac resynchronization
therapy recipients. Cardiology. 138:115–121. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang Y and Zhou ZF: Correlation analysis
of carotid intima media thickness and function in patients with
H-type hypertension and acute cerebral infarction. J Hainan Med
Univ: 23, 2017.
|
|
6
|
He X, Li DR, Cui C and Wen LJ: Clinical
significance of serum MCP-1 and VE-cadherin levels in patients with
acute cerebral infarction. Eur Rev Med Pharmacol Sci. 21:804–808.
2017.PubMed/NCBI
|
|
7
|
Wang A, Wang J, Zhang F, Han J, Sun D and
Li M: Correlation between earlyimaging and curative effect of Ⅳ
rt-PA thrombolytic therapy inacute ischemic stroke patients.
Chinese Journal of Geriatric Heart Brain and Vessel Diseases.
1054–1056. 2013.
|
|
8
|
Sun PZ, Wang Y, Mandeville E, Chan ST, Lo
EH and Ji X: Validation of fast diffusion kurtosis MRI for imaging
acute ischemia in a rodent model of stroke. NMR Biomed.
27:1413–1418. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Takahashi H, Kimura T, Yuki N and Yoshioka
A: A case of stroke-like migraine attacks after radiation therapy
(SMART) syndrome followed by cerebral infarction. Intern Med.
57:1921–1924. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hao MM, Capoccia E, Cirillo C, Boesmans W
and Vanden Berghe P: Arundic acid prevents developmental
upregulation of S100B expression and inhibits enteric glial
development. Front Cell Neurosci. 11(42)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sidoryk-Wegrzynowicz M, Gerber YN, Ries M,
Sastre M, Tolkovsky AM and Spillantini MG: Astrocytes in mouse
models oftauopathies acquire early deficits and lose
neurosupportivefunctions. Acta Neuropathol Commun.
5(89)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Esposito G, Cirillo C, Sarnelli G, De
Filippis D, D'Armiento FP, Rocco A, Nardone G, Petruzzelli R,
Grosso M, Izzo P, et al: Enteric glial-derived S100B
proteinstimulates nitric oxide production in celiac disease.
Gastroenterology. 133:918–925. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhao L, Han D and Feng J: Department of
Neurology, the Affiliated Shengjing Hospital of China Medical
University. Correlation of serum neuron specific enolase levels
with severity and prognosis of acute cerebral infarction. Chin J
Practical Internal Med. 34:484–487. 2014.(In Chinese).
|
|
14
|
Zi M and Xu Y: Involvement of cystatin C
in immunity and apoptosis. Immunol Lett. 196:80–90. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ye Q, Tian GP, Cheng HP, Zhang X, Ou X, Yu
XH, Tan RQ, Yang FY, Gong D, Huang C, et al: MicroRNA-134 promotes
the development of atherosclerosis via the ANGPTL4/LPL pathway in
apolipoprotein E knockout mice. J Atheroscler Thromb. 25:244–253.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Meng QT, Chen R, Chen C, Su K, Li W, Tang
LH, Liu HM, Xue R, Sun Q, Leng Y, et al: Transcription factors Nrf2
and NF-κB contribute to inflammation and apoptosis induced by
intestinal ischemia-reperfusion in mice. Int J Mol Med.
40:1731–1740. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen X, Wu S, Chen C, Xie B, Fang Z, Hu W,
Chen J, Fu H and He H: Omega-3 polyunsaturated fatty acid
supplementation attenuates microglial-induced inflammation by
inhibiting the HMGB1/TLR4/NF-κB pathway following experimental
traumatic brain injury. J Neuroinflammation. 14(143)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kawano H, Inatomi Y, Hirano T and Yonehara
T: Vertebral artery stump syndrome in acute ischemic stroke. J
Neurol Sci. 324:74–79. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chu J, Shen X, Fan J and Chen C: Heat rate
variability and dyssomnia and their correlations to neurological
defects in cerebral infarction patients complicated by insomnia a
concurrent on-randomized case-control study. Neural Regen Res.
3:66–70. 2008.
|
|
20
|
Finocchi C, Balestrino M, Malfatto L,
Mancardi G, Serrati C and Gandolfo C: National Institutes of Health
Stroke Scale in patients with primary intracerebral hemorrhage.
Neurol Sci. 39:1751–1755. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang XF, Li G, Fu QF, Yu XM, Zhang Z, Wang
M, Xiao Y and Shen W: Effect of butylphthalide injection on volume
of acute massive cerebral infarction and the matrix
metalloproteinase-9. Chin J Stroke. 54–57. 2018.
|
|
22
|
Onatsu J, Vanninen R, Jäkälä P, Mustonen
P, Pulkki K, Korhonen M, Hedman M, Zetterberg H, Blennow K, Höglund
K, et al: Serum neurofilament light chain concentration correlates
with infarct volume but not prognosis in acute ischemic stroke. J
Stroke Cerebrovasc Dis. 28:2242–2249. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abe A, Sakamoto Y, Nishiyama Y, Suda S,
Suzuki K, Aoki J and Kimura K: Decline in hemoglobin during
hospitalization may be associated with poor outcome in acute stroke
patients. J Stroke Cerebrovasc Dis. 27:1646–1652. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang J, Zhang Y and Xu F: Function and
mechanism of microRNA-210 in acute cerebral infarction. Exp Ther
Med. 15:1263–1268. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xi YB: Correlation of serum MCP-1 and
VE-cadherin levels with neural function and carotid atherosclerosis
in patients with acute cerebral infarction. J Hainan Med Univ.
23:129–133. 2017.PubMed/NCBI
|
|
26
|
Wang XM, Zhang GY, Xu K, et al: Changes of
serum neuron specific enolase, protein S-100 and myelin basic
protein levels in patients with cerebral infarction. Zhongguo Wei
Zhong Bing Ji Jiu Yi Xue. 17:572–573. 2005.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
27
|
Xu Z, Leng C, Yang B, Wang H, Sun J, Liu
Z, Yang L, Ge W and Zhu J: Serum cystatin C is associated with
large cerebral artery stenosis in acute ischemic stroke.
Oncotarget. 8:67181–67188. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang B, Zhu J, Miao Z, Zhou B, Ge W, Zhao
H and Xu X: Cystatin C is an independent risk factor and
therapeutic target for acute ischemic stroke. Neurotox Res. 28:1–7.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chan LP, Liu C, Chiang FY, Wang LF, Lee
KW, Chen WT, Kuo PL and Liang CH: IL-8 promotes inflammatory
mediators and stimulates activation of p38 MAPK/ERK-NF-κB pathway
and reduction of JNK in HNSCC. Oncotarget. 8:56375–56388.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shi LH, Zhou Y, Guo MF, Liu JS, Li CX,
Wang GF, Liu W and Tian L: Serum levels of S-100β correlate with
the clinical status and severity of hypoxic-ischemic encephalopathy
in neonates. Genet Mol Res. 14:14760–14771. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang J and Jiao Y: Effects of Maixuekang
capsules combined with edaravone on serum MMP-9, S-100β protein
levels and neurological functions in patients with hemorrhagic
cerebral infarction. World J Integrated Tradit Western Med.
5:40–45. 2019.
|
|
32
|
Jiang Y and Lian YJ: Effects of Danhong
injection on hemodynamics and the inflammation-related NF-κB
signaling pathway in patients with acute cerebral infarction. Genet
Mol Res. 14:16929–16937. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang S, Cai J, Lu R, Wu J, Zhang M and
Zhou X: Association between serum cystatin C level and total
magnetic resonance imaging burden of cerebral small vessel disease
in patients with acute lacunar stroke. J Stroke Cerebrovasc Dis.
26:186–191. 2017.PubMed/NCBI View Article : Google Scholar
|