Introduction
One of the best documented mechanisms of immune
evasion of cancer cells is inactivation of the immune response via
programmed cell death protein 1 (PD-1)/programmed death ligand 1
(PD-L1) (1).
PD-1 is a membrane immunoglobulin present on the
surface of T and pro-B lymphocytes, with a role in silencing the
immune response by suppressing (following PD-L1 binding) T cell
proliferation and activation. Nivolumab is an Ig4 monoclonal
antibody that blocks PD-1/PD-L1 signaling by specific binding to
PD-1; it disinhibits the antigen-dependent antitumor immune
response. The use of nivolumab was approved by the US Food and Drug
Administration in 2014 for cases of metastatic melanoma and in 2015
for cases of non-small cell lung cancer (NSCLC) and kidney cell
cancer (2-4).
The activity of stimulating T cell reactivity by
nivolumab is conditioned by the presence of antigenic stimuli for T
cell receptors and starts from a dose of 1.5 ng/ml (5). Compared to chemotherapy, the wide
therapeutic range of anti-PD-1 molecules has allowed the escalation
of nivolumab doses, although the therapeutic efficacy also
manifests at dosages of one tenth of those used in current practice
(6).
To date, no maximum tolerated dose for nivolumab has
been identified and toxicity profiles are similar over the dose
range of 0.1-10 mg/kg q2w, although a dose-effect relationship is
manifested for NSCLC up to a dose of 3 mg/kg q2w (7). However, recent studies have shown that
dose reduction (20 and 100 mg, fixed doses every 3 weeks,
respectively) does not affect progression-free survival (PFS) or
overall survival (OS) compared to 3 mg/kg q2w in patients with
NSCLC (8).
The clearance of nivolumab does not depend on the
type of tumor; it is increased in combination with ipilimumab and
tends to decrease inversely proportional to body mass index (BMI)
and albuminemia (9). In the case of
NSCLC, one of the negative predictors for the response to
immunotherapy (IT) is sarcopenia, one of the manifestations of
cachexia (10). It is important to
note that albumin deficiency may induce apparent hypocalcemia
(11).
In the same context, low creatinine levels are
associated with sarcopenia and cachexia and indicate a mediocre
response to immunotherapy.
It is interesting to note that the serum level of
nivolumab at 14, 45 and 60 days after the beginning of treatment
correlates with the plasma level of 25-hydroxycholecalciferol
(12).
Patients and methods
The present study was approved by the Oncohelp
Clinic Ethics Committee; all 78 patients included in the study
voluntarily agreed to participate and provided written consent. We
screened 692 admission charts/medical files (from January 1, 2019
to August 31, 2020) and created a MySQL database with
anthropometric (sex, age, height, BMI), imagistic (metastases
topography), hematological (red and white blood cell count),
biochemical [creatinine, calcium, alanine aminotransferase (ALT),
aspartate aminotransferase (AST)] and therapeutic (the use of
opioids) variables.
The statistical analysis of the association between
the overall time on treatment with the anthropometric, imagistic,
hematological, biochemical and therapeutic variables was performed
using the Cox Proportional Hazards Survival Regression (CPHSR) test
available at https://statpages.info/prophaz.html) and included both
past and ongoing immunotherapy cases.
Results
There were no statistically significant differences
found between the age and duration of immunotherapy in our study
group, analysed by gender (28.2% women) (Table I).
 | Table IAge and duration of treatment by
sex. |
Table I
Age and duration of treatment by
sex.
| Variables | Males and
females | Males | Females | P-value |
|---|
| Age (years), average
± SD | 63.6±8.4 | 64.3±7.5 | 62.0±10.3 | 0.509 |
| Duration of
immunotherapy (days), average ± SD | 130.5±140.7 | 129.8±144.0 | 132.9±132.3 | 0.689 |
In terms of age group distribution, we found no
significant differences between males and females (Table II).
 | Table IIAge group distribution of the patients
by sex. |
Table II
Age group distribution of the patients
by sex.
| Age group, years | 40-49 | 50-59 | 60-69 | 70-79 | ≥80 |
|---|
| Males and females
(%) | 9.0 | 20.5 | 43.6 | 24.4 | 2.5 |
| Females (%) | 18.2 | 18.2 | 40.9 | 18.2 | 4.5 |
| Males (%) | 5.4 | 21.4 | 44.6 | 26.8 | 1.8 |
| z-test (males vs.
females) | 0.075 | 0.748 | 0.764 | 0.423 | 0.496 |
Analysis of anthropometric variables showed a
statistically significant difference between height of the males
and females, but not body mass index (BMI) (Table III).
 | Table IIIHeight and BMI of patients by sex. |
Table III
Height and BMI of patients by sex.
| Sex distribution | Height (cm) | BMI
(kg/m2) |
|---|
| Males and
females | 169.7±9.2 | 25.2±5.9 |
| Females | 174.0±6.4 | 26.2±6.0 |
| Males | 159.3±6.0 | 24.6±5.9 |
| P-value (males vs
females) | <0.00001 | 0.453 |
Most of the patients included in the study exhibited
a good biological status, all with Eastern Cooperative Oncology
Group Performance Score (ECOG PS) <3 at the time of
immunotherapy initiation (Table
IV).
 | Table IVPatient distribution by ECOG and
sex. |
Table IV
Patient distribution by ECOG and
sex.
| | ECOG PS |
|---|
| Sex distribution | 0 | 1 | 2 |
|---|
| Males and females
(%) | 38.5 | 50.0 | 11.5 |
| Females (%) | 39.3 | 50.0 | 10.7 |
| Males (%) | 36.4 | 50.0 | 13.6 |
| z-test (males vs.
females) | 0.81 | 1.00 | 0.72 |
In contrast with the female patients, more male
patients (76.9 vs. 55%) showed various degrees of anemia (grade 1
and 2, P=0.067) (Table V). On
average, the hemoglobin (Hb) level did not change significantly at
6 weeks upon therapy (the average change was +0.09 g/dl) (Table VI). However, we noted wide
individual variations [standard deviation (SD)=1.30 g/dl for Hb
variation]. Of note, there were no statistically significant
differences between Hb levels at initiation and the fourth cycle of
immunotherapy (P=0,6891, Mann Whitney test).
 | Table VHematological parameters at
immunotherapy initiation. |
Table V
Hematological parameters at
immunotherapy initiation.
| Parameters | Males and females
(%) | Males (%) | Females (%) | P-value (males vs.
females) |
|---|
| Hemoglobin
(Hb) | | | | |
|
Grade 2
anemia | 15.3 | 15.4 | 15.0 | 0.97 |
|
Grade 1
anemia | 55.6 | 61.5 | 40.0 | 0.10 |
|
Normal Hb
level | 29.1 | 23.1 | 40.9 | 0.13 |
| ALC
(x109/l) | | | | |
|
<1 | 25.0 | 25.0 | 25.0 | 1 |
|
1-4.8 | 73.6 | 73.1 | 75.0 | 0.87 |
|
≥4.8 | 1.2 | 1.9 | 0.0 | 0.52 |
| ANC
(x109/l) | | | | |
|
<2 | 1.4 | 0 | 5.0 | 0.11 |
|
2-9.9 | 87.5 | 86.5 | 90.0 | 0.69 |
|
≥10 | 11.1 | 13.5 | 5.0 | 0.30 |
 | Table VIPercent distribution of cases by
hemoglobin at 6 weeks. |
Table VI
Percent distribution of cases by
hemoglobin at 6 weeks.
| | Males and females
(%) | Males (%) | Females (%) | P-value (males vs.
females) |
|---|
| Hemoglobin
(Hb) | | | | |
|
Grade 3
Anemia | 1.9 | 2.6 | 0 | 0.45 |
|
Grade 2
anemia | 9.4 | 7.9 | 13.3 | 0.47 |
|
Grade 1
anemia | 66 | 71.1 | 53.3 | 0.14 |
|
Normal Hb
level | 22.6 | 18.4 | 33.3 | 0.16 |
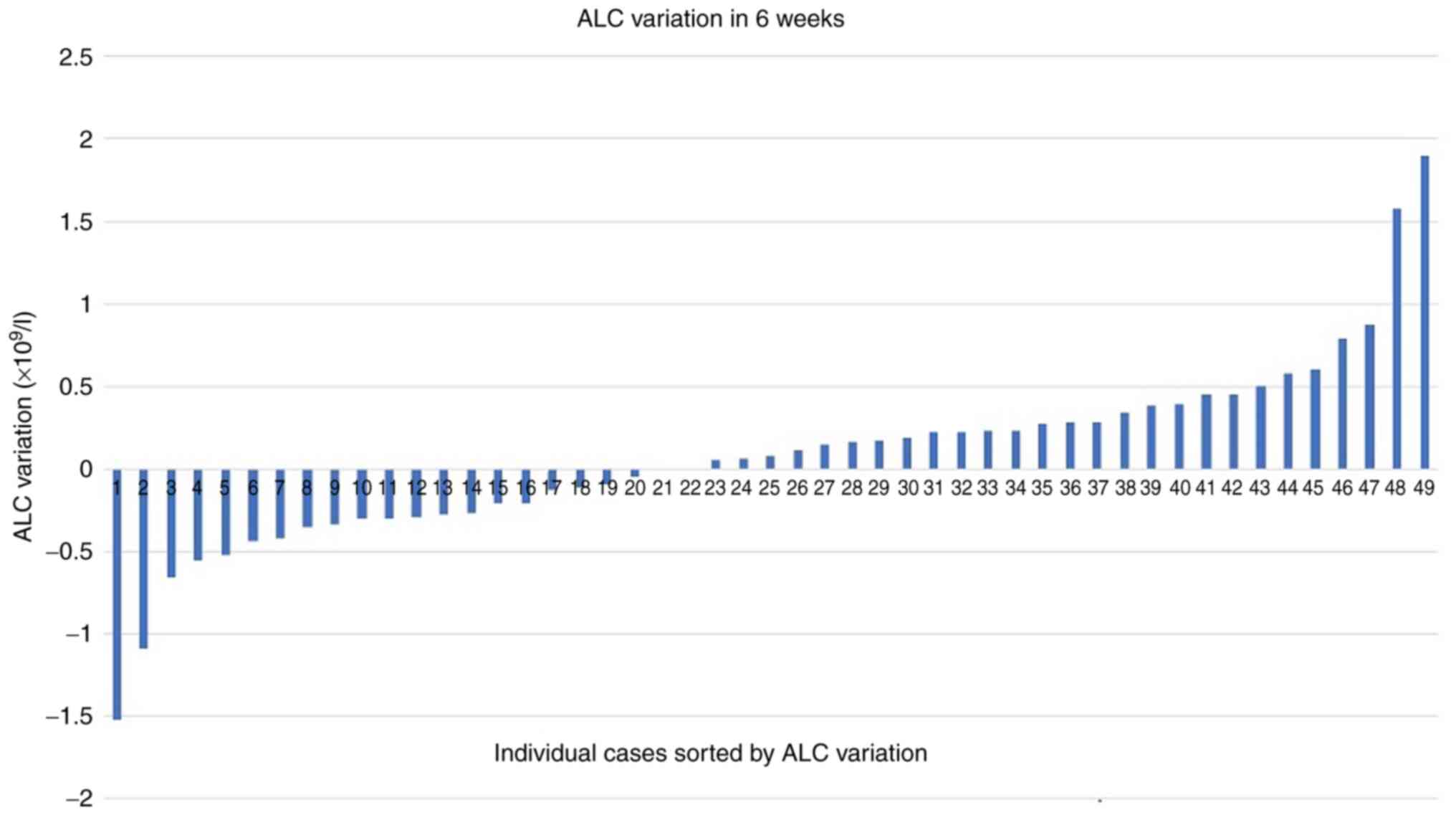
Almost 26% of the patients showed an abnormal number
of lymphocytes at baseline, while in the case of neutrophils, the
percentage of anomalies was much lower (12.5%). Similarly to the Hb
variable, there were minimal global variations (on average
+0.06x109/l) for absolute lymphocyte count (ALC)
(Fig. 1) and absolute neutrophil
count (ANC) (on average +0.16x109/l) at six weeks of
immunotherapy, with wide individual variations
(SD=0.55x109/l for ALC variation and SD=4.06 for ANC
variation). Neutropenic cases were absent at 6 weeks and
neutrophilia was present in 7.5% of cases (maximum
value=39x109/l). Of note, there were few cases of status
reversals (e.g. neutrophilia turned to normal range
neutrophils).
Leukocyte count and platelet (PLT) count showed
significant differences in regards to sex distribution at
initiation (Table VII).
 | Table VIIOther hematological parameters at
immunotherapy initiation. |
Table VII
Other hematological parameters at
immunotherapy initiation.
| Parameters | Males and females
(average ± SD) | Males (average ±
SD) | Females (average ±
SD) | P-value (males vs.
females) |
|---|
| MCV (fL) | 91.7±7.2 | 91.6±6.8 | 91.5±8.0 | 0.180 |
| Leukocyte count
(x109/l) | 9.1±4.0 | 9.9±4.3 | 7.1±2.1 | 0.0214 |
| PLT count
(x109/l) | 313.9±123.3 | 332.5±126.0 | 265.4±100.9 | 0.020 |
Creatinine, total calcium, ALT and AST showed no
significant sex differences at initiation (Table VIII). There was a slight
(statistically not significant) decrease in both creatinine (5.4%)
and calcium (1.7%) levels during the first six weeks of
therapy.
 | Table VIIIBiochemical parameters at
immunotherapy initiation. |
Table VIII
Biochemical parameters at
immunotherapy initiation.
| Parameters | Males and females
(mean ± SD) | Males (mean ±
SD) | Females (mean ±
SD) | P-value (males vs.
females) |
|---|
| Creatinine
(mg/dl) | 0.91±0.44 | 0.92±0.45 | 0.90±0.42 | 0.872 |
| Calcium (total)
(mg/dl) | 9.63±0.89 | 9.64±1.0 | 9.6±0.5 | 0.441 |
| ALT (IU/l) | 21.6±29.6 | 22.1±33.3 | 20.6±17.5 | 0.880 |
| AST (IU/l) | 27.5±31.4 | 28.8±36.7 | 24.3±8.3 | 0.342 |
Next, we evaluated the overall number and the
topography of metastases and found statistically significant
differences in males vs. females, with female predilection towards
brain and lung metastases (Table
IX). In regard to brain metastases, the frequency in patients
<60 years was significantly higher than in patients ≥60 years
(P=0.00032). In addition, liver metastases were more frequent in
patients <60 years of age (P=0.048). Moreover, there was a clear
predilection of both the 40-49 and 50-59 age groups for brain
metastasis (Table X).
Interestingly, in our cohort, more males than females had no
metastasis among evaluated sites (brain, lungs, liver, adrenal and
bones), while females tend to be overrepresented in the 2
metastatic site subgroup (Table
XI).
 | Table IXSex distribution of metastases at
immunotherapy initiation. |
Table IX
Sex distribution of metastases at
immunotherapy initiation.
| | Metastases |
|---|
| Sex
distribution | Brain (%) | Lung (%) | Liver (%) | Adrenal (%) | Bone (%) |
|---|
| Males +
females | 21.8 | 25.6 | 14.1 | 19.2 | 17.9 |
| Females | 40.9 | 40.9 | 13.6 | 4.5 | 22.7 |
| Males | 14.3 | 19.6 | 14.3 | 25 | 16.1 |
| z-test (males vs.
females) | 0.0104 | 0.0523 | 0.9442 | 0.0384 | 0.490 |
 | Table XAge distribution of metastases at
baseline. |
Table X
Age distribution of metastases at
baseline.
| | Metastases |
|---|
| Age
distribution | Brain | Lung | Liver | Adrenal | Bone |
|---|
| 40-49 years
(%) | 57 | 29 | 14 | 29 | 29 |
| 50-59 years
(%) | 44 | 25 | 31 | 31 | 19 |
| 60-69 years
(%) | 15 | 26 | 9 | 15 | 26 |
| 70-79 years
(%) | 5 | 16 | 11 | 16 | 10 |
| ≥80 years (%) | 0 | 100a | 0 | 0 | 0 |
 | Table XIOverall sex distribution in regards
to metastatic site count at baseline. |
Table XI
Overall sex distribution in regards
to metastatic site count at baseline.
| | Metastatic site
number |
|---|
| Sex
distribution | 0a | 1 | 2 | 3 | 4 | 5 |
|---|
| Males and
females | 35.8 | 37.1 | 20.5 | 5.1 | 1.2 | 0 |
| Females (%) | 22.7 | 40.9 | 31.8 | 4.5 | 0 | 0 |
| Males (%) | 41.1 | 35.7 | 16.1 | 5.4 | 1.8 | 0 |
| z-test (males vs.
females) | 0.13 | 0.67 | 0.12 | 0.87 | 0.53 | - |
Evaluation of the immunotherapy status at 20 months
showed that 15.7% of the patients underwent a single administration
of nivolumab and the treatment was discontinued immediately. A
total of 14.1% of the patients were treated for at least 2 cycles,
but for less than 2 months and discontinued. A total of 21.8% of
patients underwent at least 6 months of treatment (and from these
patients, 14.1% are still in treatment) (Fig. 2).
The results of our CPHSR analysis are summarized
[risk ratios (RR), 95% CI intervals, P-values] (Table XII), with a focus on initiation
and at six weeks of immunotherapy time points (Fig. 3).
 | Table XIIParameters at initiation and 6 weeks
of immunotherapy as analyzed by Cox proportional hazards survival
regression. |
Table XII
Parameters at initiation and 6 weeks
of immunotherapy as analyzed by Cox proportional hazards survival
regression.
| | Baseline
parameters | Parameter variation
after 6 weeks of nivolumab treatment |
|---|
| Parameter | RR (95% CI) | P-value | No. of cases | RR (95% CI) | P-value | No. of cases |
|---|
| Number of organs
with metastases | 1.5569 | 0.0035 | 76 | NA | - | - |
|
(BRA+PUL+HEP+SR+OSS) (values from 0 to 5,
average 1.01) | (1.156-2.095) | | | NA | | |
| Hepatic metastases
present | 2.6651 | 0.0097 | 76 | NA | - | |
| (0/1, average
0.14) | (1.268-5.599) | | | NA | | |
| Adrenal metastases
present, males | 3.5232 | 0.0257 | 25 | NA | - | |
| <65 years (0/1,
average 0.24) | (1.165-10.629) | | | NA | | |
| ANC >8
(x109/l)(0/1, average 0.18) | 2.3863 | 0.0277 | 72 | 2.2495 | 0.0580 | 53 |
| | (1.100-5.176) | | | (0.972-5.201) | | |
| ALC
(x109/l) | 0.9261 | 0.7202 | 72 | 2.5236 | 0.0394 | 49 |
| (average 1.61) | (0.61-1.41) | | | (1.046-6.087) | | |
| ANC
(x109/l) (average 6.37) | 1.1003 | 0.0406 | 72 | 1.0169 | 0.0569 | 49 |
| | (1.004-1.205) | | | (0.99-1.22) | | |
| Brain metastases
present at initiation | 1.9332 | 0.0440 | 76 | NA | - | - |
| (0/1, average
0.22) | (1.017-3.671) | | | NA | | |
| Lung metastases
present at initiation | 1.7943 | 0.0562 | 76 | NA | - | - |
| (0/1, average
0.26) | (0.98-3.26) | | | NA | | |
| Leukocyte count (±
x109/l) | 1.066 | 0.0637 | 72 | 1.0877 | 0.3920 | 49 |
| (average 9.13) | (0.996-1.140) | | | (0.89-1.31) | | |
| Calcium total <9
mg/dl at initiation | 2.1237 | 0.1267 | 67 | NA | - | - |
| | (0.80-5.58) | | | NA | | |
| Male sex | 1.6421 | 0.1647 | 76 | 1.6421 | 0.1647 | 76 |
| | (0.81-3.30) | | | (0.81-3.30) | | |
| AST IU/l at
initiation | 1.0052 | 0.21 | 70 | NA | - | - |
| | (0.99-1.01) | | | NA | | |
| Hb at initiation
(g/dl) | 1.0108 | 0.9053 | 72 | 0.803 | 0.2212 | 49 |
| | (0.85-1.21) | | | (0.56-1.14) | | |
| ALT IU/l at
initiation | 1.0053 | 0.2447 | 70 | NA | - | - |
| | (0.996-1.014) | | | NA | | |
| Opioid usage at
initiation | 1.604 | 0.3233 | 76 | 1.1406 | 0.8591 | 53 |
| | (0.62-4.09) | | | (0.267-4.873) | | |
| Adrenal metastases
present at initiation | 1.4187 | 0.3332 | 76 | NA | - | - |
| | (0.69-2.88) | | | NA | | |
| MCV (fL) at
initiation | 1.0165 | 0.3864 | 72 | 1.0036 | 0.906 | 49 |
| | (0.97-1.05) | | | (0.94-1.06) | | |
| PLT count
(x109/l) at initiation | 1.001 | 0.4456 | 72 | 1.0032 | 0.2157 | 49 |
| | (0.998-1.003) | | | (0.998-1.008) | | |
| Creatinine (mg/dl)
at initiation | 0.7559 | 0.4473 | 71 | 0.6269 | 0.644 | 49 |
| | (0.36-1.55] | | | (0.08-4.54) | | |
| Height, male
patients (cm) | 0.9874 | 0.6353 | 52 | 0.9874 | 0.6353 | 52 |
| | (0.93-1.04) | | | (0.93-1.04) | | |
| Bone metastases
present at initiation | 0.848 | 0.6892 | 76 | NA | - | - |
| | (0.37-1.90) | | | NA | | |
| ECOG PS (0-4) at
initiation | 0.9133 | 0.6895 | 76 | NA | - | - |
| | (0.58-1.42) | | | NA | | |
| Age (years) | 0.994 | 0.7360 | 76 | 0.994 | 0.7360 | 76 |
| | (0.95-1.03) | | | (0.95-1.03) | | |
| Calcium total at
initiation (mg/dl) | 1.0713 | 0.7445 | 67 | NA | | |
| | (0.71-1.62) | | | NA | | |
| BMI at initiation
(kg/m2) | 0.9917 | 0.7621 | 70 | NA | | |
| | (0.93-1.04) | | | NA | | |
Discussion
Following international incidence data on lung
cancer (13), our cohort was formed
mostly by men (71.8%); there were no significant differences found
between male and female patients in terms of age and age group
distribution (yet to mention the 40-49 group, where females were
predominant, P=0.075). There were no statistically significant
differences between the body mass indices (BMIs) of the male and
female patients, although males were significantly taller
(P<0.00001). The vast majority of our patients (88.5%) were in
good biological status, with ECOG PS below 2; there were no
differences between ECOG PS of the male and female patients
(P=0.741).
The analysis of hematologic parameters showed a
surprisingly higher percentage of anemia in men vs. women (which
could be explained by wider interval for grade I anemia in men),
while leukocyte and platelet counts were significantly higher in
male vs. female patients. There were no significant differences
between male and female biochemical parameters at baseline.
The mean variation in hemoglobinemia (Hb) revealed
that a significant recovery from hematopoiesis from previous toxic
chemotherapeutic effect only occurred in some of the patients.
The prevalence of lung and brain metastases was
double and more than double, respectively, among female patients;
differences reached statistical significance for brain metastases
(P=0.0104) and were close to the limit for lung metastases
(P=0.0523), while the male population showed a 5-time higher
prevalence for adrenal metastases (P=0.0394). Most women enrolled
(72.7%) had 1 or 2 metastatic sites, as compared to 51.8% in the
male subgroup.
CPHSR analysis indicated that each additional
baseline metastatic site (aggregated data for brain, lung, liver,
adrenal, bone topography) increased the patient risk of treatment
exit by 56% (P=0.0035). Hepatic metastasis at baseline translated
into a risk ratio (RR) of 2.67 (P=0.0097), an important negative
predictive value.
Baseline adrenal metastasis was a significant
negative predictor only in the <65-year old male subgroup, for
which a high 3.52 RR (P=0.0257) was calculated; this was in full
agreement with published data showing a significant decrease in
median overall survival (OS) in the presence of adrenal metastases
(14). Of note, in our cohort, the
adrenal metastasis prevalence was almost double in the 40-49/50-59
age groups in comparison with the other age groups.
As expected, cerebral metastasis has a negative
impact on the overall time of treatment (RR=1.93, P=0.044). Crinò
et al showed a 39% disease control rate with a median OS of
8.6 months in NSCLC patients under therapy with nivolumab (15).
A baseline neutrophil count over 8x109/l
and an ALC variation of +1x109/l at six weeks on therapy
were both negative predictive factors, with comparable RR=2.39
(P=0.027) and 2.52 (P=0.0394), respectively. Of note, baseline ANC
per se was found to be a much weaker negative predictor
(RR=1.10, P=0.0406).
Our data concerning ALC variation as a negative
predictor may come as a surprise, since previously published data
describe a positive correlation of ALC (at baseline and at 6 weeks
on treatment) with OS upon nivolumab therapy (16). While Karantanos et al
described static data, our approach emphasized a novel, more
dynamic parameter: The absolute change in ALC between baseline and
6-week time point. Why a positive ALC variation at 6 weeks of
therapy exerts a negative effect on overall time on treatment
remains to be explored on much wider cohorts of patients.
Other authors investigated 50 possible predictors of
disease-specific survival during nivolumab treatment for NSCLC.
Correlations with disease-specific survival were proven for ECOG
PS, size of the largest brain metastasis, number of metastatic
sites, toxicity, and malignant pleural effusion and correlations
with time to treatment failure were confirmed for malignant pleural
effusion, number of metastatic sites and number of liver metastases
(17).
As lung and breast cancers metastasize to the eye
and, although rare, metastatic choroid tumors are the most common
type of intraocular malignancy, the patients were screened for
associated ocular changes and, if necessary, for treatment options
(18-22).
The value of total calcium <9 mg/dl (lower normal
or hypocalcemia) is shown as a possible negative predictive factor
for the duration of nivolumab immunotherapy. It should be noted
that hypoalbuminemia is associated with poor immunotherapy results
(23) (possibly by increasing the
degradation of antibodies) and that hypoalbuminemia is a cause for
apparently lower values of total calcium; in conditions of
hypoalbuminemia, serum calcium values should be corrected.
Previous studies have shown that before starting
nivolumab therapy, 17% of NSCLC patients present with
hypoalbuminemia and 37% have lost more than 5% of their weight in
the last 6 months. Progression-free survival (PFS) and OS are
strongly influenced by albumin levels, hypo- vs. normal albuminemia
differences being significant: 5.2 vs. 8.5 months in the case of
PFS, respectively 6.9 vs. 18.5 months in the case of OS (23).
Besides the common limitations of a retrospective
study, our analysis was hindered by the low number of probands in
the subgroups, leading to uncomfortably wide confidence intervals
for many investigated variables.
In conclusion, negative predictive factors were
identified for the duration of nivolumab treatment: The presence of
adrenal metastases (in men under 65 years of age), the presence of
liver metastases, neutrophilia at the beginning of treatment
(expressed both as ANC and as a value exceeding
8x109/l), absolute variation (increase) of lymphocytes
at 6 weeks of treatment, the presence of brain metastases and the
number of metastatic affected organs.
It is important to report early evolutive parameters
that are predictive for the total duration of nivolumab treatment
as demonstrated for circulating lymphocyte variation in the first 6
weeks.
Acknowledgements
Professional editing, linguistic and technical
assistance was performed by Irina Radu, individual service
provider.
Funding
Funding: The present study did not receive specific funding.
Availability of data and materials
The data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
SoS organized the study, analyzed and interpreted
the study data and wrote the manuscript. SN, SV, DP, VC, SiS, HF,
RD and DM analyzed the data and helped to draft the output and
critically reviewed the manuscript; CV interpreted the data and
critically reviewed the manuscript for intellectual content. All
the authors read and approved the final version of the manuscript
for publication.
Ethics approval and consent to
participate
All patients gave their informed consent for the
procedure. The study protocol was conducted according to the
principles of the Declaration of Helsinki after the approval of the
Oncohelp Clinic Ethics Committee (3b/15.09.2020) (Timisoara,
Romania). All patients provided written informed consent for the
study participation and data collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Niezgoda A, Niezgoda P and Czajkowski R:
Novel approaches to treatment of advanced melanoma: A review on
targeted therapy and immunotherapy. Biomed Res Int.
2015(851387)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bansal P, Osman D, Gan GN, Simon GR and
Boumber Y: Recent advances in immunotherapy in metastatic NSCLC.
Front Oncol. 6(239)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gill DM, Agarwal N and Vaishampayan U:
Evolving treatment paradigm in metastatic renal cell carcinoma. Am
Soc Clin Oncol Educ Book. 37:319–329. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang C, Thudium KB, Han M, Wang XT, Huang
H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, et al: In
vitro characterization of the anti-PD-1 antibody nivolumab,
BMS-936558, and in vivo toxicology in non-human primates. Cancer
Immunol Res. 2:846–856. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Renner A, Burotto M and Rojas C: Immune
checkpoint inhibitor dosing: Can we go lower without compromising
clinical efficacy? J Glob Oncol. 5:1–5. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Agrawal S, Feng Y, Roy A, Kollia G and
Lestini B: Nivolumab dose selection: Challenges, opportunities, and
lessons learned for cancer immunotherapy. J Immunother Cancer.
4(72)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yoo SH, Keam B, Kim M, Kim SH, Kim YJ, Kim
TM, Kim DW, Lee JS and Heo DS: Low-dose nivolumab can be effective
in non-small cell lung cancer: Alternative option for financial
toxicity. ESMO Open. 3(e000332)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang J, Sanghavi K, Shen J, Zhao X, Feng
Y, Statkevich P, Sheng J, Roy A and Zhu L: Population
pharmacokinetics of nivolumab in combination with ipilimumab in
patients with advanced malignancies. CPT Pharmacometrics Syst
Pharmacol. 8:962–970. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cortellini A, Verna L, Porzio G, Bozzetti
F, Palumbo P, Masciocchi C, Cannita K, Parisi A, Brocco D, Tinari N
and Ficorella C: Predictive value of skeletal muscle mass for
immunotherapy with nivolumab in non-small cell lung cancer
patients: A ‘hypothesis-generator’ preliminary report. Thorac
Cancer. 10:347–351. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Payne RB, Little AJ, Williams RB and
Milner JR: Interpretation of serum calcium in patients with
abnormal serum proteins. Br Med J. 4:643–646. 1973.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cusato J, Genova C, Tomasello C, Carrega
P, Ottonello S, Pietra G, Mingari MC, Cossu I, Rijavec E, Leggieri
A, et al: Influence of vitamin D in advanced non-small cell lung
cancer patients treated with nivolumab. Cancers (Basel).
11(125)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Barta JA, Powell CA and Wisnivesky JP:
Global epidemiology of lung cancer. Ann Glob Health.
85(8)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tamura T, Kurishima K, Nakazawa K,
Kagohashi K, Ishikawa H, Satoh H and Hizawa H: Specific organ
metastases and survival in metastatic non-small-cell lung cancer.
Mol Clin Oncol. 3:217–221. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Crinò L, Bronte G, Bidoli P, Cravero P,
Minenza E, Cortesi E, Garassino MC, Proto C, Cappuzzo F, Grossi F,
et al: Nivolumab and brain metastases in patients with advanced
non-squamous non-small cell lung cancer. Lung Cancer. 129:35–40.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Karantanos T, Karanika S, Seth B and
Gignac G: The absolute lymphocyte count can predict the overall
survival of patients with non-small cell lung cancer on nivolumab:
A clinical study. Clin Transl Oncol. 21:206–212. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pantano F, Russano M, Berruti A, Mansueto
G, Migliorino MR, Adamo V, Aprile G, Gelibter A, Ficorella C,
Falcone A, et al: Prognostic clinical factors in patients affected
by non-small-cell lung cancer receiving nivolumab. Expert Opin Biol
Ther. 20:319–326. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Preda MA, Popa G, Karancsi OL, Musat O,
Popescu SI, Munteanu M and Popa Z: Effectiveness of subconjunctival
bevacizumab associated with a laser-based procedure in the
treatment of neovascular glaucoma. Farmacia. 66:621–626. 2018.
|
|
19
|
Boruga O, Balasoiu AT, Giuri S, Munteanu
M, Stanca HT, Iovanescu G and Preda MA: Caruncular late-onset
junctional nevus: Apropos of an anatomo-clinical observation. Rom J
Morphol Embryol. 58:1461–1464. 2017.PubMed/NCBI
|
|
20
|
Preda MA, Karancsi OL, Munteanu M and
Stanca HT: Clinical outcomes of micropulse transscleral
cyclophotocoagulation in refractory glaucoma-18 months follow-up.
Lasers Med Sci. 35:1487–1491. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Stanca HT, Munteanu M, Jianu DC, Motoc
AGM, Tăbăcaru B, Stanca S, Ungureanu E, Boruga VM and Preda MA: New
perspectives in the use of laser diode transscleral
cyclophotocoagulation. A prospective single center observational
cohort study. Rom J Morphol Embryol. 59:869–872. 2018.PubMed/NCBI
|
|
22
|
Stanca HT, Petrović Z and Munteanu M:
Transluminal Nd:YAG laser embolysis-a reasonable method to
reperfuse occluded branch retinal arteries. Vojnosanit Pregl.
71:1072–1077. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee CS, Devoe CE, Zhu X, Fishbein JS and
Seetharamu N: Pretreatment nutritional status and response to
checkpoint inhibitors in lung cancer. Lung Cancer Manag.
9(LMT31)2020.PubMed/NCBI View Article : Google Scholar
|