Introduction
Gastric carcinoma is a common type of malignant
tumor worldwide and it is estimated that newly diagnosed cases in
China annually account for 43% of the global cases, with ~400,000
cases (1). Due to its high rate of
mortality, gastric carcinoma poses a serious threat to human health
and life (2-4).
The etiology of gastric carcinoma is complicated, and the vast
majority of gastric carcinomas are diagnosed in the advanced stages
because only a small percentage of patients can be diagnosed and
treated at an early stage (5).
Previous studies have reported that the occurrence of the majority
of gastric carcinoma cases were the result of the combined action
of multiple environmental factors, such as dietary, lifestyle and
environmental influential factors, and tumor susceptibility
factors, such as the influencing factors of blood vessel invasion
(6,7). It was previously identified that
gastric carcinoma caused by Helicobacter pylori was closely
associated with the immune response of the body (8,9).
Moreover, cellular immunity dominated by cytokines was discovered
to be involved in the occurrence of gastric carcinoma (10-12).
Cytokines, such as ILs, IFNs, colony stimulating
factor, chemokines and growth factors, not only serve important
roles in immune regulation, but also participate in the occurrence
and development of various types of carcinoma (13,14).
Liu et al (13) demonstrated
that the potency of IL-34, macrophage colony stimulating factor,
tumour-associated macrophages (TAMs) and the combination of
IL-34/TAMs as novel biological markers for gastric carcinoma. ILs
are mainly involved in the activation and regulation of immune
cells in the body (15). IL-17
consists of six protein members (16-18),
and has been revealed to serve a vital role in the development of
numerous types of malignant cancer, including colorectal cancer
(19). Furthermore, the
upregulation of IL-17 was observed in various types of tumor
tissue, such as breast carcinoma and gastric carcinoma (20, 21),
which suggested that IL-17 may be associated with the development
of tumors.
At present, the standard therapy for gastric
carcinoma includes surgical intervention followed by combination
chemotherapy (22). While
chemotherapy has become the primary treatment option for advanced
gastric carcinoma (23), the effect
of traditional chemotherapy is not ideal. Targeted therapy is a
novel cancer treatment at the cellular or molecular level (24), in which specially designed drugs can
target and act on the specific genes or proteins necessary for
tumor growth. The adverse reactions of targeted therapy in patients
are usually more easily tolerated compared with those of
chemotherapy (25). Therefore,
targeted therapies for various types of solid tumor have gained
increased attention. As a popular anti angiogenic targeting drug,
apatinib can block the formation of new blood vessels in tumor
tissue and thus, inhibit the progression of tumors (26). Notably, apatinib has previously
demonstrated efficacy in patients with metastatic gastric carcinoma
(27).
Although both IL-17 and apatinib have been closely
associated with the process of tumor angiogenesis, to the best of
our knowledge, the potential effect of apatinib on IL-17 expression
levels in the development of gastric carcinoma has been rarely
reported. In the present study, the effects of IL-17 and apatinib
on gastric carcinoma were investigated. Overall, the present study
may be of great significance to provide novel specific targets for
the treatment of gastric carcinoma.
Materials and methods
Clinical sample collection
A total of 30 tumor and para-carcinoma tissues were
obtained from 30 patients with gastric carcinoma (aged between 55
and 73 years, who received surgery at Tianjin Nankai Hospital
(Tianjin, China) between January 2019 and December 2019 (Table I). Tissue samples were immediately
stored in liquid nitrogen at -80˚C after resection. Written
informed consent was obtained from each participant before surgery.
The study conformed to the Declaration of Helsinki and was approved
by Tianjin Nankai Hospital ethics committee (approval no.
NKYY_YXKT_IRB_2019_101_01).
 | Table IClinicopathological information of
patients with gastric carcinoma used in the present study (n=30,
mean ± SD). |
Table I
Clinicopathological information of
patients with gastric carcinoma used in the present study (n=30,
mean ± SD).
| Variable | Parameters |
|---|
| Age, years | 65.32±9.33 |
| Number of male
patients (%) | 17 (56.67) |
| BMI,
kg/m2 | 25.30±4.66 |
| Urea nitrogen,
mmol/l | 5.62±1.53 |
| Creatinine,
µmol/l | 79.3±8.21 |
| Low density
lipoprotein, mmol/l | 3.63±1.38 |
| Triglyceride,
mmol/l | 1.96±0.74 |
| Total cholesterol,
mmol/l | 4.77±1.24 |
| Number of diabetic
patients (%) | 20 (66.67) |
| Number of patients
with hypertension (%) | 11 (36.67) |
| Number of patients
with a smoking history (%) | 19 (63.33) |
Cell culture and treatment
The human gastric carcinoma cell line, HGC-27, was
obtained from the American Type Culture Collection. Cells were
cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (HyClone;
Cytiva), 100 U/ml penicillin and 100 U/ml streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.) in an incubator with 5%
CO2 at 37˚C. Cell propagation was performed every 24 h.
The cells in the logarithmic phase were used for further
analysis.
After cells were fully adhered to the well, they
were cultured at 37˚C at a density of 2x103
cells/cm2 and divided into the following groups: i)
Control group; ii) IL-17 group; iii) IL-17-apatinib group; and iv)
apatinib group. Cells in the IL-17 group were treated with 10 ng/ml
IL-17 (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h. The
apatinib group was treated with 50 ng/ml apatinib (cat. no. S7297,
Selleck Chemicals) for 48 h. For the IL-17-apatinib group, cells
were cultured with 10 ng/ml IL-17 and 50 ng/ml apatinib for 48 h.
Cells in the Control group were treated with same volume of DMEM.
All treatments were performed at 37˚C.
Reverse transcription-quantitative PCR
(RT-qPCR)
The expression levels of IL-17 in the para-carcinoma
and carcinoma tissues and the expression levels of Bax, Bcl-2 and
caspase-3 in HGC-27 cells among the four different groups were
analyzed using RT-qPCR. Total RNA was extracted from the cells and
tissues using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA was reverse transcribed into cDNA
using the PrimeScript™ One Step RT-PCR kit (Takara Biotechnology
Co, Ltd.) at 37˚C. qPCR was subsequently performed using the
SYBR® Premix Dimmer Eraser kit (Takara Biotechnology
Co., Ltd.). The following thermocycling conditions were used for
the qPCR: Initial denaturation at 94˚C for 5 min; followed by 35
cycles at 94˚C for 30 sec, 57˚C for 30 sec and 72˚C for 30 sec; and
a final cycle at 72˚C for 5 min. The following primers pairs were
used for the qPCR: IL-17 forward, 5'-CTGGGACGTACCGGGTCGGT-3' and
reverse, 5'-GTCTGTCGCCTGAACAACGTCT-3'; Bcl-2 forward,
5'-TGGGATGCCTTTGTGGAAC-3' and reverse, 5'-CATATTTGTTTGGGGCAGGTC-3';
Bax forward, 5'-TTCCGAGTGGCAGCTGAGATGTTT-3' and reverse,
5'-TGCTGGCAAAGTAGAAGAGGGCAA-3'; caspase-3 forward,
5'-GCAAACCTCAGGGAAACATT-3' and reverse,
5'-TTTTCAGGTCAACAACAGGTCCA-3'; and GAPDH forward,
5'-GGAAAGCTGTGGCGTGAT-3' and reverse, 5'-AAGGTGGAAGAATGGGAGTT-3'.
The relative expression levels of the target genes were quantified
using the 2-∆∆Cq method (28). Each experiment was repeated ≥3
times. GAPDH was used as the internal loading control. The target
expressions were normalized using the expression levels of GAPDH as
a reference. All kits were used according to the manufacturer's
protocols.
Western blotting
The protein expression levels of IL-17, Bax, Bcl-2
and caspase-3 in HGC-27 cells were analyzed using western blotting.
Cells were collected and total protein was extracted using RIPA
lysis buffer (Beyotime Institute of Biotechnology) on ice. The
supernatant was retained to detect protein concentration of IL-17,
Bax, Bcl-2 and caspase-3 after high-speed centrifugation (10,000 x
g, 4˚C; 60 min) using BCA. Equivalent amounts of protein/lane (30
µg/lane) in RIPA lysis buffer was separated via 10% SDS-PAGE
(Sigma-Aldrich; Merck KGaA). The separated proteins were
subsequently transferred onto a PVDF membrane (Sigma-Aldrich; Merck
KGaA) and blocked with 5% milk in Tris-buffered saline for 1 h at
25˚C. Then, the membranes were incubated with the following primary
antibodies overnight at 4˚C: Anti-Bax (1:1,000; cat. no. B3428
Sigma-Aldrich; Merck KGaA), anti-Bcl-2 (1:1,000; cat. no. B3170,
Sigma-Aldrich; Merck KGaA), anti-caspase-3 (1:1,000; cat. no.
ABC495, Sigma-Aldrich; Merck KGaA), anti-IL-17 (1:1,000; cat. no.
PRS4887, Sigma-Aldrich; Merck KGaA) and anti-GAPDH (1:1,000; cat.
no. G8795, Sigma-Aldrich; Merck KGaA). Following the primary
antibody incubation, the membranes were incubated with a secondary
antibody (1:5,000; cat. no. ZB-2301; goat anti-rabbit; Bejing
Zhongshan Jinqiao Biotechnology Co., Ltd.) for 1 h. The protein
bands were visualized using an ECL kit (Beijing Solarbio Science
& Technology Co, Ltd.). The band intensity was semi-quantified
using Image-Pro Plus 6.0 analysis software (Media Cybernetics,
Inc.). Blots were repeated ≥3 times for every condition.
MTT assay
Cell proliferation assay was performed using MTT
reagent (Shanghai Macklin Biochemical Co., Ltd.). HGC-27 cells in
the logarithmic phase were seeded into 96-well plates at a density
of 5x103 cells/well and incubated for 24 h. After the
cells had fully adhered to the well, cell suspension (100 µl/well)
of IL-17, apatinib or IL-17-apatinib were added into the test
wells. Following 12, 24 or 48 h incubation at 37˚C, 20 µl MTT (5
mg/ml) solution was added to each well and incubated for another 4
h. After removing the supernatant, 100 µl per well dimethyl
sulfoxide (DMSO) (Shanghai Macklin Biochemical Co., Ltd.) was used
to dissolve the formazan crystals. The absorbance values of each
sample were measured with a microplate reader at 490 nm.
Experiments were repeated ≥3 times and data are expressed as the
mean ± standard error of the mean.
Flow cytometric analysis of
apoptosis
HGC-27 cells in the four groups were collected
(1,000 x g, 5 min, 4˚C) and resuspended in 0.5 ml binding solution
(Annexin V-FITC apoptosis detection kit; Beyotime Institute of
Biotechnology) at a density of 5.0x105 cells/ml. The
cells were transferred to a flow tube (100 µl/tube) and then
incubated with 5 µl Annexin-V allophycocyanin and 5 µl
7-Aminoactinomycin D at 4˚C for 5 min. At the end of the
incubation, the cells were washed with ice-cold PBS three times and
the fluorescence intensity was measured using a FACSCalibur flow
cytometer (BD Biosciences) with FlowJo software (version 7.6, Tree
Star, Inc.) The apoptotic cells were combined with Annexin V
labeled with FITC, and the percentage of early and late apoptotic
cells was calculated.
Transwell invasion assays
Transwell assays were performed with 8-µm pore
Transwell plates (EMD Millipore). Briefly, 5x103 HGC-27
cells in 100 µl DMEM (Gibco; Thermo Fisher Scientific, Inc.) medium
were seeded into the upper chamber of the Transwell plate in
serum-free DMEM. The lower chamber was filled with DMEM
supplemented with 10% FBS. The cells were allowed to migrate across
a polycarbonate filter precoated with Matrigel. Following 24 h of
incubation at 37˚C, the invasive cells were in the lower chamber
were fixed in 5% glutaraldehyde for 10 min at 4˚C, stained using
0.5% crystal violet solution for 15 min at 37˚C and counted with an
inverted phase-contrast microscope (magnification, x200; Nikon
TE-2000U, Nikon Corporation). Each experiment was repeated ≥ 3
times.
Statistical analysis
All data were analyzed using SPSS 18.0 software
(SPSS, Inc.). Continuous variables were expressed as mean ± SD and
categorical data were expressed as numbers and percentages. A
paired Student's t-test (two-tailed) or one-way ANOVA followed by a
Tukey's post hoc test were used as appropriate to determine the
statistical differences between groups. Each experiment was
repeated ≥ 3 times. P<0.05 was considered to indicate a
statistically significant difference.
Results
Analysis of the data from patients
with gastric carcinoma
The basic information of the patients with gastric
carcinoma, including age, sex, BMI, smoking and hypertension
history, and biochemical indexes, are presented in Table I. The patients had a mean age of
65.32±9.33 years and a mean BMI of 25.30±4.66 kg/m2. In
total, 56.67% of the patients were male. Gastric carcinoma and
para-carcinoma tissues were obtained from the 30 patients.
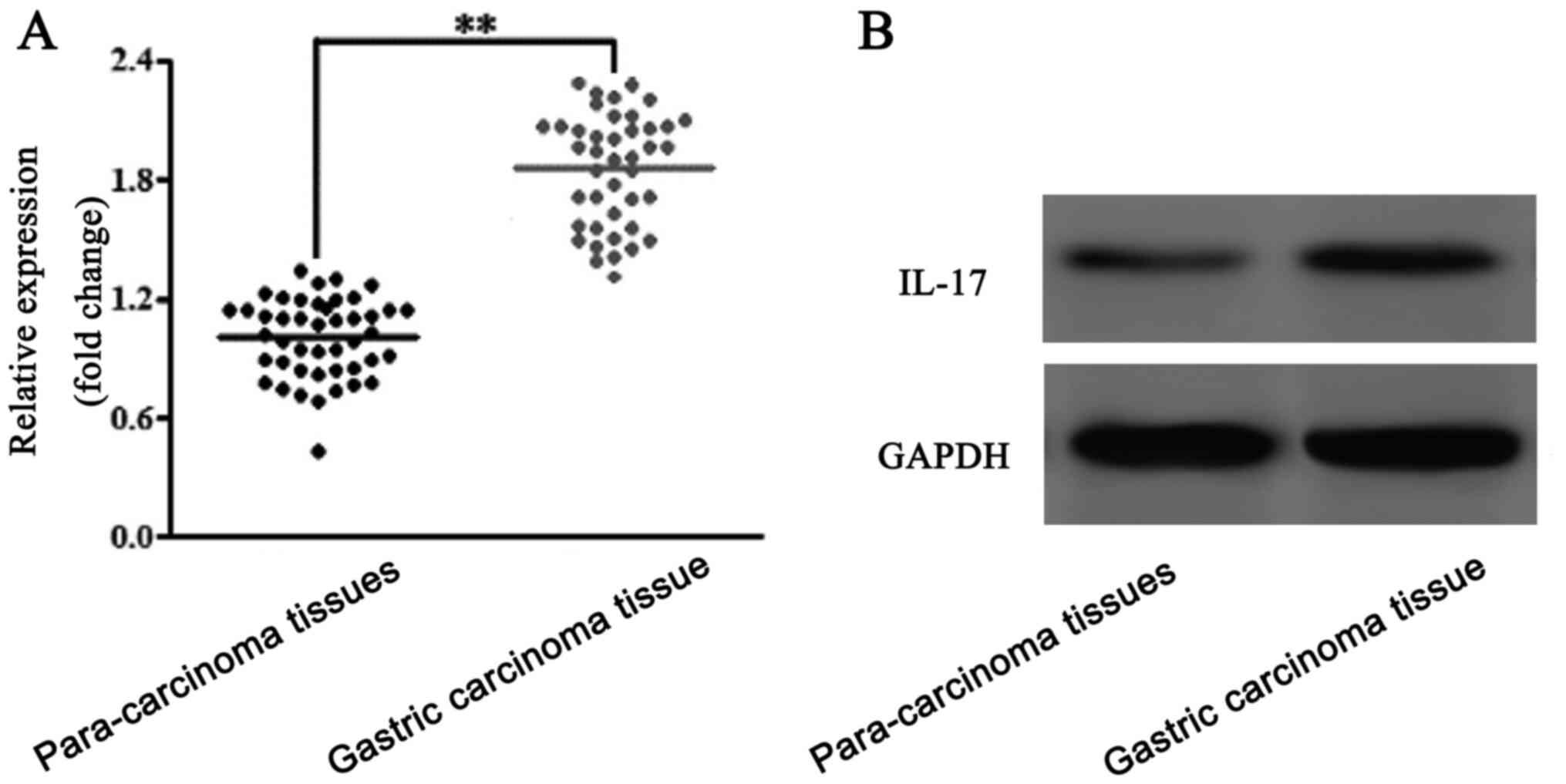
Expression levels of IL-17 in the
gastric carcinoma tissues
The expression levels of IL-17 in the gastric
carcinoma and para-carcinoma tissues were analyzed using RT-qPCR
(Fig. 1A) and western blotting
(Fig. 1B). The results of the
analyses revealed that IL-17 expression levels were upregulated in
gastric carcinoma tissues compared with para-carcinoma tissues.
Thus, IL-17 was suggested to be upregulated in patients with
gastric carcinoma.
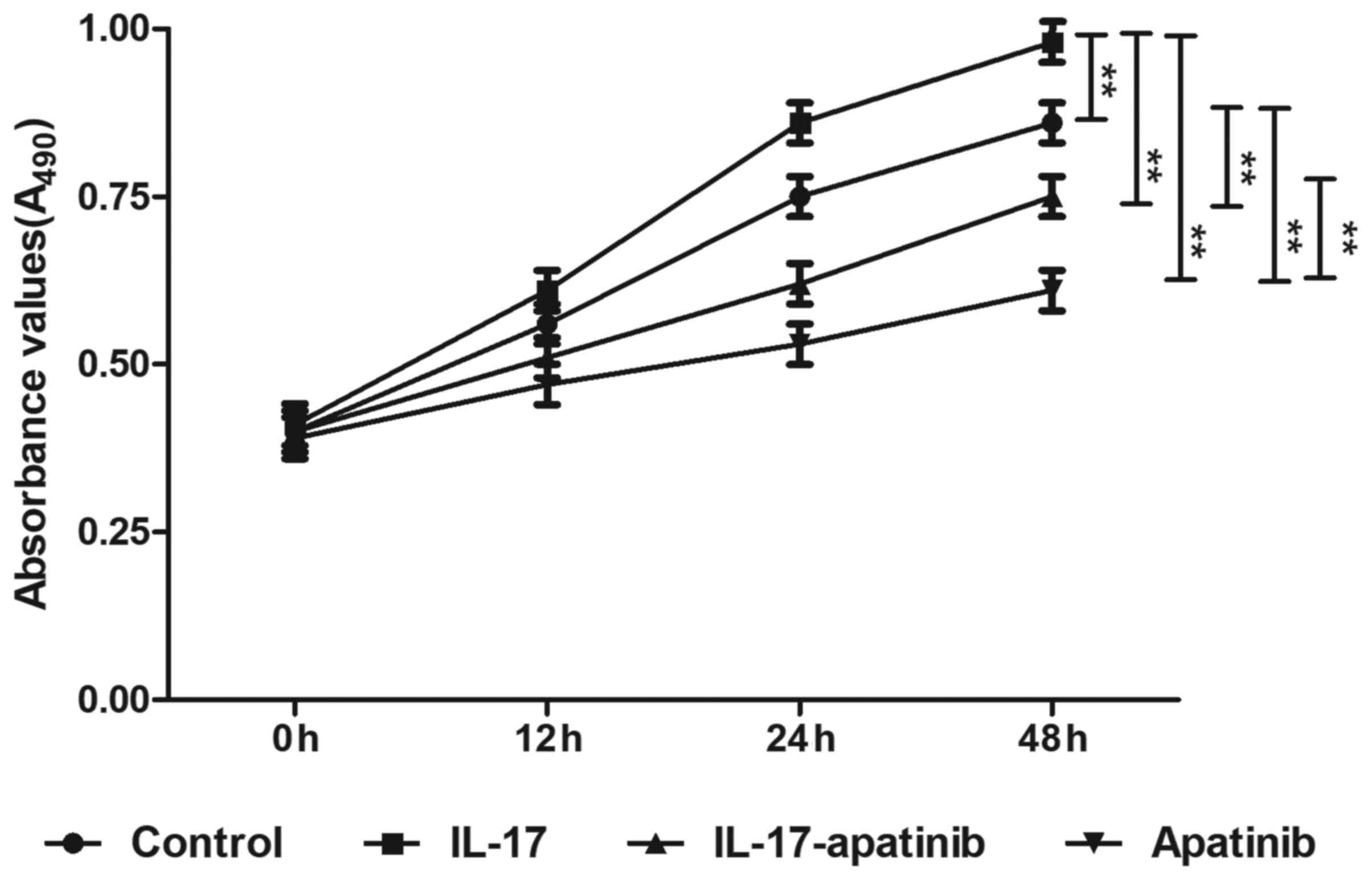
Effect of apatinib and IL-17 on the
proliferative ability of HGC-27 cells
To determine the effect of apatinib and IL-17 on the
proliferation of HGC-27 cells, an MTT assay was performed (Fig. 2). Following incubation for 48 h,
IL-17 stimulation significantly promoted the proliferation in the
IL-17 group compared with the control group, while apatinib
treatment significantly inhibited the proliferation of HGC-27 cells
compared with the control group, particularly in the apatinib group
following incubation for 48 h. Compared with the IL-17 group, the
absorbance values were significantly decreased in IL-17-apatinib
group, most notable in the apatinib group following incubation for
48 h. These results suggested that IL-17 may promote cell
proliferation, while apatinib may effectively suppress the
proliferation of HGC-27 cells.
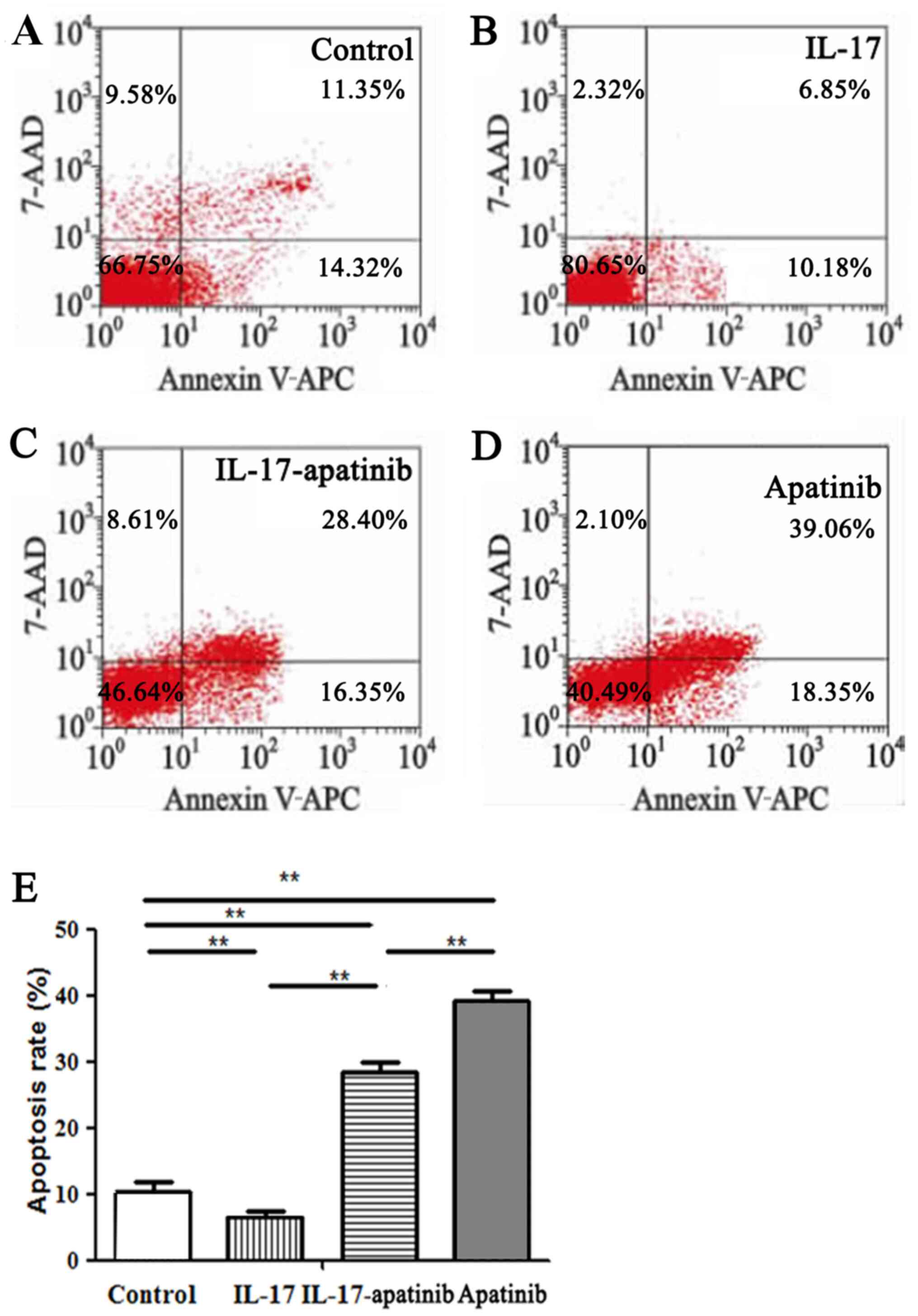
Effect of apatinib and IL-17 on the
apoptosis of HGC-27 cells
The effect of apatinib and IL-17 on the apoptosis of
HGC-27 cells was further investigated (Fig. 3). The results revealed that IL-17
significantly inhibited the apoptotic rate of HGC-27 cells in the
IL-17 group compared with the control group (Fig. 3A, B
and E). Moreover, apatinib reversed
the inhibitory effect of IL-17 and promoted the apoptosis of HGC-27
cells. Compared with in the IL-17 group, the cell apoptotic rate
was significantly increased in the IL-17-apatinib group (Fig. 3B, C
and E). Compared with the
IL-17-apatinib group, the cell apoptotic rate in the apatinib group
was significantly increased (Fig.
3C-E).
Effect of apatinib and IL-17 on the
expression levels of Bax, Bcl-2 and caspase-3 in HGC-27 cells
The expression levels of the apoptosis-related
factors Bax, caspase-3 and Bcl-2 were also analyzed using RT-qPCR
and western blotting (Fig. 4). The
RT-qPCR results revealed that the expression levels of the
proapoptotic genes Bax and caspase-3 were significantly
downregulated in IL-17 group compared with the control group
(Fig. 4A and C). However, the expression levels of the
anti-apoptotic gene Bcl-2 in the IL-17 group were significantly
upregulated compared with the control group (Fig. 4B). Moreover, the expression levels
of Bax and caspase-3 were significantly upregulated, while the
expression levels of Bcl-2 were significantly downregulated, in the
IL-17-apatinib and apatinib groups compared with the IL-17 and
control groups. Compared with the IL-17-apatinib, the apatinib
group revealed the upregulated expression levels of Bax and
caspase-3 and the downregulated expression levels of Bcl-2. Western
blotting demonstrated similar results to the RT-qPCR results
(Fig. 4D). Therefore, it these
findings suggested that apatinib and IL-17 may serve important
roles during the progression of cell apoptosis.
Invasive abilities of HGC-27
cells
A Transwell assay was performed to evaluate the
invasive ability of HGC-27 cells (Fig.
5). A significant increase in the invasive cell number was
observed in the IL-17 group compared with the control group
(Fig. 5A, B and E),
which indicated that IL-17 may promote the HGC-27 cell invasive
ability. In addition, the invasive ability of HGC-27 cells was
significantly impaired in the apatinib group compared with the
control group (Fig. 5A, D and E).
Moreover, the invasive cell number in the apatinib group was
reduced compared with the IL-17 and IL-17-apatinib groups (Fig. 5B-E). Notably, compared with the
IL-17 group, the IL-17-apatinib groups also showed a significantly
decreased number of invasive cell (Fig.
5B and C).
Discussion
IL-17 is mainly produced by CD4+ T helper 17 cells
(29). Previous studies have
reported that CD8+ T cells also produced IL-17, which performed
similar functions to the IL-17 produced by CD4+ cells (30,31).
Compared with healthy tissues, significantly upregulated expression
levels of IL-17 in gastric carcinoma tissues were discovered to be
associated with the lymphatic vascular invasion of tumors,
suggesting that IL-17 may promote the progression of tumors
(20,32). However, Iida et al (33) revealed that the survival time of
patients with high IL-17 mRNA expression levels was significantly
longer compared with those with low IL-17 mRNA expression levels by
detecting the IL-17 mRNA expression levels in the peritoneal lavage
of patients with gast fric carcinoma following surgery. Previous
studies have also demonstrated that tumor growth and distant
metastasis in IL-17 deficient mice were significantly increased
compared with those in the control group, suggesting that IL-17
could enhance the antitumor immunity of local T cells in tumors
(34,35).
The present study investigated the effects of IL-17
in gastric carcinoma. The expression levels of IL-17 were analyzed
between gastric carcinoma tissues and para-carcinoma tissues. IL-17
was originally identified as a proinflammatory cytokine that
induced inflammatory cells and factors, and exerted crucial roles
in promoting and maintaining tumor activities by regulating the
promotion of angiogenesis and tumor cell migration (32). The current results revealed that the
expression levels of IL-17 were upregulated in gastric carcinoma
tissues compared with paired para-carcinoma tissues. Thus, it was
indicated that IL-17 may promote the occurrence and be associated
with the development of gastric carcinoma.
Apatinib is a popular anti-angiogenic targeting drug
(36,37) and a small molecule tyrosine kinase
inhibitor of VEGFR (38). Apatinib
has been reported to inhibit the progression of tumors by blocking
the formation of new blood vessels in tumor tissues, and in the
past, it has been used as a combination drug for the treatment of
gastric carcinoma (39). A previous
study reported that apatinib improved the efficacy of fluorouracil
and paclitaxel both in in vitro and in vivo,
suggesting that apatinib may be an efficient and acceptably safe
treatment for late-stage gastric carcinoma (40). The clinical efficacy of apatinib in
treating metastatic gastric carcinoma was also confirmed (27). Thus, the present study further
investigated the effect of apatinib on IL-17 and the potential
mechanism during the development of gastric carcinoma using an in
in vitro model of gastric carcinoma with HGC-27 cells.
In the present study, apatinib demonstrated a
significant suppressive effect on the proliferation of HGC-27 cells
in the IL-17-apatinib and apatinib groups compared with the IL-17
and control groups, which suggested that apatinib may help to
suppress the development of gastric carcinoma. Moreover, the
results of the flow cytometric analysis demonstrated that apatinib
promoted the apoptosis of HGC-27 cells. The expression levels of
Bax and caspase-3 in the IL-17-apatinib group were significantly
upregulated, while Bcl-2 expression levels were significantly
downregulated compared with the control group, which indicated that
the use of apatinib may promote cell apoptosis in gastric
carcinoma. In addition, the results of the Transwell assay
demonstrated that IL-17 promoted the invasive ability of HGC-27
cells, while apatinib inhibited the invasive ability of HGC-27
cells. Apatinib presented an antagonistic effect with IL-17, which
effectively helped to suppress the invasive ability of HGC-27 cells
and thus, by extension, can be hypothesized to inhibit the
development of gastric carcinoma. Therefore, apatinib was suggested
to serve an important role in suppressing the development of
gastric carcinoma by promoting apoptosis and inhibiting the
proliferative and invasive abilities of gastric carcinoma
cells.
Nonetheless, there are some limitations to the
current study. For instance, the present study initially analyzed
the expression levels of IL-17 in 30 paired gastric carcinoma and
para-carcinoma tissues from 30 patients with gastric carcinoma;
this sample size is relatively small, which may cause bias to the
results. Therefore, studies with larger cohorts are required to
verify the findings of the present study. Secondly, the present
study only examined the potential mechanism of IL-17 and apatinib
in the development of gastric carcinoma in an in in vitro
model of HGC-27 cells, and thus additional in in vivo
research is required. Finally, the mechanism of apatinib in
inhibiting the progression of gastric carcinoma via the Bax/Bcl-2
signaling pathway remains to be fully elucidated. Therefore, how
the Bax/Bcl-2 signaling pathway functions in gastric carcinoma and
how apatinib regulates the expression of IL-17 should be further
studied. Nonetheless, the present research expanded the current
knowledge of the effect of apatinib on gastric carcinoma cells
stimulated by IL-17.
In conclusion, gastric carcinoma tissues were
revealed to have upregulated IL-17 expression levels compared with
para-carcinoma tissues. IL-17 was identified to promote the
proliferative and invasive abilities of HGC-27 cells, and inhibit
cell apoptosis, by significantly downregulating the expression
levels of Bax and caspase-3 and upregulating the expression levels
of Bcl-2. Therefore, the current findings suggested that IL-17 may
be closely associated with the occurrence and development of
gastric carcinoma. Conversely, apatinib, used as a treatment drug,
inhibited the proliferative and invasive abilities of HGC-27 cells
and promoted cell apoptosis. Thus, it was suggested that apatinib
may inhibit cell proliferation and invasion, and promote apoptosis
by regulating IL-17 expression via the Bax/Bcl-2 signaling pathway
and may serve a vital role in the development of gastric
carcinoma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ conceived and designed the study. TW and LC
performed the experiments. TW, LC and JZ analyzed and interpreted
the data. TW and JZ wrote the manuscript. JZ, TW and LC confirmed
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study conformed to the Declaration of
Helsinki and was approved by Tianjin Nankai Hospital ethics
committee (approval no. NKYY_YXKT_IRB_2019_101_01). Written
informed consent was obtained from each participant before
surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zuo CH, Xie H, Liu J, Qiu XX, Lin JG, Hua
X and Qin A: Characterization of lymph node metastasis and its
clinical significance in the surgical treatment of gastric cancer.
Mol Clin Oncol. 2:821–826. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Flores-Luna L, Bravo MM, Kasamatsu E,
Lazcano Ponce EC, Martinez T, Torres J, Camorlinga-Ponce M and Kato
I: Risk factors for gastric precancerous and cancers lesions in
Latin American counties with difference gastric cancer risk. Cancer
Epidemiol. 64:101630–101637. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Katai H, Mizusawa J, Katayama H, Morita S,
Yamada T, Bando E, Ito S, Takagi M, Takagane A, Teshima S, et al:
Survival outcomes after laparoscopy-assisted distal gastrectomy
versus open distal gastrectomy with nodal dissection for clinical
stage IA or IB gastric cancer (JCOG0912): A multicentre,
non-inferiority, phase 3 randomised controlled trial. Lancet
Gastroenterol Hepatol. 5:142–151. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li F, Chen Z, Tan B, Liu Y, Zhao Q, Fan L,
Deng H, Ma Y and Li Y: Influential factors and prognostic analysis
of blood vessel invasion in advanced gastric cancer. Pathol Res
Pract. 216:152727–152732. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kanat O, O'Neil B and Shahda S: Targeted
therapy for advanced gastric cancer: A review of current status and
future prospects. World J Gastrointest Oncol. 7:401–410.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yusefi AR, Bagheri Lankarani K, Bastani P,
Radinmanesh M and Kavosi Z: Risk Factors for Gastric Cancer: A
Systematic Review. Asian Pac J Cancer Prev. 19:591–603.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Behnampour N, Hajizadeh E, Zayeri F and
Semnani S: Modeling of influential predictors of gastric cancer
incidence rates in Golestan province, North Iran. Asian Pac J
Cancer Prev. 15:1111–1117. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pan W, Zhang H, Wang L, Zhu T, Chen B and
Fan J: Association between Helicobacter pylori infection and kidney
damage in patients with peptic ulcer. Ren Fail. 41:1028–1034.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sánchez Rodríguez E, Sánchez Aldehuelo R,
Ríos León R, Martín Mateos RM, García García de Paredes A, Martín
de Argila C, Caminoa A, Albillos A and Vázquez-Sequeiros E:
Clinical validation of Endofaster® for a rapid diagnosis
of Helicobacter pylori infection. Rev Esp Enferm Dig. 112:23–26.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liao C, Hu S, Zheng Z and Tong H:
Contribution of interaction between genetic variants of
interleukin-11 and Helicobacter pylori infection to the
susceptibility of gastric cancer. OncoTargets Ther. 12:7459–7466.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Simondurairaj C, Krishnakumar R, Sundaram
S and Venkatraman G: Interleukin-6 receptor (IL-6R) expression in
human gastric carcinoma and its clinical dignificance. Cancer
Invest. 37:293–298. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang Y, Tang M, Wang XG, Gu JH, Zhou LN,
Jin J, Li P, Wang LQ and Chen MB: Elevated serum levels of
interleukin-37 correlate with poor prognosis in gastric cancer. Rev
Esp Enferm Dig. 111:941–945. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Q, Zhang Y, Zhang J, Tao K, Hambly BD
and Bao S: Inverse correlation between Interleukin-34 and gastric
cancer, a potential biomarker for prognosis. Cell Biosci.
10(94)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu Y, Xu Y, Wang Y, Yao Y and Yang J:
Associations between interleukin gene polymorphisms and the risk of
gastric cancer: A meta-analysis. Clin Exp Pharmacol Physiol.
45:1236–1244. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bao S, Hu R and Hambly BD: IL-34, IL-36
and IL-38 in colorectal cancer-key immunoregulators of
carcinogenesis. Biophys Rev. 12:925–930. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Elshazli RM, Salman DO, Kamel MM, Toraih
EA and Fawzy MS: Genetic polymorphisms of IL-17A rs2275913,
rs3748067 and IL-17F rs763780 in gastric cancer risk: Evidence from
8124 cases and 9873 controls. Mol Biol Rep. 45:1421–1444.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li S, Cong X, Gao H, Lan X, Li Z, Wang W,
Song S, Wang Y, Li C, Zhang H, et al: Tumor-associated neutrophils
induce EMT by IL-17a to promote migration and invasion in gastric
cancer cells. J Exp Clin Cancer Res. 38:6–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhao WM, Shayimu P, Liu L, Fang F and
Huang XL: Association between IL-17A and IL-17F gene polymorphisms
and risk of gastric cancer in a Chinese population. Genet Mol Res.
15:1–7. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wu D, Wu P, Huang Q, Liu Y, Ye J and Huang
J: Interleukin-17: A promoter in colorectal cancer progression.
Clin Dev Immunol. 2013:436307–436313. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Meng XY, Zhou CH, Ma J, Jiang C and Ji P:
Expression of interleukin-17 and its clinical significance in
gastric cancer patients. Med Oncol. 29:3024–3028. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang L, Qi Y, Hu J, Tang L, Zhao S and
Shan B: Expression of Th17 cells in breast cancer tissue and its
association with clinical parameters. Cell Biochem Biophys.
62:153–159. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang FH, Shen L, Li J, Zhou ZW, Liang H,
Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, et al: The Chinese
Society of Clinical Oncology (CSCO): Clinical guidelines for the
diagnosis and treatment of gastric cancer. Cancer Commun (Lond).
39:10–40. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Batista TP, Santos CA and Almeida GFG:
Perioperative chemotherapy in locally advanced gastric cancer. Arq
Gastroenterol. 50:236–242. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hammoud MK, Yosef HK, Lechtonen T,
Aljakouch K, Schuler M, Alsaidi W, Daho I, Maghnouj A, Hahn S,
El-Mashtoly SF, et al: Raman micro-spectroscopy monitors acquired
resistance to targeted cancer therapy at the cellular level. Sci
Rep. 8:15278–15288. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bing K, Ming K and Yuan G: Efficacy and
safety of mono chemotherapy and targeted therapy for advanced non
small cell lung cancer patients over 80 years old. J Chengdu Med
Coll. 13:473–476. 2018.(In Chinese).
|
|
26
|
Lin Y, Zhai E, Liao B, Xu L, Zhang X, Peng
S, He Y, Cai S, Zeng Z and Chen M: Autocrine VEGF signaling
promotes cell proliferation through a PLC-dependent pathway and
modulates Apatinib treatment efficacy in gastric cancer.
Oncotarget. 8:11990–12002. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen R, Chen QT and Dong YH: Clinical
efficacy of apatinib in treating metastatic gastric cancer and its
effect on IL-17. Oncol Lett. 17:5447–5452. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔ C(T)) method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Okada K, Fujimura T, Kikuchi T, Aino M,
Kamiya Y, Izawa A, Iwamura Y, Goto H, Okabe I, Miyake E, et al:
Effect of interleukin (IL)-35 on IL-17 expression and production by
human CD4+ T cells. PeerJ. 5(e2999)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Perdomo-Celis F, Feria MG, Taborda NA and
Rugeles MT: A Low Frequency of il 17 producing CD8 (+) T Cells is
associated with persistent immune activation in people living with
HIV despite HAART induced viral suppression. Front Immunol.
9:2502–2515. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Volarić I, Vičić M and Prpić-Massari L:
The Role of CD8+ T cells and their cytokines in the pathogenesis of
psoriasis. Acta Dermatovenerol Croat. 27:159–162. 2019.PubMed/NCBI
|
|
32
|
Wu X, Zeng Z, Xu L, Yu J, Cao Q, Chen M,
Sung JJ and Hu P: Increased expression of IL17A in human gastric
cancer and its potential roles in gastric carcinogenesis. Tumour
Biol. 35:5347–5356. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Iida T, Iwahashi M, Katsuda M, Ishida K,
Nakamori M, Nakamura M, Naka T, Ojima T, Ueda K, Hayata K, et al:
Prognostic significance of IL-17 mRNA expression in peritoneal
lavage in gastric cancer patients who underwent curative resection.
Oncol Rep. 31:605–612. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Surendar J, Frohberger SJ, Karunakaran I,
Schmitt V, Stamminger W, Neumann AL, Wilhelm C, Hoerauf A and
Hübner MP: Adiponectin limits IFN-γ and IL-17 producing CD4 T cells
in obesity by restraining cell intrinsic glycolysis. Front Immunol.
10:2555–2571. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xu LZ, Xie RD, Xie H, Ju JY, Fu XY, Di DL,
Peng MY, Gao W, Zhang YY, Yu D, et al: Chimeric specific antigen
epitope-carrying dendritic cells induce interleukin-17(+)
regulatory T cells to suppress food allergy. Clin Exp Allergy.
50:231–243. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Deng M, Zha J, Jiang Z, Jia X, Shi Y, Li
P, Chen XL, Fang Z, Du Z and Xu B: Apatinib exhibits anti-leukemia
activity in preclinical models of acute lymphoblastic leukemia. J
Transl Med. 16:47–56. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang W, Zhang L, Xie Y, Zhen T, Su G and
Zang Q: Fatal hemoptysis in patients with advanced esophageal
cancer treated with apatinib. OncoTargets Ther. 11:2565–2570.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Peng H, Zhang Q, Li J, Zhang N, Hua Y, Xu
L, Deng Y, Lai J, Peng Z, Peng B, et al: Apatinib inhibits VEGF
signaling and promotes apoptosis in intrahepatic
cholangiocarcinoma. Oncotarget. 7:17220–17229. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wu X and Huang S: HER2-specific chimeric
antigen receptor-engineered natural killer cells combined with
apatinib for the treatment of gastric cancer. Bull Cancer.
106:946–958. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xu Z, Hu C, Chen S, Zhang C, Yu J, Wang X,
Lv H and Cheng X: Apatinib enhances chemosensitivity of gastric
cancer to paclitaxel and 5-fluorouracil. Cancer Manag Res.
11:4905–4915. 2019.PubMed/NCBI View Article : Google Scholar
|