Introduction
Periodontal disease is a chronic and non-specific
infectious disease of tooth-supporting periodontal tissues mainly
caused by Gram-negative bacterial infection. Periodontal ligament
(PDL) is a specialized soft connective tissue that connects the
tooth root surface with the alveolar bone socket. It consists of
different cell populations, including endothelial cells,
fibroblasts, epithelial cells, osteoblasts, the rest of Malassez
and cementoblasts (1).
Porphyromonas gingivalis (Pg), a Gram-negative bacterial
species, has been extensively studied as one of the putative
periodontal pathogens with sufficient evidence (2). Lipopolysaccharide (LPS) is the major
pathogenic factor on the surface of Gram-negative bacteria. LPS
from Pg (Pg-LPS), considered as one of the main virulence factors
of Pg, may induce inflammatory cytokine secretion by periodontal
cells (3,4), damaging periodontal tissue and leading
to the resorption of the alveolar bone (5-7).
Periodontitis is an independent risk factor for
coronary heart disease (8). As a
significant risk factor for cardiovascular disease, vascular
calcification is commonly present in atherosclerosis (9). In the past, vascular calcification was
considered a passive regulator of aging-related diseases; while it
is now generally recognized as an actively regulated bone-like
formation process with the transformation of vascular smooth muscle
cells(VSMCs) into osteoblast-like cells with the expression of
osteogenesis-related proteins (10). It has been indicated that vascular
calcification is correlated with inflammation and is recognized as
a procedural osteogenesis process initiated by endovascular
inflammatory factors (11). The
phenotype of VSMCs is transformed into osteoblast-like cells in the
arterial calcification process under chronic inflammatory
conditions (12). Preliminary
studies of our group suggested that Pg-LPS promoted the abnormal
proliferation and alkaline phosphatase (ALP) activity of rat aortic
smooth muscle cells; the expression of the calcification-related
genes ALP, core-binding factor α1 (Runx2), bone sialoprotein (BSP)
and osteopontin (OPN) increased, further suggesting that Pg-LPS had
a specific effect on calcification (13,14).
However, the microenvironment of the human body is
complex, where the effects of VSMCs on vascular calcification in
the setting of periodontal disease remained to be fully determined.
In the present study, a three-dimensional co-culture model of human
periodontal ligament cells (HPDLCs) and human umbilical artery
smooth muscle cells (HUASMCs) was established by using Transwell
inserts to simulate the complex microenvironment including the
coexistence of periodontitis and vascular calcification. The
present study explored the effects of Pg-LPS on the proliferation,
ALP activity, formation of calcified nodules and mRNA expression of
related calcification genes (ALP, Runx2 and BSP) in HUASMCs under
co-culture conditions.
Materials and methods
Cell culture and characterization
The present study was approved by the Medical Ethics
Committee of the Affiliated Hospital of Qingdao University
(Qingdao, China; no. QYFYWZLL26135). Written informed consent was
obtained from each patient or from the parents/guardians of those
participants who were minors. To obtain HPDLCs, premolars (healthy
orthodontic tooth extractions) without caries, periodontal disease
nor periapical periodontitis freshly extracted between December
2017 and December 2018 were selected. The patients (20 males and 20
females) were between 12 and 25 years old. All the cells from 60
teeth were pooled for different assays. The primary culture of
HPDLCs was established according to a method described previously
(15). First, the cells were
cultured in Dulbecco's Modified Eagle Medium (DMEM; HyClone;
Cytiva) containing 20% fetal bovine serum (FBS) (Gibco; Thermo
Fisher Scientific, Inc.) in an atmosphere under 5% CO2
at 37˚C. The morphology and growth of the cells were observed.
After the cells reached 80% confluency, they were passaged by
trypsinization (Beijing Solarbio Science & Technology Co.,
Ltd.). After passaging, the medium was replaced with medium
containing 10% FBS. The third-generation cells were identified by
vimentin (cat. no. PB9359; Boster Biological Technology) and
keratin (cat. no. BM0031; Boster Biological Technology) staining
with the streptavidin-biotin complex (cat. no. SA1021, Boster
Biological Technology) immunohistochemical method. Cells in the
third to fifth generation with good viability were used for the
subsequent experiments.
Primary HUASMCs (cat. no. 8030) were purchased from
ScienCell Research Laboratories, Inc. and subcultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C with 5%
CO2 after recovery. The third generation HUASMCs were
prepared on cell slides at 1x104 cells/ml for α-smooth
muscle actin (α-SMA; cat. no. BS70000; Bioworld Technology, Inc.)
immunofluorescence staining. The cells in the exponential growth
phase from the third generation to the eighth generation were used
for the subsequent experiments.
Co-culture
HUASMCs were seeded into six-well microplates
(Corning, Inc.) in DMEM containing 10% FBS at 1x104
cells/ml. After 4 h, the cell took adhesion at 37˚C with 5%
CO2, fully expanded to a confluency of 70%. The medium
was then refreshed and the HPDLCs were seeded into Transwell
inserts in DMEM containing 10% FBS, at 1x104 cells/ml.
The non-contact HPDLCs-HUASMCs co-culture system was established
using Transwell inserts (Corning, Inc.) with a pore size of 0.4 µm
(16). Co-cultured cells were not
in direct contact but were able to interact with each other via the
culture fluid for 48 h 37˚C with 5% CO2.
Cell viability assay
The cells were divided into four experimental groups
as follows: i) HUASMC group, which was cultured normally (group C);
ii) HPDLCs-HUASMCs co-culture group (group CO); iii) HUASMCs group
cultured with Pg-LPS (group CP; InvivoGen); and iv) HPDLCs-HUASMCs
co-culture group cultured with Pg-LPS (group CO-P). In addition,
the four groups as above were set up in calcification-inducing
culture medium (groups C-C, CO-C, CP-C, CO-CP, respectively). The
final concentration of Pg-LPS added in the experiment was 1 µg/ml
(13,17). The cells were inoculated according
to the experimental groups. HUASMCs were inoculated in 24-well
microplates (Corning, Inc.) and Transwell inserts containing HPDLCs
were placed on top of the 24-well microplates to establish the
HPDLCs-HUASMCs co-culture system. HUASMCs alone were first cultured
without HPDLCs in DMEM containing 10% FBS for 24 h, followed by
synchronization in serum-free medium for 12 h, and subsequently,
culture was performed for 48 h according to each group's treatment
requirements. The viability of the HUASMCs as a confluence of
70-90% was measured by a Cell Counting Kit-8 (Dojindo Molecular
Technologies, Inc.). For the cell viability assay, 50 µl CCK-8
solution was added to each well containing 500 µl medium (volume
ratio, 10%) for different doses or varying time points; air bubbles
were avoided. The plates were incubated at 37˚C with 5%
CO2 for 2 h, according to the manufacturer's protocol.
The absorbance was measured with a spectrophotometer at a
wavelength of 450 nm. The experimental results were expressed as
the optical density.
ALP assay
The effects of normal medium or
calcification-inducing culture medium on ALP activity of HUASMCs
were measured by an ALP kit (cat. no. A059-2-2, Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's protocol.
The experimental groups and culture methods were the same as those
in the above experiment; the cells for the relevant groups were
inoculated and cultured for 48 h at 37˚C with 5% CO2.
The ALP activity and protein concentration in the supernatant were
measured using a microplate reader at a wavelength of 520 nm. The
results were normalized to the total protein content per sample
using a bicinchoninic acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.) and expressed in fold induction of ALP activity
(18).
Calcification-inducing culture
Calcification-inducing culture medium was prepared
as follows: β-glycerophosphate, vitamin C and dexamethasone (all
from Sigma-Aldrich; Merck KGaA), with final concentrations of 1, 50
and 10 mM, respectively, were added to DMEM containing 5% FBS.
This part of the experiment consisted of eight
groups, which were all cultured in Transwell plates at
1x104 cells/ml. In four groups, the treatment was
performed with normal culture medium (groups C, CO, CP and CO-P)
whereas the four other groups were cultured as follows: HUASMCs
incubated with the calcification-inducing culture medium (group CC)
or with Pg-LPS-containing calcifying culture medium (group C-CP);
HPDLCs-HUASMCs co-cultured cultured in calcifying medium (group
CO-C) or with Pg-LPS-containing calcified medium (group CO-CP). The
cultured cells were inoculated to set up the groups and the medium
was changed every 3 days up to 21 days.
Expression of calcification-related
factors (ALP, Runx2 and BSP) determined by reverse
transcription-quantitative (RT-q) PCR
The different groups were set up according to the
pre-set experimental conditions and cultured for 48 h. The total
cellular RNA was extracted using TRIzol® (Thermo Fisher
Scientific, Inc.) according to the instructions of the
manufacturer. The concentration and purity of RNA were measured
using NanoDrop spectrophotometer (Thermo Fisher Scientific, Inc.).
For each group, 1.0 µg of RNA was reversely transcribed into
complementary (c)DNA in a 20-µl reaction system using PrimerScript™
RT reagent Kit (cat. no. RR047A, Takara Bio, Inc.) a kit with gDNA
Eraser. The total reaction mixture was incubated at 50˚C for 15 min
and 85˚C for 5 sec. The PCR amplification reaction was performed on
1.0 µl of cDNA in a 20-µl reaction system using TB Green™ Premix Ex
Taq™ II(Tli RNaseH Plus) (cat. no. RR820A; Takara Bio, Inc.) on a
7500 qPCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The thermocycling conditions were as follows: Initial
denaturation at 95˚C for 10 min; followed by 40 cycles of 95˚C for
1 min and 60˚C for 1 min. Specific primer sequences for ALP, Runx2,
BSP and GAPDH were designed and synthesized by Sangon Biotech, Co.,
Ltd. and their sequences are provided in Table I. The GAPDH gene expression levels
were calculated using the 2-ΔΔCq
method (19).
 | Table IPrimers used for real-time PCR
analyses. |
Table I
Primers used for real-time PCR
analyses.
| Gene/sequence (5'
to 3') | Product length
(bp) | GenBank accession
no. |
|---|
| Alkaline
phosphatase | 122 | NM_001632.4 |
|
Forward,
AGAATCTGGTGCAGGAATGG | | |
|
Reverse,
AGGCTCAAAGAGACCCATGA | | |
| Runx2 | 107 | NM_004348.3 |
|
Forward,
GTGGACGAGGCAAGAGTTTC | | |
|
Reverse,
TTCCCGAGGTCCATCTACTG | | |
| Bone
sialoprotein | 123 | NM_004967.3 |
|
Forward,
CGGAGGAGACAATGGAGAAG | | |
|
Reverse,
CCACCATTTGGAGAGGTTGT | | |
| GAPDH | 138 | NM_002046.6 |
|
Forward,
GTCTCCTCTGACTTCAACAGCG | | |
|
Reverse,
ACCACCCTGTTGCTGTAGCCAA | | |
Calcification-specific alizarin red S
staining and quantitative analysis of calcified nodules
After culturing for 3 weeks, the Transwell inserts
were removed and the culture medium was discarded, followed by
rinsing twice with pre-warmed PBS, addition of 4% paraformaldehyde
and keeping at room temperature for 30 min. The cells were rinsed
with distilled water twice. Subsequently, 1% alizarin S red dye
liquor (Beijing Solarbio Science & Technology Co., Ltd.) was
added to cover the culture plate's bottom, with microscopic
observation and gentle shaking by hands and keeping at room
temperature for 15 min. After rinsing with distilled water four
times, the solution was gently shaken for 5 min and incubated at
-20˚C for 10 min.
The results were observed under an inverted
microscope. The quantitative analysis method for calcium nodules
was according to Gregory et al (20), with 150 µl of the liquid pipetted
from each tube and transferred to a 96-well microplate (Corning,
Inc.) to determine the absorbance at a wavelength of 405 nm using a
microplate reader.
Statistical analysis
Values are expressed as the mean ± standard
deviation from at least 3 independent experiments. One-way analysis
of variance was performed for comparisons among the groups and
intergroup comparisons were performed using Bonferroni's correction
using SPSS statistical software (version 22.0; IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of the cells
The HPDLCs were positive and negative for
anti-vimentin (Fig. 1A) and
anti-keratin staining (Fig. 1B),
respectively. α-SMA was highly expressed in HUASMCs (Fig. 1C). The HPDLCs in the Transwell
inserts were adherent and they exhibited long spindle or radial
arrangements after being inoculated for ~12 h (Fig. 2A). The HUASMCs in the culture plate
were adherent and were polygonal or spindle-shaped after
inoculation for 16 h (Fig. 2B).
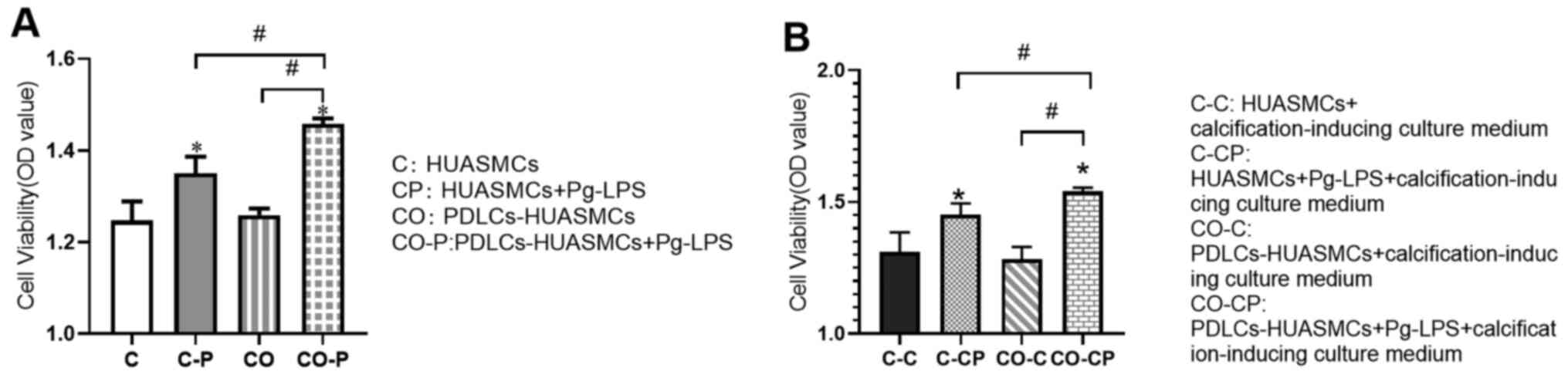
Pg-LPS-stimulated HUASMC proliferation
is increased in the co-culture system
The effect of Pg-LPS treatment for 48 h on HUASMCs
to increase the proliferation of co-cultured HUASMCs was greater
than that of monoculture HUASMCs and the difference was
statistically significant. However, there was no significant
difference in the proliferation of HUASMCs in mono- vs. co-culture
without Pg-LPS stimulation (Fig.
3A). The trends for the co-culture system were similar in the
calcification-inducing culture medium (Fig. 3B).
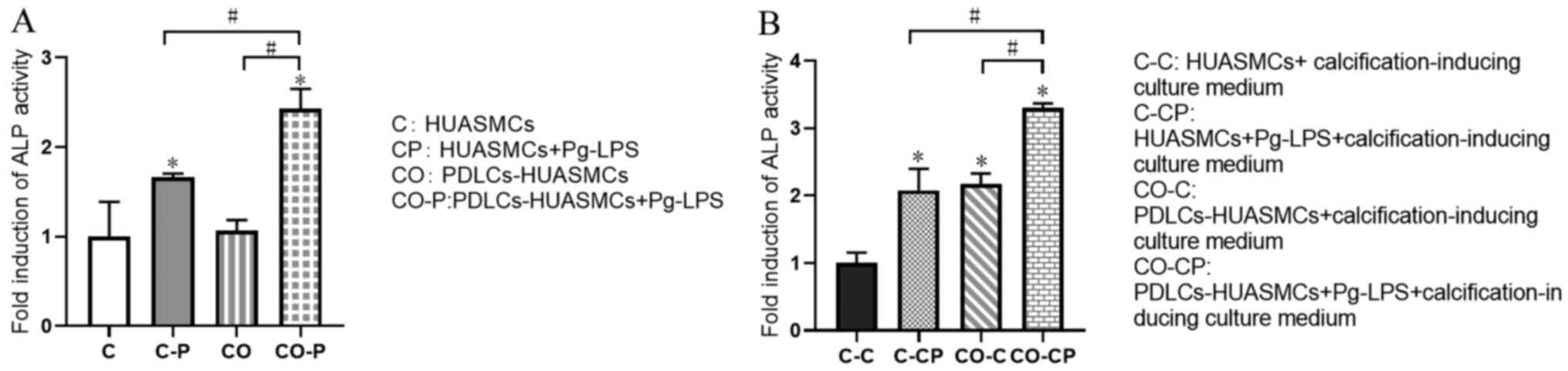
Effects of normal medium and
calcification-inducing culture medium on ALP activity of
HUASMCs
After Pg-LPS stimulation for 48 h, the ALP activity
in the supernatant of HUASMCs under co-culture conditions was
enhanced and higher than that of the HUASMCs in monoculture, with a
significant difference. However, there was no significant
difference in the ALP activity in the supernatant of HUASMCs in
mono- vs. co-culture without Pg-LPS stimulation (Fig. 4A). This phenomenon was similar in
the groups with Pg-LPS stimulation as in the calcification-inducing
culture medium. However, co-culturing the cells in
calcification-inducing culture medium exhibited significantly
higher ALP activity compared with HUASMCs in calcification-inducing
culture medium, with a significant difference. (Fig. 4B).
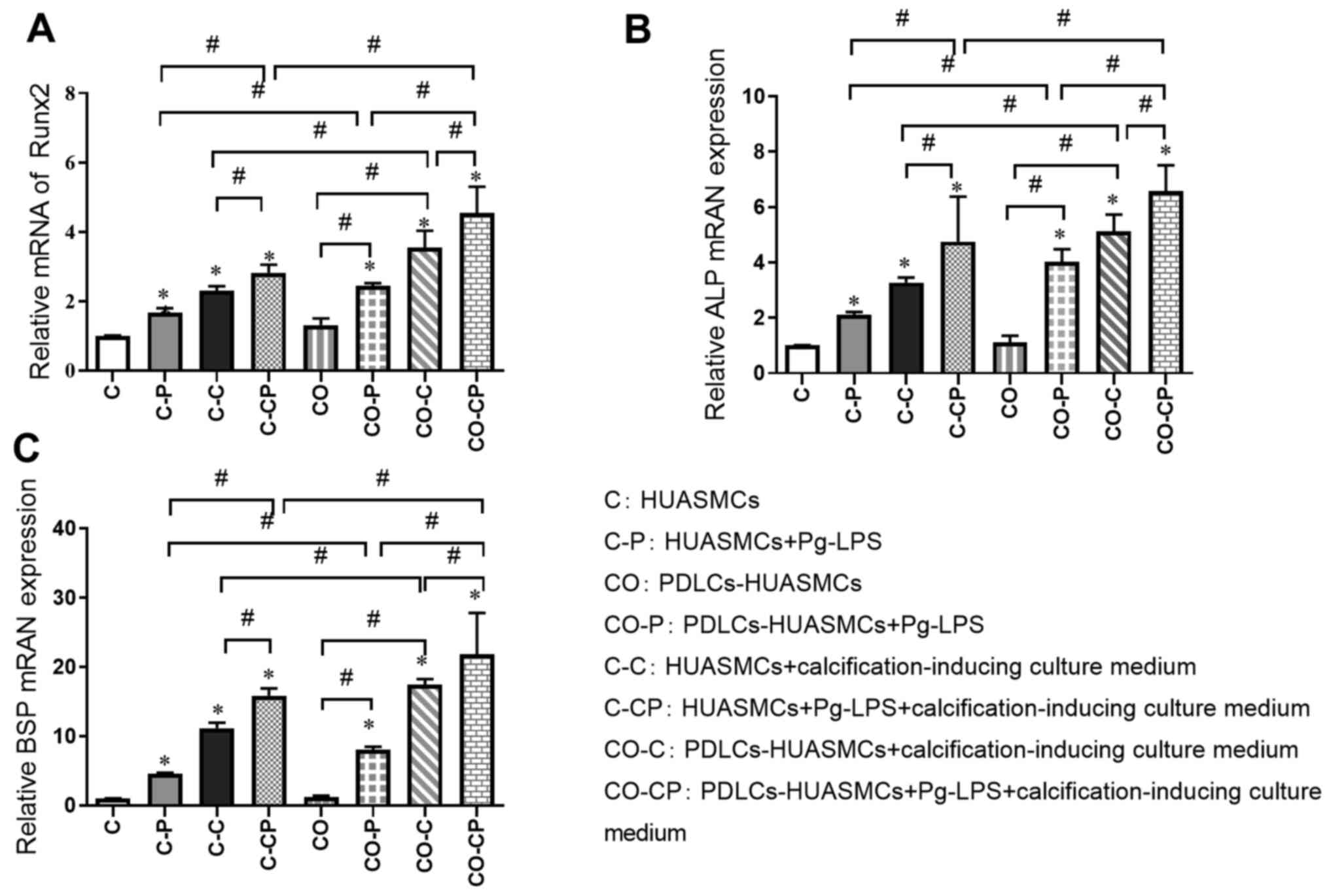
Relative quantification of osteogenic
gene induction by Pg-LPS in HUASMCs in the co-culture system
The relative expression of calcification-related
factors was detected by RT-qPCR in the different experimental
groups after incubation for 48 h. The expression of Runx2, ALP and
BSP was significantly higher in the groups with calcification
induction medium (C-C, C-CP, CO-C and CO-CP) than in the groups
with normal culture medium (C, C-P, CO and CO-P, respectively) and
significantly higher in groups with Pg-LPS (C-P, C-CP, CO-P and
CO-CP) than in the groups without Pg-LPS (C, C-C, CO and CO-C,
respectively). In addition, the expression of those
calcification-related factors in group CO was slightly higher than
that in group C, but the difference was not significant. The
calcification effect in each co-culture group (CO-P, CO-C and
CO-CP) was higher than that in each monoculture group (C-P, C-C and
C-CP, respectively; Fig. 5).
Effects of Pg-LPS on calcified nodule
formation by HUASMCs in the co-culture system
The cells were co-cultured with Pg-LPS and
calcification-inducing medium and then stained with alizarin red S
to detect the formation of calcified nodules. In the inverted
phase-contrast microscopy images, the calcified nodules were
scattered in the C-P and CO-P groups and were almost absent in the
C and CO groups. All of the groups under calcification induction or
calcification induction + Pg-LPS (1 µg/ml) conditions exhibited
many calcified nodules, whilst the co-cultured CO-C and CO-CP
groups had slightly more calcified nodules than those cultured in
DMEM medium (Fig. 6).
The quantitative detection of calcified nodules
indicated that calcium nodules formation in the group with general
culture in DMEM medium and Pg-LPS was more extensive than that in
the corresponding control group. Each calcification induction group
exhibited significantly more calcium nodule formation than the
corresponding general culture group in DMEM medium. In the normal
culture medium, the extent of calcium nodule formation by HUASMCs
in group C and group CO was not significantly different. However,
under the calcification induction conditions, calcium nodule
formation in group CO-C was more extensive than in group C-C,
whilst the largest amount of calcium nodules was detected in the
CO-CP group (Fig. 6).
Discussion
Vascular calcification is a common pathological
process of aging and various conditions including atherosclerotic
coronary artery disease and diabetes. As one of the primary risk
factors and early signs of cardiovascular disease, it mainly
includes intima and tunica media calcification, both of which
frequently occur at the same time. VSMCs were identified as the
major cell type involved in the process of vascular calcification,
possessing the ability to differentiate into osteoblast-like
phenotype under the stimulation of pathological factors, and to
synthesize and secrete related proteins and transcription factors
promoting bone formation, as well as to express active ALP
(21,22). The specific phenotypic
transformation mechanism in VSMCs has remained to be fully
elucidated, but inflammation and immune response may have an
important role (23).
A comprehensive study suggested that periodontitis
has a particular role in promoting vascular calcification of
coronary heart disease, and coronary heart disease, in turn,
aggravates the destruction of periodontal tissue to a certain
extent (24-28).
As one of the major pathogenic bacteria in periodontitis, Pg has a
higher detection rate in the atherosclerotic plaques from patients
with severe periodontitis and coronary heart disease than in
patients with mild periodontitis and coronary heart disease. Yang
et al (28) reported that Pg
induced VSMC calcification and activity in mice and directly
stimulated in vitro cultured aortic vessels to cause
vascular wall calcinosis. Pg-LPS is an important pathogenic factor
of bacteria. Han et al (29)
demonstrated that low concentrations (<100 µg/ml) of
Escherichia coli LPS promoted the abnormal proliferation of
VSMCs in rats. A previous study by our group suggested that one day
of culture with Pg-LPS at each concentration between 1 ng/ml and
µg/ml was able to promote the abnormal proliferation and ALP
activity of VSMCs, affecting the expression of
calcification-related genes (Runx2, ALP, BSP and OPN) (13). These results indicated that Pg-LPS
affected VSMC calcification and provided indirect evidence of
Pg-LPS promoting vascular tube calcification. Since the body's
internal environment is complex, the effect of Pg-LPS on VSMCs
in vivo requires further study.
At present, co-culture is a commonly used cell
biology technique (30,31). Cell co-culture using Transwell
inserts is able to achieve the interaction effect of cells in the
upper and lower culture medium through a porous membrane, which
more fully simulates the in vivo environment. In the present
experiments, HUASMCs were selected as a cell model, and they were
more convincing in the study of the relationship between human
periodontitis and cardiovascular disease than the rat VSMCs used in
the past (13). The co-culture
system model of HPDLCs and HUASMCs using a Transwell chamber was
selected to simulate the coexistence of periodontitis and vascular
calcification in vivo. Of note, the morphology and growth
rate of the two types of cells in the co-culture system did not
change significantly compared with HUASMCs in monoculture, and this
model more fully simulated the environment in vivo. Pg-LPS
is an independent factor in periodontal disease and the co-culture
system was able to simulate the environment of periodontal disease
more realistically. It was reported that Pg-LPS stimulated HPDLCs
to secrete IL-6, IL-8, TGF and other inflammatory factors (32-34).
Thammasitboon et al (35)
indicated that macrophages had an important role in LPS-induced
osteoblast and PDLC apoptosis. Therefore, it was speculated in the
present study that one of the reasons of co-cultured HUASMCs
exhibiting abnormal proliferation was due to the effect of the
HPDLCs. Periodontitis may promote the abnormal proliferation of
VSMCs, whereby the abnormal proliferation of VSMCs may result in
the formation of new intima, arteriosclerosis and restenosis
(21,36).
The expression of calcification-related genes was
detected in the present study to further investigate the effect of
Pg-LPS on HUASMC calcification. ALP is an early differentiation
marker of osteoblasts and one of the confirmed phenotypic markers
of osteoblast-like cells. It is also indispensable for osteoblast
activity and bone formation (37).
BSP is one of the acid glycoproteins secreted mainly by
osteoblasts. It is able to activate osteoblasts or guide them to
promote calcification. Its expression is an essential marker of
calcification and matrix deposition (38). Runx2 is the initiation factor
regulating the differentiation of undifferentiated mesenchymal stem
cells into osteoblasts. Studies have indicated that the cranial
cells of Runx2-/- mice are not
able to differentiate into osteoblasts when cultured in
vitro (39). Runx2 is also a
key transcription factor regulating the directional differentiation
of osteoblasts and chondrocytes and has a critical regulatory role
in calcification. The expression of Runx2 in the atherosclerotic
plaque is considered a sign for the initiation of vascular
calcification (13,40).
The present study suggested that ALP activity in
HUASMCs increased after the action of Pg-LPS for 48 h, and the ALP
activity of HUASMCs in the co-culture group was stronger than that
in the monoculture group. Pg-LPS and calcification-inducing medium
promoted the expression of ALP, BSP and Runx2 in HUASMCs, and the
calcification-inducing medium had a more obvious promoting effect.
In addition, when both factors were combined, the promotion effect
was the strongest. This was similar to a previous study by our
group, where Pg-LPS promoted the abnormal proliferation and ALP
activity of VSMCs and affected the expression of
calcification-related genes (Cbfα1, ALP, BSP and OPN). Yin et
al (41) indicated that LPS
stimulated VSMC proliferation via phenotypic modulation and LPS
promoted VSMC proliferation through the Toll-like receptor 4/Rac
family small GTPase 1/Akt signaling pathway. ALP promotes
ossification by dephosphorylation, destroying mineralization
inhibitors and acting as a calcium-binding protein or phosphate
transporter. Higher ALP activity suggests ossification and
transformation (42). In addition,
the promoting effect of Pg-LPS and calcification-inducing solution
in the co-culture group was significantly higher than that in the
monoculture group. It may be speculated that HPDLCs have a
synergistic effect with Pg-LPS. HPDLCs may secrete certain
inflammatory factors under the effect of Pg-LPS and aggravate the
calcification of SMCs, and the calcification effect of co-culture
HUASMCs was not only due to Pg-LPS but also because of the effect
of HPDLCs. HPDLCs were able to promote the differentiation of
HUASMCs into the osteoblast-like phenotype under the stimulation of
Pg-LPS, indicating that vascular calcification may be induced and
aggravated by periodontitis. Further studies by our group will
examine the specific regulatory mechanism of LPS on ALP activity
and the expression of calcification-related factors.
Alizarin red S staining is an effective method for
determining the formation of calcified nodules. After 21 days of
cell culture, calcium nodule staining and quantitative analysis
indicated that both Pg-LPS and calcification-inducing solution
promoted the formation of calcified nodules. Regarding the groups
cultured in calcification-inducing medium, Pg-LPS increased the
calcium nodules in the co-culture group compared to the HUASMCs in
monoculture. These results indicated that Pg-LPS was able to
promote HUASMC calcification, and in the presence of Pg-LPS and the
calcification-inducing medium, the co-culture system had a more
significant effect to stimulate HUASMC calcification. These results
also confirmed that HPDLCs had a specific role in promoting HUASMC
calcification under the effect of Pg-LPS.
In the present study, under the inflammatory
conditions due to Pg-LPS stimulation, the proliferation and ALP
activity of HUASMCs in the co-culture group increased significantly
and the expression of calcification-related genes (ALP, Runx2 and
BSP) was also higher compared to the HUASMCs in monoculture. The
high immune activity of Pg-LPS may explain these results. It
changed the survival micro-environment of the two cell types and
caused them to produce various inflammatory factors, with a
synergistic role of the pro-inflammatory factors, aggravating the
calcification of HUASMCs. The lack of western blot analysis being
performed in the present study to investigate protein expression
was a limitation of the present study and future studies should
perform this to verify the present results. However, the human body
is a complex organism and the growth and reproduction of cells are
affected by numerous factors, such as the microenvironment and
intercellular interactions. Therefore, the in vitro culture
environment differed significantly from the body's internal
environment. The in vitro experiments alone were not able to
fully explain the mechanisms and signaling pathways responsible for
the effect of Pg-LPS on VSMCs, necessitating further studies. The
co-culture system was used to simulate the environment in
vitro, which has the advantage of laying a better foundation
for subsequent in vivo experiments compared with single-cell
culture in vitro. Our group also considers constructing a
better co-culture system or animal model to study the relationship
of periodontitis and vascular calcification. The co-culture system
may have an important role to stimulate HPDLCs to secrete IL-6,
IL-8, TGF and other inflammatory factors. In the future, a better
co-culture system may be established to study the relationship of
inflammation factors with Pg-LPS in terms of protein and RNA
expression.
In conclusion, Pg-LPS was able to induce the
proliferation of HUASMCs and its effect was more significant in the
HPDLCs-HUASMC co-culture system. Pg-LPS may stimulate calcification
by increasing the ALP activity of HUASMCs and upregulating the
expression of its calcification-related genes (ALP, Runx2 and BSP).
This effect was greater in the co-culture system. The present
results also indirectly demonstrated that eliminating inflammation
may be an influential factor to inhibit vascular calcification.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant no. 81100755), the Health Department of
Shandong Province (grant no. 2016WS0252) and the Key Laboratory of
Stomatology and Biomedicine of Shandong Province (grant no.
SDKQ201401).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL, SS and GL contributed equally to the study
design, manuscript preparation and data collection. WS, PS and WJ
made contributions to the study design and data acquisition and
drafted the manuscript. JD contributed to the study conception and
critically revised the manuscript. KP supervised the research,
oversaw the collection of results and data interpretation and
critically revised the manuscript. JL and KP check and approve the
authenticity of the raw data. All authors read and approved the
final manuscript as submitted and agree to be accountable for all
aspects of the work.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Affiliated Hospital of Qingdao University
(Qingdao, China; no. QYFYWZLL26135). Written informed consent was
obtained from each patient or from the parents/guardians of those
participants who were minors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Safi IN, Mohammed Ali Hussein B and
Al-Shammari AM: In vitro periodontal ligament cell expansion by
co-culture method and formation of multi-layered periodontal
ligament-derived cell sheets. Regen Ther. 11:225–239.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gorasia DG, Glew MD, Veith PD and Reynolds
EC: Quantitative proteomic analysis of the type IX secretion system
mutants in Porphyromonas gingivalis. Mol Oral Microbiol.
35:78–84. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Maldonado RF, Sá-Correia I and Valvano MA:
Lipopolysaccharide modification in Gram-negative bacteria during
chronic infection. FEMS Microbiol Rev. 40:480–493. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Le Sage F, Meilhac O and Gonthier MP:
Porphyromonas gingivalis lipopolysaccharide induces
pro-inflammatory adipokine secretion and oxidative stress by
regulating Toll-like receptor-mediated signaling pathways and redox
enzymes in adipocytes. Mol Cell Endocrinol. 446:102–110.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhao M, Dai W, Wang H, Xue C, Feng J, He
Y, Wang P, Li S, Bai D and Shu R: Periodontal ligament fibroblasts
regulate osteoblasts by exosome secretion induced by inflammatory
stimuli. Arch Oral Biol. 105:27–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Naruishi K: Carotenoids and periodontal
infection. Nutrients. 12(269)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Groeger S, Jarzina F, Windhorst A and
Meyle J: Influence of retinoic acid on human gingival epithelial
barriers. J Periodontal Res. 51:748–757. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ehara S and Yoshiyama M: Quantitative
analyses of coronary artery calcification by using clinical
cardiovascular imaging. Clin Calcium. 20:1686–1692. 2010.PubMed/NCBI
|
|
9
|
Kendrick J and Chonchol M: The role of
phosphorus in the development and progression of vascular
calcification. Am J Kidney Dis. 58:826–834. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lanzer P, Boehm M, Sorribas V, Thiriet M,
Janzen J, Zeller T, St Hilaire C and Shanahan C: Medial vascular
calcification revisited: Review and perspectives. Eur Heart J.
35:1515–1525. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bessueille L and Magne D: Inflammation: A
culprit for vascular calcification in atherosclerosis and diabetes.
Cell Mol Life Sci. 72:2475–2489. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ruiz JL, Hutcheson JD and Aikawa E:
Cardiovascular calcification: current controversies and novel
concepts. Cardiovasc Pathol. 24:207–212. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu G, Deng J, Zhang Q, Song W, Chen S,
Lou X, Zhang P and Pan K: Porphyromonas gingivalis
Lipopolysaccharide stimulation of vascular smooth muscle cells
activates proliferation and calcification. J Periodontol.
87:828–836. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li S, Fan SX and McKenna TM: Vascular
smooth muscle cells on Matrigel as a model for LPS-induced
hypocontractility and NO formation. Am J Physiol. 272:H576–H584.
1997.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tang XL and Meng HX: The inhibitory effect
of calcitriol on the proliferation of hPDLCs populations of
different vitamin D receptor Fok I genotypes. Shanghai Kou Qiang Yi
Xue. 18:422–426. 2009.PubMed/NCBI(In Chinese).
|
|
16
|
Dong X, Wang YS, Dou GR, Hou HY, Shi YY,
Zhang R, Ma K, Wu L, Yao LB, Cai Y, et al: Influence of Dll4 via
HIF-1α-VEGF signaling on the angiogenesis of choroidal
neovascularization under hypoxic conditions. PLoS One.
6(e18481)2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang YL, Pan KQ, Sun Y and Deng J: Effect
of lipopolysaccharide on the expression of ALP, BSP, DSPP in rat
dental pulp cells. Shanghai Kou Qiang Yi Xue. 23:431–435.
2014.PubMed/NCBI(In Chinese).
|
|
18
|
Young N, Mikhalkevich N, Yan Y, Chen D and
Zheng WP: Differential regulation of osteoblast activity by Th cell
subsets mediated by parathyroid hormone and IFN-gamma J. Immunol.
175:8287–8295. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Aonuma H, Ogura N, Takahashi K, Fujimoto
Y, Iwai S, Hashimoto H, Ito K, Kamino Y and Kondoh T:
Characteristics and osteogenic differentiation of stem/progenitor
cells in the human dental follicle analyzed by gene expression
profiling. Cell Tissue Res. 350:317–331. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gregory CA, Gunn WG, Peister A and Prockop
DJ: An Alizarin red-based assay of mineralization by adherent cells
in culture: Comparison with cetylpyridinium chloride extraction.
Anal Biochem. 329:77–84. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liberman M, Pesaro AE, Carmo LS and
Serrano CV Jr: Vascular calcification: Pathophysiology and clinical
implications. Einstein (Sao Paulo). 11:376–382. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Burton DG, Matsubara H and Ikeda K:
Pathophysiology of vascular calcification: Pivotal role of cellular
senescence in vascular smooth muscle cells. Exp Gerontol.
45:819–824. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Davis-Dusenbery BN, Wu C and Hata A:
Micromanaging vascular smooth muscle cell differentiation and
phenotypic modulation. Arterioscler Thromb Vasc Biol. 31:2370–2377.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bahekar AA, Singh S, Saha S, Molnar J and
Arora R: The prevalence and incidence of coronary heart disease is
significantly increased in periodontitis: A meta-analysis. Am Heart
J. 154:830–837. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Humphrey LL, Fu R, Buckley DI, Freeman M
and Helfand M: Periodontal disease and coronary heart disease
incidence: A systematic review and meta-analysis. J Gen Intern Med.
23:2079–2086. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Khader YS, Albashaireh ZS and Alomari MA:
Periodontal diseases and the risk of coronary heart and
cerebrovascular diseases: A meta-analysis. J Periodontol.
75:1046–1053. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang YM, Zhong LJ, He BX, Nie J, Wang X
and Li WC: Study on the correlation between coronary heart disease
and chronic periodontitis. Zhonghua Liu Xing Bing Xue Za Zhi.
27:256–259. 2006.PubMed/NCBI(In Chinese).
|
|
28
|
Yang WW, Guo B, Jia WY and Jia Y:
Porphyromonas gingivalis-derived outer membrane vesicles
promote calcification of vascular smooth muscle cells through
ERK1/2-RUNX2. FEBS Open Bio. 6:1310–1319. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Han F, Hou N, Liu Y, Huang N, Pan R, Zhang
X, Mao E and Sun X: Liraglutide improves vascular dysfunction by
regulating a cAMP-independent PKA-AMPK pathway in perivascular
adipose tissue in obese mice. Biomed Pharmacother.
120(109537)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gharaibeh B, Lu A, Tebbets J, Zheng B,
Feduska J, Crisan M, Péault B, Cummins J and Huard J: Isolation of
a slowly adhering cell fraction containing stem cells from murine
skeletal muscle by the preplate technique. Nat Protoc. 3:1501–1509.
2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Saldarriaga Fernández IC, Busscher HJ,
Metzger SW, Grainger DW and van der Mei HC: Competitive time- and
density-dependent adhesion of staphylococci and osteoblasts on
crosslinked poly(ethylene glycol)-based polymer coatings in
co-culture flow chambers. Biomaterials. 32:979–984. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sun Y, Shu R, Li CL and Zhang MZ:
Gram-negative periodontal bacteria induce the activation of
Toll-like receptors 2 and 4, and cytokine production in human
periodontal ligament cells. J Periodontol. 81:1488–1496.
2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tintut Y, Patel J, Parhami F and Demer LL:
Tumor necrosis factor-alpha promotes in vitro calcification of
vascular cells via the cAMP pathway. Circulation. 102:2636–2642.
2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yamamoto T, Kita M, Oseko F, Nakamura T,
Imanishi J and Kanamura N: Cytokine production in human periodontal
ligament cells stimulated with Porphyromonas gingivalis. J
Periodontal Res. 41:554–559. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Thammasitboon K, Goldring SR and Boch JA:
Role of macrophages in LPS-induced osteoblast and PDL cell
apoptosis. Bone. 38:845–852. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sadowitz B, Seymour K, Gahtan V and Maier
KG: The role of hyaluronic acid in atherosclerosis and intimal
hyperplasia. J Surg Res. 173:e63–e72. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Boström KI, Rajamannan NM and Towler DA:
The regulation of valvular and vascular sclerosis by osteogenic
morphogens. Circ Res. 109:564–577. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Khan SZ, Kokubu E, Matsuzaka K and Inoue
T: Behaviour of rat-cultured dental pulp cells in three-dimensional
collagen type-1 gel in vitro and in vivo. Aust Endod J. 39:137–145.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ciceri P, Elli F, Cappelletti L, Tosi D,
Savi F, Bulfamante G and Cozzolino M: Osteonectin (SPARC)
expression in vascular calcification: In vitro and ex vivo studies.
Calcif Tissue Int. 99:472–480. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pang J, Zhang Y, Ke J, Yu Q, He W and Wu
B: Upregulation of dentin matrix protein 1 promoter activities by
core binding factor alpha1 in human dental pulp stem cells. Biochem
Biophys Res Commun. 357:505–510. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yin Q, Jiang D, Li L, Yang Y, Wu P, Luo Y,
Yang R and Li D: LPS promotes vascular smooth muscle cells
proliferation through the TLR4/Rac1/Akt signalling pathway. Cell
Physiol Biochem. 44:2189–2200. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lv X-C, Bi L-J, Jiang Y and Wang X:
Effects of icariin on the alkline phosphatase activity of human
periodontal ligament cells inhibited by lipopolysaccharide. Mol Med
Rep. 8:1411–1415. 2013.PubMed/NCBI View Article : Google Scholar
|