Introduction
Depression is a mental health condition with a high
clinical incidence. The prevalence of depression in younger
patients (age, 12-17 years; incidence rate >12% in the USA in
2015) is higher compared with elderly ones (age, ≥18 years;
incidence rate <10% in the USA in 2015) (1,2).
Globally, depression is considered to be one of the single largest
contributors to non-fatal health losses, accounting for 7.5% of all
years lived with disability (YLD). It has been reported that ~80%
of depression cases occur in low to middle-income countries
(3). There are several typical
antidepressant drugs in common usage, including selective serotonin
reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs),
serotonin-noradrenergic reuptake inhibitors (SNRIs) and monoamine
oxidase inhibitors (MAOIs) (4).
However, ~30-40% of patients do not respond to these drugs during
the first 4-6 weeks of treatment. In addition, the efficacy of
these drugs is often controversial, while a number of them are
accompanied by numerous side effects, including apathy, sedation
and cognitive and sleep disorders (5,6).
Therefore, more effective and better tolerated antidepressants are
urgently needed to treat depression.
Angelica sinensis (Oliv.) Diels (AS) is a
well-known traditional Chinese medicine, which has been applied as
a treatment for gynecological diseases. The antidepressant-like
effects of AS extracts have also been reported (7,8).
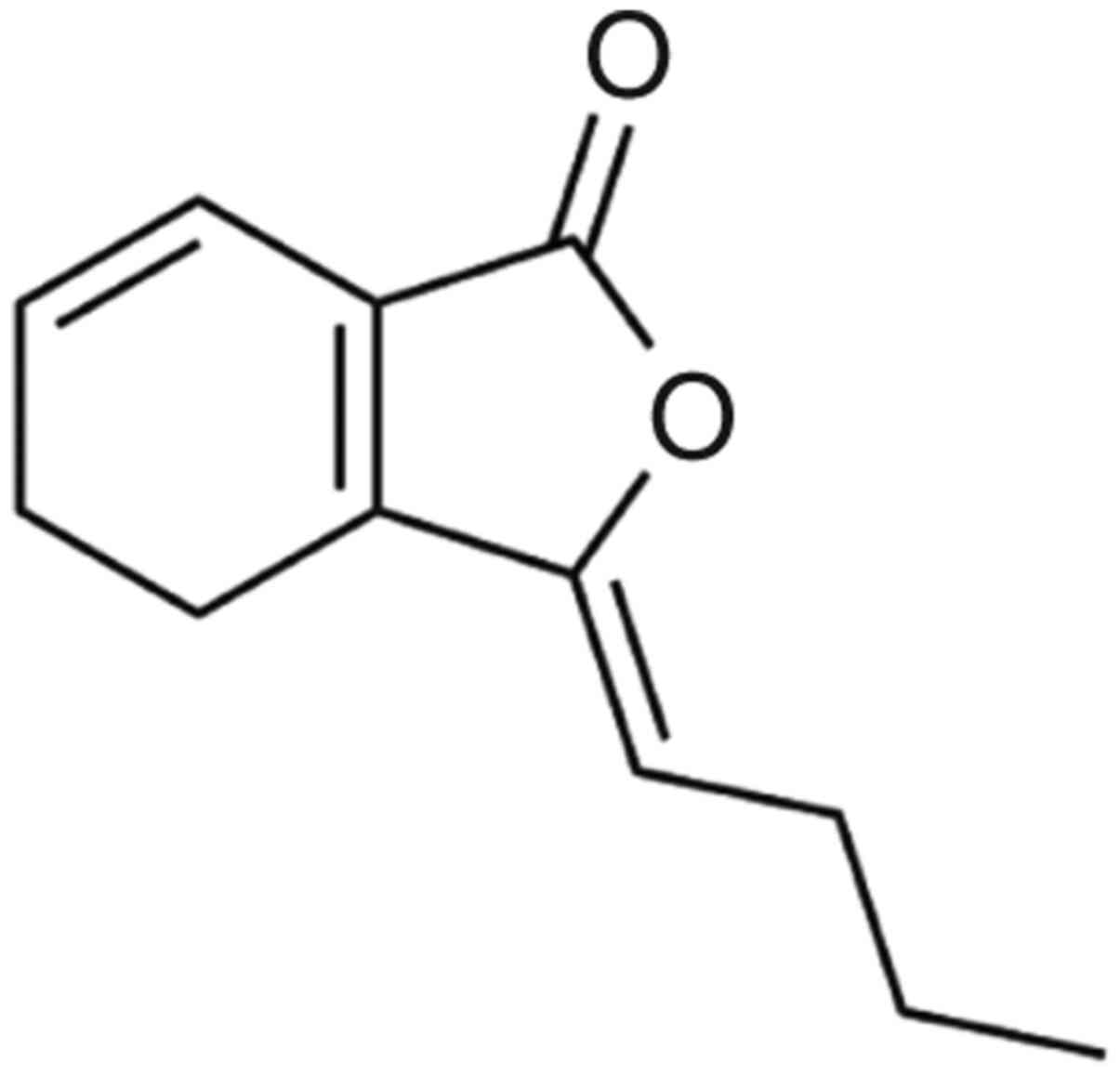
Z-ligustilide (LIG; Fig.
1) is the main component of AS, accounting for 2-3.5% of all
composite compounds (9-13).
Studies have indicated that LIG may significantly improve blood
circulation, protect against nerve damage and attenuate painful
behavior (11,14-17).
The neuroprotective effects of LIG have also been reported in
several animal models of cerebral ischemia, indicating that LIG may
reduce the infarct size in the ischemic area, decrease edema in the
brain and improve neurobehavioral defects (18). Furthermore, LIG is thought to
protect against hypoperfusion-induced nerve damage in the cerebral
cortex, inhibit cortical neuron apoptosis and maintain the
integrity of the neuronal structure (19).
The active antidepressant constituents of AS have
been identified and LIG is thought to be one of the most important
(20,21). A previous study demonstrated that
LIG easily penetrates the blood-brain barrier and quickly enters
the brain (22). By applying
pharmaceutical analysis and pharmacokinetics, LIG is expected to be
a major constituent of antidepressants (23,24).
Progesterone and allopregnanolone are neurosteroids,
mediating both the transport of cholesterol from the outer to the
inner mitochondrial membrane and the activation of a series of
enzymatic reactions to regulate the biosynthesis of neurosteroids
(25,26). Studies indicate that reduced levels
of progesterone and allopregnanolone are associated with the
development of various mental disorders, including anxiety and
depression (27,28). Conversely, exogenous administration
of progesterone and allopregnanolone may significantly improve
depression (29-32).
The present study aimed to evaluate the
antidepressant-like effects of LIG and assess its association with
the secreted levels of neurosteroids (progesterone and
allopregnanolone) in the brain using a rat model.
Materials and methods
Animals
A total of 80 male Sprague-Dawley (SD) rats (weight,
160-200 g; age, 7 weeks) were purchased from the Guangdong Medical
Laboratory Animal Center (Guangzhou, China). Prior to experimental
procedures, all animals were maintained under controlled
environmental conditions for ≥7 days (temperature, 22±2˚C;
humidity, 45-65%; 12-h light/dark cycle) with free access to food
and water. All animal experiments were conducted according to the
Guidelines for the Management and Use of Experimental Animals
(33). The experimental procedures
were approved by the Animal Care Research Council of Guangdong
Pharmaceutical University and complied with the principles of
Laboratory Animal Care (34) to
minimize the suffering of animals.
Drug preparation and
administration
LIG (purity, ≥98%) was obtained from Nanjing Dasf
Biotech Ltd. Sertraline (Ser) was purchased from Sigma-Aldrich
(Merck KGaA). Mifepristone (Mp) and finasteride (Fin) were acquired
from Shanghai Yien Chemical Technology Co., Ltd, and chloral
hydrate (CH) from Qingdao Yulong Algae Co., Ltd. Rats were
randomized into eight groups. Rats in the normal [not exposed to
chronic unpredictable mild stress (CUMS)] and vehicle-treated
groups (exposed to CUMS) were treated with 0.9% physiological
saline. For the behavioral tests, rats in the Ser positive control
group were treated with 15 mg/kg Ser (35,36).
Each behavioral trial consisted of two parts: The
pharmacodynamic evaluation of LIG and the evaluation of the effect
of Mp and Fin antagonists on LIG. For the pharmacodynamic
evaluation of LIG, rats were divided into three groups, where rats
were intraperitoneally (i.p.) injected with 10, 20 or 40 mg/kg LIG,
as previously described (21,37).
For the evaluation of the effect of Mp and Fin antagonists on LIG,
rats were divided into the following two groups: i) LIG (20 mg/kg)
+ Mp (30 mg/kg) (38,39); and ii) LIG (20 mg/kg) + Fin (50
mg/kg) (40,41). LIG, Ser, Mp and Fin were diluted in
0.9% physiological saline. LIG and Ser were administered once
daily, while Mp and Fin were administered 1 h prior to the
behavioral tests.
CUMS
For CUMS, all rats were treated as previously
described (21,42). Briefly, single housed rats were
exposed to one stress stimuli per day, which could not be predicted
by the animal (Table I). The full
experimental procedure lasted for 41 days. Stress stimuli induction
occurred from day 1-35 followed by drug administration from day
36-48. The forced swimming test (FST), sucrose preference test
(SPT) and open field test (OFT) were performed 1 h following drug
administration on day 43-48 (Fig.
2).
 | Figure 2Schematic depiction of the
application of CUMS and behavioral tests. Following CUMS and drug
administration, the animals underwent FST (day 43), SPT (day 46)
and OFT (day 48). Ser (15 mg/kg; i.p.) and LIG (10, 20, 40 mg/kg;
i.p.) were administered daily from day 36 to 48. CUMS, chronic
unpredictable mild stress; FST, forced swimming test; SPT, sucrose
preference test; OFT, open field test; Ser, sertraline; LIG,
Z-ligustilide. |
 | Table ISchedule of chronic unpredictable
mild stressors applied. |
Table I
Schedule of chronic unpredictable
mild stressors applied.
| Treatment week | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday |
|---|
| 1st | 3 | 1 | 4 | 5 | 2 | 7 | 8 |
| 2nd | 1 | 8 | 2 | 6 | 4 | 5 | 7 |
| 3rd | 2 | 7 | 3 | 1 | 8 | 6 | 4 |
| 4th | 4 | 5 | 1 | 8 | 7 | 3 | 2 |
| 5th | 5 | 2 | 6 | 7 | 1 | 4 | 8 |
FST (day 43)
FST is a behavioral test used to evaluate the effect
of antidepressants. This test was carried out as previously
described (21,43,44).
Briefly, all rats were placed in separated glass cylinders filled
with 20 cm of freshwater at 24±2˚C and forced to swim. Each animal
was forced to swim for 6 min and the duration of immobility in the
last 4 min was recorded. A rat was considered immobile when
stationary or when making only the necessary moves to keep its head
above the water.
SPT (day 46)
Low SPT is utilized to evaluate the state of
depression (21,43,45).
Prior to SPT, two bottles (volume, 200 ml) with 1% sucrose solution
(w/v) were provided to each rat for 24 h. The following day, the
sucrose solution in one bottle was replaced with pure water. During
the test, rats were allowed to drink 1% sucrose solution or pure
water for 3 h, and the consumed volume was recorded. Sucrose
preference was calculated according to the following equation:
Sucrose preference (%)=sucrose intake (g)/[sucrose intake (g) +
water intake (g)] x100.
OFT (day 48)
OFTis a behavioral test for evaluating locomotor
activity. Due to chronic stress, rats show an unavoidable tendency
to reduce their locomotor activity in the open field (21,43,46).
The apparatus was placed in a plastic enclosure (dimensions,
100x100x60 cm), with floor and walls painted black. The floor was
divided into 25 equal squares, and a 60-W light bulb was hung 40 cm
above the center. During the experiment, incidences of crossings
(each 25 square crossed with all four paws) and rearings (vertical
activity with rats standing on hind legs) were recorded for 3
min.
Determination of progesterone and
allopregnanolone levels
Emerging evidence has suggested that the pathogenic
mechanisms of depression areassociated withdysfunction
ofprogesterone and allopregnanolone biosynthesis (31,32).
Following the behavioral tests, rats were anesthetized with CH (400
mg/kg; i.p.) (47) prior to
decapitation. Subsequently, the brain regions of interest were
removed and dissected on ice. The prefrontal cortex and hippocampus
were extracted using 1 ml extraction buffer[containing 50 mM
Tris-HCl (pH, 7.4), 150 mM NaCl, 1% NP-40]/100 mg of tissue. To
collect the supernatants, all brain samples were homogenized in a
tissue homogenizer 20 times with ice-cold lysis buffer [containing
137 mM NaCl, 20 mM Tris-HCl (pH, 8.0), 1% NP40, 10% glycerol, 1 mM
PMSF 10 µg/ml aprotinin, 1 µg/mlleupetin and 0.5 mM sodium
vanadate]. The homogenate supernatants were centrifuged for 25 min
at 4,360 x g and 4˚C. The levels of progesterone (cat. no.
ADI-900-011; Enzo Life Sciences, Inc.) and allopregnanolone (cat.
no. E1963Ge; EIAab) were determined in the supernatants using
ELISA. ELISA was performed in accordance with the manufacturers'
instructions and optical density (OD) was measured at a wavelength
of 450 nm.
Statistical analysis
All data are presented as the mean ± SEM.
Differences among groups were analyzed by one-way analysis of
variance (ANOVA) followed by Bonferroni's multiple comparison tests
using GraphPad prism 5.0 software (GraphPad Software Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of LIG on FST in rats
As shown in Fig. 3,
following CUMS, the immobility duration of rats was significantly
increased. Consistent with Ser treatment (15 mg/kg; i.p.), LIG
administration (20 and 40 mg/kg; i.p.) exhibited
antidepressant-like effects on rats, as demonstrated by the reduced
immobility time (P<0.0001 for both 20 and 40 mg/kg).
Furthermore, treatment with Mp (P=0.0270) and Fin (P=0.0246)
significantly reversed the LIG-mediated reduction in immobility
time. These results suggested that LIG may produce
antidepressant-like effects.
Effect of LIG on SPT in rats
The results of SPT are presented in Fig. 4. Treatment of CUMS rats with Ser (15
mg/kg; i.p.) and LIG (20 and 40 mg/kg; i.p.) notably increased
sucrose preference (P=0.0284, 20 mg/kg; P=0.0061, 40 mg/kg).
However, Mp (P=0.0209) and Fin (P=0.0238) could significantly
reduce sucrose preference in comparison with LIG alone. These
findings further confirmed the antidepressant-like effects of
LIG.
Effect of LIG on OFT in rats
The antidepressant-like effects of LIG confirmed by
OFT are shown in Fig. 5. Following
CUMS, rats in the vehicle-treated group showed markedly reduced
crossings and rearing time compared with the normal group
(P=<0.0001). Similarly to the effects of Ser (15 mg/kg; i.p.),
treatment with LIG (20 and 40 mg/kg; i.p.) reversed the number of
crossings (20 mg/kg, P=0.0068; 40 mg/kg; P=0.0005; Fig. 5A) and the rearing time (20 mg/kg,
P=0.0036; 40 mg/kg, P=0.0013; Fig.
5B) of CUMS rats. In addition the increased number of crossings
and rearing time mediated by LIG (20 mg/kg; i.p.) was inhibited by
Mp (crossings, P=0.0467; rearing, P=0.0158) and Fin (crossings,
P=0.0447; rearing, P=0.0058). The aforementioned results supported
the hypothesis that LIG had antidepressant-like effects.
Role of progesterone and
allopregnanolone on the antidepressant effects of LIG
The levels of progesterone and allopregnanolone were
evaluated at the end of each behavioral test (Fig. 6). The levels of progesterone and
allopregnanolone in the prefrontal cortex and hippocampus were
significantly decreased in the vehicle-treated group compared with
the normal group. However, treatment with Ser (15 mg/kg; i.p.) or
LIG, restored the levels of progesterone in the prefrontal cortex
(20 mg/kg, P=0.0440; 40 mg/kg, P=0.0024; Fig. 6A) and hippocampus (20 mg/kg,
P=0.0020; 40 mg/kg, P=0.0033; Fig.
6B). Similar results were observed in the levels of
allopregnanolone in the prefrontal cortex (20 mg/kg, P=0.0033; 40
mg/kg, P<0.0001; Fig. 6C) and
hippocampus (20 mg/kg, P=0.0166; 40 mg/kg, P<0.0001; Fig. 6D). Following treatment with Mp,
levels of progesterone were decreased in the prefrontal cortex
(P=0.0088) and hippocampus (P=0.0017), while Fin notably attenuated
the increased levels of allopregnanolone in the prefrontal cortex
(P=0.0434) and hippocampus (P=0.0224) compared with LIG alone.
Overall, the aforementioned findings indicated that the
antidepressant-like effects of LIG were associated with the
biosynthesis of progesterone and allopregnanolone in the prefrontal
cortex and hippocampus.
Discussion
Studies have indicated that stressful life events,
including chronic, low-intensity and long-term daily stressors are
involved in the development of depression (48-50).
CUMS is a widely used animal model, which is commonly established
to simulate the stressors of depression and investigate its
pathogenesis (51,52). Therefore, to evaluate the
antidepressant effects of LIG, a CUMS rat model was established.
FST, SPT and OFT are common behavioral tests applied to evaluate
depression in animals (43-46).
In the present study, a series of behavioral manifestations of CUMS
on FST, SPT and OFT in rats indicated that the depression model was
successfully established.
Long-term treatment with antidepressants may reverse
CUMS-induced behavioral anomalies. Ser, an SSRI, is used to treat
depression-associated symptoms, including depression with or
without history of mania (53,54).
Ser was selected as a positive control drug. In addition, the
effective doses of LIG (20 and 40 mg/kg) and Ser were selected
based on a series of experiments. The results suggested that
treatment of rats with LIG significantly reduced their immobility
and increased their frequency of crossings, rearing time and
sucrose preference in comparison with a vehicle. Therefore, the
findings of the present study further supported the hypothesis for
the antidepressant-like effects of LIG.
Studies showed that long-term treatment with Ser
exerted antidepressant-like effects, when administrated 1 h prior
to behavioral tests (55,56). Therefore, in the present study the
same treatment scheme with Ser was adopted for LIG, and the results
confirmed that this treatment approach was effective. Behavioral
tests were performed one week following drug administration,
suggesting that, similar to SSRI, the antidepressant-like effects
of LIG were time-dependent (57,58).
Mp is an anti-progesterone drug, which blocks
progesterone through binding to the progesterone receptor. The
receptor itself has no progesterone or estrogen activity (38,39).
Fin, a steroidal molecule, selectively inhibits type II
5α-reductase, a rate-limiting enzyme, which affects the
antidepressant and anti-anxiety activities of several neurosterols,
such as allopregnanolone. Treatment with Fin has been associated
with several neuropsychiatric side effects, including emotional
sensitivity, depression and anxiety (40,41).
In the present study the levels of progesterone and
allopregnanolone were reduced in the Mp- and Fin-treated groups,
respectively. In addition, Mp and Fin antagonized the
antidepressant-like effects of LIG, suggesting that Mp and Fin were
antagonized the LIG receptor.
Several functional abnormalities have been found in
the brain regions implicated in depression, including the
prefrontal cortex and hippocampus. These brain regions are
comprised of multiple neuron networks, which serve an important
role in several processes, including emotional regulation,
self-reference processing, memory and internal psychological
activities (59-62).
Therefore, in the present study, the effect of LIG on the
prefrontal cortex and hippocampus was investigated. The results
indicated that treatment with LIG significantly increased the
levels of progesterone and allopregnanolone in both of these brain
areas.
Allopregnanolone is a well-known positive allosteric
modulator of γ-aminobutyric acid (GABA) A receptors and is
considered to be a selective endogenous modulator of the effects of
GABA A on GABA A receptors (63,64).
Progesterone upregulates the expression of the GABA A receptor α2,
α3, α4 and δ subunits, which are associated with
antidepressant-like activities. It has been suggested that the
effects of GABA A receptors may be mediated by their conversion to
allopregnanolone (65).
Several studies have confirmed an association
between reduced levels of neurosteroids (progesterone and
allopregnanolone) and depression (66,67).
Clinical studies have demonstrated that the levels of progesterone
and allopregnanolone are significantly decreased in patients with
depression. Therefore, it is considered that the reduced
biosynthesis of progesterone and allopregnanolone may be involved
in the pathogenesis of depression (30-32).
A limited number of studies have focused on the role of
progesterone and allopregnanolone in the antidepressant effects of
LIG. In the present study levels of progesterone and
allopregnanolone were increased following treatment of CUMS rats
with LIG, suggesting that the biosynthesis of both neurosteroids
could play an important role in the antidepressant effects of LIG.
However, more experiments are needed to further elucidate this
mechanism. This study could provide novel perspectives on the
antidepressant-like effects of LIG and its possible underlying
mechanisms.
In the present study the levels of progesterone and
allopregnanolone were determined using ELISAs and certain chemicals
in the lysis buffer may affect the results of ELISA experiments .
Therefore, more accurate methods, such as liquid
chromatography-tandem mass spectrometry (LC-MS/MS), should be
applied in future studies to determine the levels of neurosteroids
in the brain.
The present study evaluated the antidepressant-like
effects of LIG on rats by measuring immobility time by FST, sucrose
preference by SPT as well as locomotion and rearing time by OFT.
Furthermore, the levels of progesterone and allopregnanolone in the
prefrontal cortex and hippocampus were evaluated. The results
demonstrated that the antidepressant-like effects of LIG could be
promoted by the biosynthesis of progesterone and allopregnanolone
in the brain. The current study preliminarily explored the
antidepressant effects and possible mechanisms of LIG, therefore,
further studies should be performed in the future to evaluate the
antidepressant-like effects of LIG using more animal models, in
order to provide a safe and effective treatment for depression.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by a grant from the Guangdong
Medical Science and Technology Research Fund Project (grant no.
A2018352).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JCM and ZKQ conceptualized the study, designed the
experiments and wrote the manuscript; HPH, ZLM and SFC performed
the experiments. JSC and HLZ analyzed the data. JCM and KSC confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Experimental procedures were approved by the Animal
Care Research Council of Guangdong Pharmaceutical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Weinberger AH, Gbedemah M, Martinez AM,
Nash D, Galea S and Goodwin RD: Trends in depression prevalence in
the USA from 2005 to 2015: Widening disparities in vulnerable
groups. Psychol Med. 48:1308–1315. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang L, Feng Z, Yang G, Yang Y, Wang K,
Dai Q, Zhao M, Hu C, Zhang R, Liu K, et al: Depressive symptoms
among children and adolescents in western china: An epidemiological
survey of prevalence and correlates. Psychiatry Res. 246:267–274.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
GBD 2015 Disease and Injury Incidence and
Prevalence Collaborators. Global, regional, and national incidence,
prevalence, and years lived with disability for 310 diseases and
injuries, 1990-2015: A systematic analysis for the Global Burden of
Disease Study 2015. Lancet. 388:1545–1602. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhao ZQ, Chiechio S, Sun YG, Zhang KH,
Zhao CS, Scott M, Johnson RL, Deneris ES, Renner KJ, Gereau RW IV
and Chen ZF: Mice lacking central serotonergic neurons show
enhanced inflammatory pain and an impaired analgesic response to
antidepressant drugs. J Neurosci. 27:6045–6053. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yang X, Guo Z, Lu J, Zhao B, Fei Y, Li J,
Jiang H, Sun L, Wang Y, Sun Y and Bao T: The role of MAPK and
dopaminergic synapse signaling pathways in antidepressant effect of
electroacupuncture pretreatment in chronic restraint stress rats.
Evid Based Complement Alternat Med. 2017:1–9. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ma Z, Ji W, Qu R, Wang M, Yang W, Zhan Z,
Fu Q and Ma S: Metabonomic study on the antidepressant-like effects
of banxia houpu decoction and its action mechanism. Evid Based
Complement Alternat Med. 2(213739)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shen J, Zhang J, Deng M, Liu Y, Hu Y and
Zhang L: The antidepressant effect of Angelica sinensis extracts on
chronic unpredictable mild stress-induced depression is mediated
via the upregulation of the BDNF signaling pathway in rats. Evid
Based Complement Alternat Med. 2:1–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Champakaew D, Junkum A, Chaithong U,
Jitpakdi A, Riyong D, Wannasan A, Intirach J, Muangmoon R, Chansang
A, Tuetun B and Pitasawat B: Assessment of Angelica sinensis
(Oliv.) Diels as a repellent for personal protection against
mosquitoes under laboratory and field conditions in northern
Thailand. Parasit Vectors. 9(373)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen XP, Li W, Xiao XF, Zhang LL and Liu
CX: Phytochemical and pharmacological studies on Radix Angelica
sinensis. Chin J Nat Med. 11:577–587. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jin M, Zhao K, Huang Q, Xu C and Shang P:
Isolation, structure and bioactivities of the polysaccharides from
angelica sinensis (oliv.) diels: A review. Carbohydr Polym.
89:713–722. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chao WW and Lin BF: Bioactivities of major
constituents isolated from Angelica sinensis (Danggui). Chin Med.
6(29)2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jeong SY, Kim HM, Lee KH, Kim KY, Huang
DS, Kim JH and Seong RS: Quantitative analysis of marker compounds
in angelica gigas, angelica sinensis, and angelica acutiloba by
hplc/dad. Chem Pharm Bull (Tokyo). 63:504–511. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yi LZ, Liang YZ, Wu H and Yuan DL: The
analysis of radix angelicae sinensis (danggui). J Chromatogr A.
1216:1991–2001. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yin J, Wang CY, Mody A, Bao L, Hung SH,
Svoronos SA and Tseng Y: The effect of Z-ligustilide on the
mobility of human glioblastoma T98G cells. PLoS One.
8(e66598)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang L, Du JR, Wang J, Yu DK, Chen YS, He
Y and Wang CY: Z-ligustilide extracted from radix angelica sinensis
decreased platelet aggregation induced by adp ex vivo and
arterio-venous shunt thrombosis in vivo in rats. Yakugaku Zasshi.
129:855–859. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang J, Du JR, Wang Y, Kuang X and Wang
CY: Z-ligustilide attenuates lipopolysaccharide-induced
proinflammatory response via inhibiting nf-κb pathway in primary
rat microglia. Acta Pharmacol Sin. 31:791–797. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gong WX, Zhou YZ, Li X, Gao L, Wang YH,
Tian JS and Du GH: Antidepression constituents from angelica
sinensis radix in xiaoyao powder. Chin Tradit Herb Drugs.
46:2856–2862. 2015.
|
|
18
|
Long FY, Shi MQ, Zhou HJ, Liu DL, Sang N
and Du JR: Klotho upregulation contributes to the neuroprotection
of ligustilide against cerebral ischemic injury in mice. Eur J
Pharmacol. 820:198–205. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Du JR, Yu Y, Ke Y, Wang CY, Zhu L and Qian
ZM: Ligustilide attenuates pain behavior induced by acetic acid or
formalin. J Ethnopharmacol. 112:211–214. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou Y, Ren Y, Ma Z, Jia G, Gao X, Zhang L
and Qin X: Identification and quantification of the major volatile
constituents in antidepressant active fraction of xiaoyaosan by gas
chromatography-mass spectrometry. J Ethnopharmacol. 141:187–192.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xu F, Peng D, Tao C, Yin D, Kou J, Zhu D
and Yu B: Anti-depression effects of danggui-shaoyao-san, a fixed
combination of traditional Chinese medicine, on depression model in
mice and rats. Phytomedicine. 18:1130–1136. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li J, Yu J, Ma H, Yang N, Li L, Zheng DD,
Wu MX, Zhao ZL and Qi HY: Intranasal pretreatment with
Z-ligustilide, the main volatile component of rhizoma chuanxiong,
confers prophylaxis against cerebral ischemia via nrf2 and hsp70
signaling pathways. J Agric Food Chem. 65:1533–1542.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lu J, Fu L, Qin G, Shi P and Fu W: The
regulatory effect of xiaoyao san on glucocorticoid receptors under
the condition of chronic stress. Cell Mol Biol (Noisy-le-grand).
64:103–109. 2018.PubMed/NCBI
|
|
24
|
Zhu X, Jing L, Chen C, Shao M, Fan Q, Diao
J, Liu Y, Lv Z and Sun X: Danzhi xiaoyao san ameliorates
depressive-like behavior by shifting toward serotonin via the
downregulation of hippocampal indoleamine 2,3-dioxygenase. J
Ethnopharmacol. 160:86–93. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Frye CA, Koonce CJ and Walf AA:
Involvement of pregnane xenobiotic receptor in mating-induced
allopregnanolone formation in the midbrain and hippocampus and
brain-derived neurotrophic factor in the hippocampus among female
rats. Psychopharmacology (Berl). 231:3375–3390. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Droogleever Fortuyn HA, van Broekhoven F,
Span PN, Bäckström T, Zitman FG and Verkes RJ: Effects of phd
examination stress on allopregnanolone and cortisol plasma levels
and peripheral benzodiazepine receptor density.
Psychoneuroendocrinology. 29:1341–1344. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tomaselli G and Vallée M: Stress and drug
abuse-related disorders: The promising therapeutic value of
neurosteroids focus on pregnenolone-progesterone-allopregnanolone
pathway. Front Neuroendocrinol. 55(100789)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Barak Y and Glue P: Progesterone loading
as a strategy for treating postpartum depression. Hum
Psychopharmacol. 35(e2731)2020.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Niwa T, Okada K, Hiroi T, Imaoka S,
Narimatsu S and Funae Y: Effect of psychotropic drugs on the
21-hydroxylation of neurosteroids, progesterone and
allopregnanolone, catalyzed by rat cyp2d4 and human cyp2d6 in the
brain. Biol Pharm Bull. 31:348–351. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dominique CM: Progesterone and
allopregnanolone enhance the miniature synaptic release of glycine
in the rat hypoglossal nucleus. Eur J Neurosci. 30:2100–2111.
2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Osborne L, Gispen F, Meilman S, Almatrafi
M and Payne J: Allopregnanolone and progesterone in pregnancy
predict postpartum depression and anxiety. F1000Posters.
6(514)2015.
|
|
32
|
Schüle C, Nothdurfter C and Rupprecht R:
The role of allopregnanolone in depression and anxiety. Prog
Neurobiol. 113:79–87. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
He ZM, Li GP, Zhu DS and Lu SM: Guidelines
for the management and use of experimental animals. Beijing,
Science and Technology Publications, 2016.
|
|
34
|
National Research Council US Institute for
Laboratory Animal Research: Guide for the Care and Use of
Laboratory Animals. Astronomy and Astrophysics, 1996.
|
|
35
|
Zhang LM, Zhou WW, Ji YJ, Li Y, Zhao N,
Chen HX, Xue R, Mei XG, Zhang YZ, Wang HL and Li YF: Anxiolytic
effects of ketamine in animal models of posttraumatic stress
disorder. Psychopharmacology (Berl). 232:663–672. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Miao YL, Guo WZ, Shi WZ, Fang WW, Liu Y,
Liu J, Li BW, Wu W and Li YF: Midazolam ameliorates the behavior
deficits of a rat posttraumatic stress disorder model through dual
18 kda translocator protein and central benzodiazepine receptor and
neurosteroidogenesis. PLoS One. 9(e101450)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yu J, Jiang Z, Ning L, Zhao Z, Yang N,
Chen L, Ma H, Li L, Fu Y, Zhu H and Qi H: Protective HSP70
induction by Z-ligustilide against oxygen-glucose deprivation
injury via activation of the MAPK pathway but not of HSF1. Biol
Pharm Bull. 38:1564–1572. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Huang J, Zhang Y, Huang Y, Zhang X and
Xiao J: Effect of mifepristone on adriamycin resistance in human
breast cancer cell line mcf-7/adm in vitro and in vivo. Zhong Nan
Da Xue Xue Bao Yi Xue Ban. 35:576–583. 2010.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
39
|
Fiancette JF, Balado E, Piazza PV and
Deroche-Gamonet V: Mifepristone and spironolactone differently
alter cocaine intravenous self-administration and cocaine-induced
locomotion in C57BL/6J mice. Addict Biol. 15:81–87. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gorin RE, Crabbe JC, Tanchuck MA, Long SL
and Finn DA: Effects of finasteride on chronic and acute ethanol
withdrawal severity in the wsp and wsr selected lines. Alcohol Clin
Exp Res. 29:939–948. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mladenović D, Hrnčić D, Petronijević N,
Jevtić G, Radosavljević T, Rašić-Marković A, Puškaš N, Maksić N and
Stanojlović O: Finasteride improves motor, EEG, and cellular
changes in rat brain in thioacetamide-induced hepatic
encephalopathy. Am J Physiol Gastrointest Liver Physiol.
307:931–40. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wu X, Tang B, Liao X, Su Z, Lee SM, Cai Y
and Li C: Suppressive effects of the supercritical-carbon dioxide
fluid extract of chrysanthemum indicum on chronic unpredictable
mild stress-induced depressive-like behavior in mice. Food Funct.
10:1212–1224. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yan S, You ZL, Zhao QY, Peng C, He G, Gou
XJ and Lin B: Antidepressant-like effects of Sanyuansan in the
mouse forced swim test, tail suspension test, and chronic mild
stress model. Kaohsiung J Med Sci. 31:605–612. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Huang HL, Lim SL, Lu KH and Sheen LY:
Antidepressant-like effects of Gan-Mai-Dazao-Tang via monoamine
regulatory pathways on forced swimming test in rats. J Tradit
Complement Med. 8:53–59. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gao X, Zhuang FZ, Qin SJ, Zhou L, Wang Y,
Shen QF, Li M, Villarreal M, Benefield L, Gu SL and Ma TF:
Dexmedetomidine protects against learning and memory impairments
caused by electroconvulsive shock in depressed rats: Involvement of
the NMDA receptor subunit 2B (NR2B)-ERK signaling pathway.
Psychiatry Res. 243:446–452. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Schulz D: Acute food deprivation separates
motor-activating from anxiolytic effects of caffeine in a rat open
field test model. Behav Pharmacol. 29:543–546. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Choi YB, Kim YI, Lee KS, Kim BS and Kim
DJ: Protective effect of epigallocatechin gallate on brain damage
after transient middle cerebral artery occlusion in rats. Brain
Res. 1019:47–54. 2004.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Fahey AG and Cheng HW: Effects of social
disruption on physical parameters, corticosterone concentrations,
and immune system in two genetic lines of white leghorn layers.
Poult Sc. 87:1947–1954. 2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kessler RC: The effects of stressful life
events on depression. Ann Rev Psychol. 48:191–214. 1997.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kendler KS, Karkowski LM and Prescott CA:
Causal relationship between stressful life events and the onset of
major depression. Am J Psychiatry. 156:837–841. 1999.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Qiao H, Li MX, Xu C, Chen HB, An SC and Ma
XM: Dendritic spines in depression: What we learned from animal
models. Neural Plast. 2016(8056370)2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Antoniuk S, Bijata M, Ponimaskin E and
Wlodarczyk J: Chronic unpredictable mild stress for modeling
depression in rodents: Meta-analysis of model reliability. Neurosci
Biobehav Rev. 99:101–116. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
De Vane CL, Liston HL and Markowitz JS:
Clinical pharmacokinetics of sertraline. Clin Pharmacokinet.
41:1247–1266. 2002.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Gilliam FG, Black KJ, Carter J, Freedland
KE, Sheline YI, Tsai WY and Lustman PJ: A trial of sertraline or
cognitive behavior therapy for depression in epilepsy. Ann Neurol.
86:552–560. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Qiu ZK, Zhang LM, Zhao N, Chen HX, Zhang
YZ, Liu YQ, Mi TY, Zhou WW, Li Y, Yang RF, et al: Repeated
administration of AC-5216, a ligand for the 18 kDa translocator
protein, improves behavioral deficits in a mouse model of
post-traumatic stress disorder. Prog Neuropsychopharmacol Biol
Psychiatry. 45:40–46. 2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang LM, Qiu ZK, Zhao N, Chen HX, Liu YQ,
Xu JP, Zhang YZ, Yang RF and Li YF: Anxiolytic-like effects of
YL-IPA08, a potent ligand for the translocator protein (18 kDa) in
animal models of post-traumatic stress disorder. Int J
Neuropsychopharmacol. 17:1659–1669. 2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Oh SJ, Cheng J, Jang JH, Arace J, Jeong M,
Shin CH, Park J, Jin J, Greengard P and Oh YS: Hippocampal mossy
cell involvement in behavioral and neurogenic responses to chronic
antidepressant treatment. Mol Psychiatry. 25:1215–1228.
2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Artigas F: Developments in the field of
antidepressants, where do we go now? Eur Neuropsychopharmacol.
25:657–670. 2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Rauch SL, Shin LM and Wright CI:
Neuroimaging studies of amygdala function in anxiety disorders. Ann
NY Acad Sci. 985:389–410. 2003.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Bremner JD: Traumatic stress: Effects on
the brain. Dialogues Clin Neurosci. 8(445)2006.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Frodl T, Reinhold E, Koutsouleris N,
Reiser M and Meisenzahl EM: Interaction of childhood stress with
hippocampus and prefrontal cortex volume reduction in major
depression. J Psychiatr Res. 44:799–807. 2010.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Tebartz van Elst L, Hesslinger B, Thiel T,
Geiger E, Haegele K, Lemieux L, Lieb K, Bohus M, Hennig J and Ebert
D: Frontolimbic brain abnormalities in patients with borderline
personality disorder: A volumetric magnetic resonance imaging
study. Biol Psychiatry. 54:163–171. 2003.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Viero C and Dayanithi G: Neurosteroids are
excitatory in supraoptic neurons but inhibitory in the peripheral
nervous system: It is all about oxytocin and progesterone
receptors. Prog Brain Res. 170:177–192. 2008.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Qiu ZK, Zhang GH, Zhong DS, He JL, Liu X,
Chen JS and Wei DN: Puerarin ameliorated the behavioral deficits
induced by chronic stress in rats. Sci Rep. 7(6266)2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Peng HY, Chen GD, Lee SD, Lai CY, Chiu CH,
Cheng CL, Chang YS, Hsieh MC, Tung KC and Lin TB: Neuroactive
steroids inhibit spinal reflex potentiation by selectively
enhancing specific spinal GABA(A) receptor subtypes. Pain.
143:12–20. 2009.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Klatzkin RR, Morrow AL, Light KC, Pedersen
CA and Girdler SS: Histories of depression, allopregnanolone
responses to stress, and premenstrual symptoms in women. Biol
Psychol. 71:2–11. 2006.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Hellgren C, Comasco E, Skalkidou A and
Sundström-Poromaa I: Allopregnanolone levels and depressive
symptoms during pregnancy in relation to single nucleotide
polymorphisms in the allopregnanolone synthesis pathway. Horm
Behav. 94:106–113. 2017.PubMed/NCBI View Article : Google Scholar
|