Introduction
Skin is the largest organ in the body. It is the
first barrier that protects the body from external agents. The skin
consists of a stratum corneum composed of keratinized epithelial
cells, an epidermis composed of keratinocytes, and a dermis
containing fibrous collagen and elastin (1). Collagen is a major matrix protein
produced by fibroblasts. It is particularly rich in the skin
(dermis). Collagen contributes to mechanical firmness of the skin.
It helps cell adhesion and induction of cell division and
differentiation (2). Skin aging and
damage are caused by UV light, genetic factors, oxidative stress,
and environmental exposure. Skin aging is divided into photoaging
caused by UV exposure and endogenous aging caused by physiological
factors. However, UV exposure is the most common cause of skin
damage and aging (3,4). UV spectrum is divided into UV-A
(320-400 nm), UV-B (290-320 nm), and UV-C (290-100 nm). In
particular, UV-B causes oxidative stress such as reactive oxygen
species (ROS) on the skin. It can result in transient and
persistent DNA damage with increased expression of aging factors
such as matrix metalloproteinase (MMPs) (5,6).
Increased expression of MMPs can degrade collagen in the dermis and
reduce its production. Inhibited expression of MMPs is an important
factor in regulating collagen metabolism and promoting collagen
production (7,8). MMP-1 is called collagenase. It mainly
decomposes type 1 collagen in the dermis. Expression of MMPs is
initiated by the activation of the MAPK signaling pathway by ROS
(9). UVB stimulation can activate
phosphorylation of ERK1/2, JNK, and p38 kinase. Activated MAP
kinase can promote phosphorylation of p65 and p50 proteins as
important subunits of the NF-κB transcription factor, thereby
increasing the amount of transcription factors translocated into
the nucleus. Activated and translocated into the nucleus, NF-κB can
promote the transcription of proteins such as MMP-1, resulting in
collagen degradation (10,11).
Limonium tetragonum is a biennial plant of
the Plumbaginaceae family. This plant contains active ingredients
such as myricetin, myricetin glycosides, tannins, and caprolactam.
It has been used in folk medicine to treat uterine bleeding,
oligomenorrhea, and dysgalactia (12). Triglochin maritimum is a
perennial plant that has been reported to have antioxidant and
anti-inflammatory effects (13).
Artemisia scoparia is a perennial plant in the Asteraceae
family found mainly in India and Pakistan. Its main chemical
components have been reported to be flavonoids, coumarin, ketone,
and chromogen (14). A.
scoparia has been used as a folk remedy for its antipyretic,
anticholesterol, antiseptic, antibacterial, diuretic, and
vasodilator properties (15). Red
ginseng is a herbal medicine that has been used for a long time in
oriental medicine. Its main chemical component is ginsenoside. It
has been reported to be effective in alleviating diseases related
to oxidative stress (16). The
purpose of this study was to investigate inhibitory effects of
L. tetragonum, T. maritimum, A. scoparia, and
red ginseng complex against UVB-induced photoaging and the
mechanism of action involved in such effects.
Materials and methods
Materials
Dulbecco's modified Eagle medium (DMEM) and fetal
bovine serum were purchased from Gibco; Thermo Fisher Scientific,
Inc. Penicillin/streptomycin antibiotics came from Invitrogen;
Thermo Fisher Scientific, Inc. EZ-Cytox reagent and EZ-western Lumi
Pico Alpha were obtained from DoGenBio. Protease inhibitors,
tert-butyl hydroperoxide (tBHP), L-ascorbic acid, and o-toluidine
blue were purchased from Sigma-Aldrich. Radio-immunoprecipitation
assay buffer (RIPA buffer) was purchased from Thermo Fisher
Scientific, Inc. ELISA Kit for Collagen Type I was purchased from
Cloud-Clone Corp.. Collagen, elastin, MMP-1, MMP-9, JNK, p-JNK,
ERK, p-ERK, p38, p-p38, NF-κB, P-NF-κB, and HRP conjugated
secondary antibody (Santa Cruz Biotechnology, Inc.) and actin
antibody (Biosciences) were also used in this study.
Plant material and extract
Limonium tetragonum used in the experiment
was collected from Sinsido. Artemisia scoparia was collected
from Sohwangsagu. Triglochin maritimum Linnaeus was
collected from Simwon-myeon. Plants were identified by Dr Ahn
Jin-Gap. Red ginseng root was purchased from Jinandang Farming
Association Corporation. Halophyte red ginseng complex extract
(HRCE) was produced from raw materials of complex Limonium
tetragonum 2: Artemisia scoparia 1: Triglochin maritimum 1:
Red ginseng 2, and ethanol 50% with the extraction ratio of 20:1.
HRCE was filtered using a 0.45-m filter, concentrated with a rotary
pressure reducer, dried with a freeze dryer, and stored at
-20˚C.
Cell culture
Human keratinocyte (HaCaT) cell line was purchased
from CLS Cell Lines Service GmbH. These cells were cultured and
maintained in DMEM media supplemented with 10% fetal bovine serum,
100 U/ml penicillin, and 100 µg/ml streptomycin in a 5%
CO2 incubator at 37˚C.
Animal and experimental design
Male hairless mice at five weeks of age were
purchased from Orient Bio Inc. These mice were housed in an
air-conditioned room with temperature of 22±2˚C, humidity of
50-60%, and a 12/12 h day/night cycle. These mice were given a
commercial-standard laboratory diet and water at will. All
procedures were performed in compliance with guiding principles for
animal care and use committee of Jeonju University Institutional
Animal Care and Used Committee guidelines (approved no.
JJU-IACUC-2018-5). Animals were adapted to the laboratory
environment for one week prior to the experimentation. The number
of mice in each experimental group was five. HRCE (200 mg/kg), HRCE
(100 mg/kg) + L-ascorbic acid (AA) (25 mg/kg), and L-ascorbic acid
(AA) (50 mg/kg) were dissolved in saline and oral administered at
one week before UVB irradiation and continued until the termination
of the experiment. The UVB-irradiated control group was
administered with saline. Irradiated groups received saline, HRCE,
or AA. Dorsal skin area of mice was exposed to UVB radiation from
LF-215.M lamp (emission peak at 312 nm; Uvitec). Using an
electronic controller, UVB dosage at a fixed distance from lamps to
the dorsal skin surface of mice was regulated to be 300
mJ/cm2. The exposure time was 3 min thrice a week for
two weeks. At the end of the experiment, the mice were euthanized
through cervical dislocation. The dorsal dermis was collected and
stored at -80˚C for western blotting and fixed in 4%
paraformaldehyde for histological analysis.
Cell viability
Cell viability assay was measured using EZ-Cytox
reagent. HaCaT cells (1x105 cells/ml) were seeded into
96-well plates and incubated for 24 h. These cells were then
pretreated with 100 µg/ml of HRCE or 50 µg/ml of L-ascorbic acid
(AA) for 1 h and subsequently stimulated with 400 µM of tBHP for 16
h. After 16 h, 10 µl of EZ-Cytox reagent was added to each well.
Cells were then incubated for 4 h. The absorbance of each well was
then measured at 450 nm with a microplate reader (Tecan). The
concentration of tBHP treatment was determined in previous
experiments. HaCaT cells were treated with various concentrations
of tBHP for 16 h, and the survival rate was ~60% compared to the
negative control at 400 µM concentration, which was determined to
be a suitable concentration for the experiment.
ELISA assay
Culture supernatants of HaCaT cells treated with or
without HRCE or AA for 48 h after UVB radiation were used to
measure concentrations of Type I collagen. The protocol used was in
accordance with the outlined protocol of the manufacturer of the
Kit without modification.
Immunofluorescence staining
In cell culture slide chambers, HaCaT cells were
pretreated with or without HRCE or AA for 24 h and then stimulated
with or without 20 mJ/cm of UVB irradiation. These cells were fixed
and permeabilized by 100% ice-cold methanol for 10 min at -20˚C.
Cells on slides were blocked with 1% BSA for 1 h at room
temperature and incubated with collagen antibodies overnight at
4˚C. These cells were then washed with PBS and further incubated
with goat anti-mouse IgG, (H + L) Alexa Fluor™ plus 488 conjugated
secondary antibodies for 1 h. Slides were washed with PBS and
mounted with DAP mounting medium. Visualization was under a Zeiss
fluorescence Microscope (Zeiss Co.).
Protein extraction
HaCaT cells (2x105 cells/ml) were
pretreated with HRCE or AA for 1 h and then treated with UVB
irradiation (20 mJ/cm2). These cells were incubated for
15 min or 1 h, washed with ice-cold PBS, and centrifuged at 2,000
rpm at 4˚C for 2 min. The supernatant was discarded and cell
pellets were suspended in 0.1 ml of ice-cold RIPA buffer. Tubes
were vortexed and incubated on ice for 15 min with gentle shaking.
After incubation, tubes were centrifuged at 12,000 rpm for 15 min
at 4˚C to pellet cell debris. The supernatant (protein lysates) was
transferred into new tubes and stored at -80˚C for subsequent
use.
For protein extraction from tissue samples, at the
end of the UVB irradiation in animal experiment described above,
the dorsal dermis was removed and 0.2 g of the tissue from each
mouse was grounded in liquid nitrogen and placed in 0.2 ml of RIPA
buffer. Tissues were then incubated on ice for 5 min before
centrifugation at 12,000 rpm for 15 min at 4˚C. The supernatant was
transferred into new tubes and stored at -80˚C for subsequent
use.
Western blot analysis
Proteins (45 µg) were separated on 7.5% SDS-PAGE
gels and transferred onto PVDF membranes. After blocking with 5%
BSA prepared with 1% Tween-20 in 20 mmol/l TBS (pH 7.4), membranes
were then incubated overnight with specific primary antibodies to
be assessed. These membranes were washed and incubated with
appropriate horseradish peroxidase-conjugated secondary antibody.
Protein expression was detected and visualized using a
chemiluminescence detection system. The density of each band in an
immunoblot was analyzed using ImageJ gel analysis software
developed by the National Institutes of Health.
Histopathological examination
Dorsal skin tissues were fixed in 10% neutral
buffered formalin, washed with 1X PBS five times, dehydrated in
graduated ethanol, cleared in xylene, embedded in paraffin, and
sectioned at 5 µm in thickness. Sections were then stained with
H&E for measuring epidermal thickness, trichrome for collagen
fiber analysis, and toluidine blue for mast cell quantification.
All staining procedures were done using their respective protocols
with little or no modifications. Histopathological changes were
examined under a light microscope (Leica). Transepidermal water
loss (TEWL) was expressed as an average value after five repeated
measurements using a gpskin barrier (Amicogen).
Statistical analysis
Data are presented as mean ± SD. Statistically
significant differences among groups were determined by one-way
analysis of variance (ANOVA) followed by Tukey's test.
Statistically significant difference was considered at
P<0.05.
Results and Discussion
HRCE recovers tBHP-induced cell
damage
Exposure of UVB can lead to over-production of ROS
on the skin, resulting in oxidative stress. Increased intracellular
ROS can cause skin diseases, including photoaging, inflammation,
and carcinogenesis (17).
Therefore, protecting the skin against oxidative stress might be a
strategy to prevent UVB-related skin damage. The cytoprotective
effect of HRCE in tBHP-stimulated HaCaT cells was investigated. As
shown in Fig. 1, stimulated cells
without HRCE treatment showed significantly lower survival rates
than unstimulated cells. However, the survival rate was
significantly higher when cells were treated with 100 µg/ml of HRCE
or 50 µg/ml of AA. This implies that HRCE and AA can protect skin
against damaging effects of peroxide.
HRCE protects collagen and elastin
from cell damage caused by UVB
In the skin, collagen and elastin are important for
maintaining elasticity, strength, and structure. A decrease in
collagen and elastin expression has been observed in photoaged skin
(18,19). Therefore, the effect of HRCE on the
expression of collagen and elastin in cell damage induced by UVB
was investigated. As shown in Fig.
2A, the amount of collagen released into the culture medium of
UVB-stimulated cells without HRCE treatment was significantly
reduced compared to that in the unstimulated experimental group.
Results of immunofluorescence staining (Fig. 2B) also confirmed that collagen
density was decreased in cells stimulated by UVB. However, HRCE
significantly increased collagen synthesis, which was reduced by
UVB. Intracellular collagen and elastin protein expression were
examined by western blot analysis (Fig.
2C). Results showed that HRCE recovered UVB-induced collagen
and elastin degradation. AA can act as a photoprotective agent. It
can stimulate collagen synthesis, protect against damage caused by
UVB radiation, and relieve inflammation in the skin (20). In this study, AA also prevented
collagen loss. Moreover, HRCE showed similar or slightly less
inhibitory effects on collagen and elastin degradation than AA. In
general, the reduction of collagen and elastin in the skin is
driven by the expression of matrix metalloproteinases (MMP)
(21). For this reason, the
expression of MMPs was also investigated in this study.
HRCE suppress the expression of
MMPs
Exposure to UV can increase the expression of matrix
metalloproteinases (MMP) in human skin. MMPs can degrade
extracellular matrix (ECM) such as collagen, fibronectin, and
elastin and cause photoaging (21,22).
The effect of HRCE on protein expression of MMPs called collagenase
was investigated. As shown in Fig.
3, HRCE suppressed the expression of MMP-1 and MMP-9 that was
increased by UVB. In particular, inhibition of HRCE on MMP-9
expression was superior to that of AA, the reference compound.
According to previous studies, MMP-1 degrades Collagen types I and
III while MMP-9 degrades Collagen type IV and elastin (22,23).
Therefore, HRCE has collagen and elastin protective effect by
suppressing the expression of MMP-1 and MMP-9.
HRCE suppresses phosphorylation of
Mitogen-activated protein kinase (MAPK) proteins
Next, pathways stimulated by irradiation with UVB
were investigated. Fig. 4A shows
the degree of phosphorylation of MAPKs in cells exposed to UVB.
Results showed that HRCE had no inhibitory effect on
phosphorylation of c-Jun N-terminal kinases (JNK) or p38
mitogen-activated protein kinases (p38). However, the
phosphorylation inhibitory effect of extracellular signal-regulated
kinases (ERK) was significantly evident. As HRCE inhibited the
phosphorylation of ERK, the activation of NF-κB transcription
factor by HRCE was then investigated. As a result (Fig. 4B), it was confirmed that the
phosphorylation of NF-κB transcription factor of HaCaT cells
exposed to UVB was significantly inhibited by HRCE. According to
previous studies, when the skin is exposed to UV, ROS
overproduction occurs, causing MAPK (ERK, JNK, and p38)
phosphorylation and NF-κB activation, which then promotes the
expression of MMPs genes and proteins, causing collagen degradation
and inflammation (24,25). Thus, the cell protective effect from
UVB of HRCE might be due to the inhibition of ERK and NF-κB
signaling pathway.
HRCE prevents UVB-induced skin damage
and morphological changes
Because HRCE was confirmed to have preventative
effect against photoaging and skin damage at the cell level, the
efficacy of HRCE was then evaluated in in vivo studies.
Improvement of clinical signs and symptoms of UV-induced skin
damage by HRCE was evaluated by measuring epidermal thickness and
transepidermal water loss (TEWL). Results revealed that repeated
irradiation with UVB (300 mJ/cm2) caused skin edema and
dryness (Fig. 5A). As shown in
Fig. 5B and C, skin thickness values of UVB irradiated
mice were increased. However, when mice were treated with HRCE or
AA, skin thickness was reduced. TEWL values of mice in the HRCE
group were significantly reduced (Fig.
5D). TEWL is a measure of the function of the stratum corneum.
A healthy stratum corneum layer can prevent foreign substances from
penetrating the skin. It can also prevent moisture loss (26). The reduction of TEWL with HRCE
administration suggests that HRCE can maintain the function of the
stratum corneum after UV damage.
HRCE prevents UVB-induced collagen and
elastin degradation
Collagen can maintain skin's strength and elasticity
along with keratin formation (27).
Elastin is a major protein in the extracellular matrix that helps
the skin to return to its original shape when the skin is subjected
to physical pressure (28).
Collagen and elastin are main targets in studies about UV-induced
skin damage. Western blot and trichrome staining were performed to
investigate the inhibitory effect of HRCE on collagen degradation.
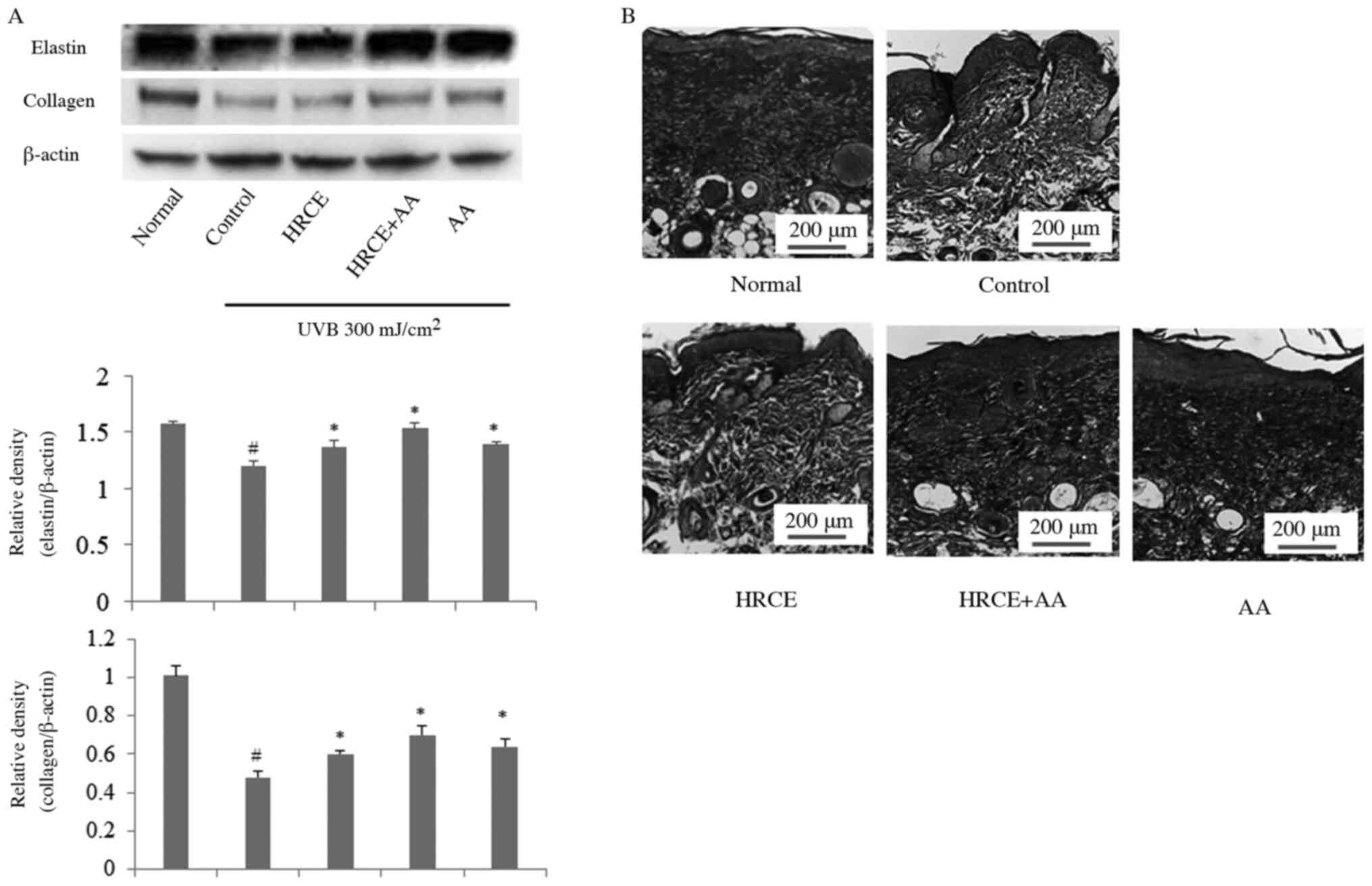
According to Fig. 6A, collagen and
elastin of mice decomposed by UVB irradiation were recovered by
administration of HRCE. Trichrome staining showed that collagen
density increased when mice were administered with HRCE (Fig. 6B). Through animal experiments, the
improvement effect of HRCE on UV-induced collagen and elastin
degradation was consistent with results from cell experiments.
HRCE prevents infiltration of mast
cells to the skin
It is known that infiltration of mast cells occurs
in skin after UVB exposure (29).
Toluidine blue staining showed that mast cells infiltrated the skin
after UVB irradiation (Fig. 7A and
B). However, the infiltration of
mast cells was significantly reduced in skin of mice of the
HRCE-administered group and the AA-administered group. Previous
studies have reported that mast cells increased by UV in the skin
might increase the risk of developing basal cell carcinoma
(30). These effects are thought to
be due to direct suppression of mast cell invasion or the reduction
of oxidative stress by HRCE. These results suggest that HRCE can be
used not only to protect collagen and elastin degradation by UVB,
but also to prevent skin inflammation and basal cell carcinoma.
Therefore, it is considered that there is a need for research on
the effects of HRCE on inflammatory cytokines and inflammatory
reactions caused by UV rays.
In conclusions, to the best of our knowledge, this
is the first study to reveal that HRCE can prevent degradation of
collagen and elastin caused by UV exposure using HaCat cells and
hairless mice. It is thought that HRCE can inhibit the expression
of MMPs by blocking the activation of MAPK and NFκB signaling
pathways at the cellular level. In addition, the collagen
protective effect of HRCE in cell experiments was consistent with
results from animal experiments. Therefore, HRCE is a potential
health functional material that can prevent skin damage caused by
UV rays such as skin aging, wrinkles, blemishes, and freckles. It
has possible application in the food and cosmetics industry.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by a grant from Jeonbuk
Research&Development Program funded by Jeonbuk Province,
Republic of Korea (grant no. RA201906-5-C4).
Availability of data and materials
The datasets used and/or analyzed data during the
study are available from the corresponding author on reasonable
request.
Authors' contributions
JYS and SIJ designed the research. JHP, JYS, DNC,
HJK and YTL performed the experiments. JYS, BOC and SIJ analyzed
the data. SIJ and JYS wrote the manuscript draft. BOC, SIJ and JYS
reviewed and edited the final manuscript. SIJ managed the research
project. JYS and SIJ confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Mice were handled and experiments were carried out
based on Jeonju University Institutional Animal Care and Use
Committee guidelines with permission to carry out the experiment
obtained from Jeonju University (approval no.
JJU-IACUC-2018-5).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Egert M, Simmering R and Riedel CU: The
association of the skin microbiota with health, immunity, and
disease. Clin Pharmacol Ther. 102:62–69. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Perlish JS, Lemlich G and Fleischmajer R:
Identification of collagen fibrils in scleroderma skin. J Invest
Dermatol. 90:48–54. 1988.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Afaq F and Mukhtar H: Botanical
antioxidants in the prevention of photocarcinogenesis and
photoaging. Exp Dermatol. 15:678–684. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rittie L and Fisher GJ: UV-light-induced
signal cascades and skin aging. Ageing Res Rev. 1:705–720.
2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ho JN, Lee YH, Park JS, Jun WJ, Kim HK,
Hong BS, Shin DH and Cho HY: Protective effects of aucubin isolated
from Eucommia ulmoides against UVB-induced oxidative stress
in human skin fibroblasts. Biol Pharm Bull. 28:1244–1248.
2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wolf ST, Kenney LE and Kenney WL:
Ultraviolet radiation exposure, risk, and protection in military
and outdoor athletes. Curr Sports Med Rep. 19:137–141.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lauer-Fields JL, Juska D and Fields GB:
Matrix metalloproteinases and collagen catabolism. Biopolymers.
66:19–32. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: They're not just for matrix anymore! Curr Opin
Cell Biol. 13:534–540. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jalmi SK and Sinha AK: ROS mediated MAPK
signaling in abiotic and biotic stress-striking similarities and
differences. Front Plant Sci. 6(769)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Khan N, Syed DN, Pal HC, Mukhtar H and
Afaq F: Pomegranate fruit extract inhibits UVB-induced inflammation
and proliferation by modulating NF-κB and MAPK signaling pathways
in mouse skin. Photochem Photobiol. 88:1126–1134. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang MH, Kim NH, Heo JD, Sung SH and Jeong
EJ: Hepatoprotective effects of Limonium tetragonum, edible
medicinal halophyte growing near seashores. Pharmacogn Mag. 10
(Suppl 3):S563–S568. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Antioxidant and Anti-inflammatory Activity
of Six Halophytes in Korea. Natural Product Sci. 24:40–46.
2018.
|
|
14
|
Wang X, Huang H, Ma X, Wang L, Liu C, Hou
B, Yang S, Zhang L and Du G: Anti-inflammatory effects and
mechanism of the total flavonoids from Artemisia scoparia
Waldst et kit. In vitro and in vivo. Biomed Pharmacother.
104:390–403. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sajid M, Khan MR, Shah NA, Ullah S, Younis
T, Majid M, Ahmad B and Nigussie D: Proficiencies of Artemisia
scoparia against CCl4 induced DNA damages and renal toxicity in
rat. BMC Complement Altern Med. 16(149)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lee YM, Yoon H, Park HM, Song BC and Yeum
KJ: Implications of red Panax ginseng in oxidative stress
associated chronic diseases. J Ginseng Res. 41:113–119.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bickers DR and Athar M: Oxidative stress
in the pathogenesis of skin disease. J Invest Dermatol.
126:2565–2575. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fisher GJ, Datta S, Wang Z, Li XY, Quan T,
Chung JH, Kang S and Voorhees JJ: c-Jun-dependent inhibition of
cutaneous procollagen transcription following ultraviolet
irradiation is reversed by all-trans retinoic acid. J Clin Invest.
106:663–670. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Kwon KR, Alam MB, Park JH, Kim TH and Lee
SH: Attenuation of UVB-induced photo-aging by polyphenolic-rich
spatholobus suberectus stem extract via modulation of
MAPK/AP-1/MMPs signaling in human keratinocytes. Nutrients.
11(1341)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Petruk G, Del Giudice R, Rigano MM and
Monti DM: Antioxidants from plants protect against skin photoaging.
Oxid Med Cell Longev. 2018(1454936)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ
and Fisher GJ: Matrix-degrading metalloproteinases in photoaging. J
Investig Dermatol Symp Proc. 14:20–24. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kim J, Lee CW, Kim EK, Lee SJ, Park NH,
Kim HS, Kim HK, Char K, Jang YP and Kim JW: Inhibition effect of
Gynura procumbens extract on UV-B-induced
matrix-metalloproteinase expression in human dermal fibroblasts. J
Ethnopharmacol. 137:427–433. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pittayapruek P, Meephansan J, Prapapan O,
Komine M and Ohtsuki M: Role of matrix metalloproteinases in
photoaging and photocarcinogenesis. Int J Mol Sci.
17(868)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ryu J, Park SJ, Kim IH, Choi YH and Nam
TJ: Protective effect of porphyra-334 on UVA-induced photoaging in
human skin fibroblasts. Int J Mol Med. 34:796–803. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tanaka K, Asamitsu K, Uranishi H,
Iddamalgoda A, Ito K, Kojima H and Okamoto T: Protecting skin
photoaging by NF-kappaB inhibitor. Curr Drug Metab. 11:431–435.
2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Thiele JJ, Dreher F, Maibach HI and Packer
L: Impact of ultraviolet radiation and ozone on the transepidermal
water loss as a function of skin temperature in hairless mice. Skin
Pharmacol Appl Skin Physiol. 16:283–290. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Parvizi J and Kim GK: -Collagen. Chapter
53. In: High Yield Orthopaedics. Parvizi J and Kim GK (eds). WB
Saunders, Philadelphia, PA, pp107-109, 2010.
|
|
28
|
Muiznieks LD, Weiss AS and Keeley FW:
Structural disorder and dynamics of elastin. Biochem Cell Biol.
88:239–250. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Alard P, Niizeki H, Hanninen L and
Streilein JW: Local ultraviolet B irradiation impairs contact
hypersensitivity induction by triggering release of tumor necrosis
factor-alpha from mast cells. Involvement of mast cells and
Langerhans cells in susceptibility to ultraviolet B. J Invest
Dermatol. 113:983–990. 1999.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Smirnova IO, Kvetnoĭ IM, Anichkov NM,
Smirnova ON and Antonova IV: Mast cells in photolesion of the skin
and basal cell cancer associated with it. Arkh Patol. 67:26–29.
2005.PubMed/NCBI
|