Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is a common
cause of stroke that usually results in undesirable outcomes and is
associated with a high mortality rate around the world of 50%
(1,2). Neuronal pyroptosis has been suggested
to be a key factor in the process of early brain injury (3). Upregulation of the melanoma 2 (AIM2)
inflammasome, which is activated by double-stranded DNA from
mitochondria and nuclei, leads to neuronal pyroptosis in a mouse
model of SAH (4). In addition,
calcium overload has also been suggested to be involved in neuronal
pyroptosis after SAH (5). Previous
studies have reported that early brain injury, which is
characterized by an inflammatory response prior to cerebral
vasospasm, leads to disability or even mortality post-SAH (6,7).
Therefore, investigations into the potential effective
anti-pyroptotic strategies have been gathering attention as therapy
for patients with SAH.
A number of studies have reported that inhalation of
hydrogen gas significantly attenuates oxidative stress, exhibits
anti-pyroptosis and anti-inflammatory activities and reduces
cerebral ischemia/reperfusion injury in rodent models (8-10).
Zhan et al (2) demonstrated
that hydrogen gas exerts protective effects against early brain
injury post-SAH, which was mediated by inhibiting oxidative stress.
It has also been reported that hydrogen gas attenuates inflammatory
responses in the heart and liver after organ transplantation
(11,12). Mitochondrial regulation, including
those of mitochondrial ATP-sensitive K+
(mitoKATP) channels, serves a role in the
neuroprotective effects of hydrogen gas (13,14).
In addition, hydrogen gas has been suggested to activate both
proliferating and pro-apoptotic signaling cascades, including the
ERK1/2 or p38 MAPK (15-17).
However, the exact mechanism by which hydrogen gas protects against
SAH-induced neurologic dysfunction remains poorly understood.
Opening of mitoKATP channels, in addition
to the activation of ERK1/2 and p38 MAPK have all been reported to
underlie the neuroprotective effects of hydrogen gas as
aforementioned. Therefore, in the present study, the potential
therapeutic effects of hydrogen gas post-conditioning on a
SAH-induced rat model of neuronal pyroptosis and the
mitoKATP/ERK1/2/p38 MAPK signal pathway induced by
intravascular perforation was investigated.
Materials and methods
Animals
In total, 137 male Sprague-Dawley rats (Liaoning
Changsheng Biotechnology Co., Ltd.) weighing 324±13 g (age, 9-10
weeks) were utilized in the present study. Under controlled
conditions, the rats were exposed to a regular 12 h light/dark
cycle (lights on at 7:00 a.m. and lights off at 7:00 p.m.). The
room temperature was maintained at 25±1˚C and the humidity was kept
at 50±10%. Rats were allowed free access to standard diet and
water.
According to a random number table, rats were
divided into the following five groups: i) Sham (n=24); ii) SAH
(n=30); iii) SAH plus treatment with 2.9% hydrogen
post-conditioning for 2 h (SAH + H2; n=25); iv) SAH
treatment with an intraperitoneal injection (i.p.) pre-injection of
5-hydroxydecanoate sodium (5-HD; 40 mg/kg; cat. no. ab141672;
Abcam) followed by 2.9% hydrogen post-conditioning for 2 h (SAH +
H2 + 5-HD; n=31); and v) SAH treatment with
pre-injection of saline containing an equivalent concentration of
DMSO and 2.9% hydrogen post-conditioning for 2 h (SAH +
H2 + saline; n=27).
SAH was initiated by intravascular perforation on
the bifurcation of the anterior cerebral artery and the middle
cerebral artery. Rats in the H2 groups inhaled 2.9%
hydrogen mixed with 20% oxygen and balanced nitrogen (flow rate, 1
l/min) immediately after SAH for 2 h. Rats in the 5-HD groups were
administered 5-HD (40 mg/kg i.p.) at 30 min before SAH. Rats in the
saline groups were administered with saline containing an
equivalent concentration of DMSO i.p. The animal protocols included
in the present study were ratified by the Institutional Animal Care
and Use Committee at The Cangzhou Central Hospital (Cangzhou,
China).
SAH model and hydrogen
administration
Intravascular perforation was performed to simulate
SAH in a rat model (18). Compared
with two injections of arterial blood solvates into the cisterna
magna, the rat endovascular perforation model was considered to be
the more representative model of human SAH (19). Briefly, under sevoflurane anesthesia
[induction (7-8%) and maintenance (3-4%)], rats were intubated and
then ventilated at a tidal volume of 4 ml/100 g (fraction of
inspired oxygen, 40%) using ventilators (Shanghai Alcott Biological
Technology Inc.). The rectal temperature was maintained at
36.0±0.5˚C using a heating pad. The left external carotid artery
was then ligated, following which a sharpened 5-0 suture line was
placed from the stump of the external carotid artery. The vessel
wall located on the bifurcation of the anterior and middle cerebral
arteries was perforated. The sham-operated rats also underwent the
same procedure but the arteries were not perforated. The rats in
the H2 groups inhaled 2.9% hydrogen mixed with 20%
oxygen and balanced nitrogen (Gilmore Liquid Air Company) at a flow
rate of 1 l/min immediately after SAH for 2 h under anesthesia. The
hydrogen concentration was discontinuously monitored using a
handheld hydrogen detector (H2scan Corporation) every 10
min. After post-conditioning of hydrogen gas, the rats were freely
allowed access to water and food in separate animal facilities. The
health and behavior including respiratory function of rats were
monitored every hour within 24 h. If any of the rats were unable to
eat food or drink water, or breathed slowly and weakly, euthanasia
was performed via cervical dislocation under sevoflurane anesthesia
within 24 h after SAH. According to a previous study (18), the severity of SAH was assessed by a
SAH grading scale. Following decapitating under anesthesia (8%
sevoflurane) and the removal of brains 24 h post-SAH, the bases of
the brains were pictured. Six segments of the basal cistern base
were allocated a grade from 0 to 3 (0, no SAH; 1, minimal
subarachnoid blood; 2, mediocre blood with visible arteries; 3,
blood clots covering all arteries). The SAH grade was calculated
using the sum of the six scores from the six segments.
Neurobehavioral test
A modified method of Garcia et al (20) was used 24 h post-SAH to evaluate
neurologic behavior in the rats (n=6 per group) (20). The modified Garcia method ranged
from 0 to 18 points, including spontaneous activity (0-3 points),
symmetry in forelimb movement (0-3 points), forepaw outstretching
(0-3 points), climbing (0-3 points), body proprioception (0-3
points) and response to vibrissae touch (0-3 points). The
conductors (ZWS and HYJ), who were blinded to the group information
of the rats, assessed the aforementioned neurologic parameters of
the rats.
Brain water content
The rats were decapitated under anesthesia (8%
sevoflurane) 24 h post-SAH before the brain tissues were
immediately isolated from the skull and dissected into right and
left hemispheres, cerebellum and brain stem (n=6 per group). After
weighing (wet weight), all tissues were dehydrated for 72 h in a
105˚C oven and weighed again (dry weight). The following formula
was used to assess brain water content: [(Wet weight-dry
weight)/wet weight] x100%.
Reactive oxygen species (ROS)
production
Rats were perfused through the left
ventricle-ascending aorta with cold saline under anesthesia (8%
sevoflurane) 24 h post-SAH (n=6 per group). Compared with right
hemisphere, left hemisphere is associated with more significant
changes in oxidative stress and inflammatory parameters (21). The cortical tissue from the left
hemisphere was lysed in RIPA buffer (cat. no. P0013C; Beyotime
Institute of Biotechnology) and quantified using the BCA protein
assay. The mixture containing cortical tissues (0.1 ml containing 1
mg), ROS assay medium (2.9 ml; D-PBS, cat. no. C0221D; Beyotime
Institute of Biotechnology) and 2',7'-dichlorofluorescin diacetate
(5 nmol/µl; cat. no. S0033S; Beyotime Institute of Biotechnology)
was incubated at 37˚C for 15 min. The fluorescence intensity was
used to assess ROS production through a microplate reader (485 nm
excitation wavelength; 525 nm emission wavelength; Bio-Rad
Laboratories, Inc.).
Immunofluorescence
Rats were perfused via the left ventricle-ascending
aorta with cold saline under anesthesia (8% sevoflurane) 24 h
post-SAH and then re-infused with 10% neutral-buffered formalin
(n=6 per group). After fixation with 10% neutral-buffered formalin
for 48 h at room temperature, the brain tissues were embedded in
paraffin. Brain tissues were then sectioned (4 µm thick), dewaxed
by xylene and gradient-hydrated by ethanol at room temperature.
After boiling with 3% sodium citrate at 100˚C for 20 min, the
slices were blocked with QuickBlock™ Blocking Buffer for Immunol
Staining (cat. no. P0260; Beyotime Institute of Biotechnology) for
1 h at room temperature and subsequently incubated with primary
monoclonal rabbit antibodies against cleaved caspase-1 (1:500; cat.
no. 4199; Cell Signaling Technology, Inc.) and polyclonal mouse
anti-rat-neuronal nuclei (NeuN; 1:500; cat. no. ab104224; Abcam) at
4˚C overnight. After rinsing with PBS three times, the sections
were incubated with the secondary antibodies (FITC-conjugated goat
anti-rabbit IgG; 1:500; cat. no. A0562; and Cy3-conjugated goat
anti-mouse IgG, 1:500; cat. no. A0521; Beyotime Institute of
Biotechnology) for 1 h at room temperature. Finally, the cell
nuclei were stained with DAPI (5 µg/ml per section; Beyotime
Institute of Biotechnology) for 5 min at room temperature. A
fluorescence microscope (MF43; Guangzhou Micro-shot Technology Co.,
Ltd.) was used to observe six fields of view at magnifications of
x200 and x1,000 in three sections randomly selected from each
group. In each field, the average density of the fluorescence
signals were measured using the Image-pro plus 6.0 software (Media
Cybernetics, Inc.). Cells labeled with cleaved caspase-1 and NeuN
were defined as pyroptotic before being counted.
Western blotting
Following ice-saline perfusion via the left
ventricle-ascending aorta under anesthesia (8% sevoflurane), the
cortical tissue from the left hemisphere was lysed in RIPA buffer
(cat. no. P0013C; Beyotime Institute of Biotechnology) and
assembled to extract total protein (n=6 per group) 24 h post-SAH.
Each sample contained 40 µg protein and was separated by 10%
SDS-PAGE. The separated protein was then transferred onto PVDF
membranes (Beyotime Institute of Biotechnology). After blocking
with buffer (cat. no. P0252, QuickBlock™ Western Blocking Buffer;
Beyotime Institute of Biotechnology) at 25˚C for 10 min, polyclonal
rabbit anti-rat IL-1β antibody (1:1,000; cat. no. K107559P; Beijing
Solarbio Science & Technology Co., Ltd.), polyclonal rabbit
anti-rat IL-18 antibody (1:1,000, cat. no. K002143P; Beijing
Solarbio Science & Technology Co., Ltd.), monoclonal rabbit
anti-rat phosphorylated (p-)-ERK1/2 antibody (1:1,000, cat. no.
AF1891; Beyotime Institute of Biotechnology), monoclonal rabbit
anti-rat ERK1/2 antibody (1:1,000, cat. no. AF1051; Beyotime
Institute of Biotechnology) antibodies, polyclonal rabbit anti-rat
p-p38 antibody (1:1,000, cat. no. AF5887; Beyotime Institute of
Biotechnology), and polyclonal rabbit anti-rat p38 antibody
(1:1,000, cat. no. AF7668; Beyotime Institute of Biotechnology)
were applied overnight at 4˚C. HRP-labeled goat anti-rabbit
secondary antibodies (1:1,000, cat. no. A0208; Beyotime Institute
of Biotechnology) were used to incubate the membranes at room
temperature for 1 h. After rinsing with Western Wash Buffer (cat.
no. P0023C; Beyotime Institute of Biotechnology), the PVDF
membranes were incubated with BeyoECL Moon reagent (cat. no.
P0018FM; Beyotime Institute of Biotechnology) for 5 min. A western
blotting detection system (Gel Doc XRS, Bio-Rad Laboratories, Inc.)
was used to visualize the density of protein bands on the PVDF
membranes. GAPDH (1:1,000; cat. no. K106389P; Beijing Solarbio
Science & Technology Co., Ltd.) was used as an internal
reference and membranes were incubated with this overnight at 4˚C
(22). All western blotting bands
were semi-quantified using Image Lab software 6.0.1 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Fisher's exact test with Bonferroni's correction was
used to assess the difference in mortality between groups (sham vs.
SAH, SAH vs. SAH + H2, SAH + H2 vs. SAH +
H2 + 5-HD, SAH + H2 + 5-HD vs. SAH +
H2 + saline and SAH + H2 vs. SAH +
H2 + saline). The data of neurobehavioral tests are
presented as the median + interquartile range and further analyzed
by Kruskal-Wallis followed by Dunn's post hoc test. The remaining
data are expressed as the mean ± SD. Statistical differences
between the various groups were evaluated by one-way analysis of
variance and Tukey's test. The statistical analysis was assessed by
the SPSS 11.0 software (SPSS, Inc.) and the level of statistically
significant difference was considered at P<0.05.
Results
Mortality
Prior to scheduled euthanasia, 17 rats died due to
cerebral hernia. A total of 5 rats were euthanized due to reaching
humane endpoints such as slow and weak breath, and the remaining 12
rats had died prior to post-operative monitoring. The mortality
rate within 24 h post-SAH was 0% (0 of 24 rats) in the sham group,
20% (6 of 30 rats) in the SAH group, 4% (1 of 25 rats) in the SAH +
H2 group, 22.6% (7 of 31 rats) in the SAH +
H2 + 5-HD group and 11.1% (3 of 27 rats) in the SAH +
H2 + saline group, and there was no significant
difference between sham and SAH, SAH and SAH + H2, SAH +
H2 and SAH + H2 + 5-HD, SAH + H2
5-HD and SAH + H2 + saline, SAH + H2 and SAH
+ H2 + saline groups (Fig.
1A). A total of 16 rats died within 6 h after SAH and 1 rat
died at 18 h after SAH. Compared with rats in the sham group, the
average SAH grades were significantly increased in rats with SAH
exposure in the other four groups (Fig.
1B). There was no significant statistical difference in the
average SAH grades among the remaining four groups based on the SAH
grade (18) (Fig. 1B). This finding indicated a similar
degree of bleeding among the groups.
Neurobehavioral test
Compared with that in rats in the sham group,
neurologic function of the rats in the SAH group was significantly
impaired 24 h after the SAH-operation (P<0.05; Fig. 2). Hydrogen gas post-conditioning
after SAH induction significantly improved the neurobehavioral
score in rats compared with that in rats treated with SAH alone 24
h post-SAH (P<0.05; Fig. 2). By
contrast, whilst 5-HD partially but significantly reversed the
changes mediated by hydrogen gas in the neurobehavioral score in
rats (P<0.05; Fig. 2). There was
no statistically significant difference in the neurological scores
of rats between the SAH + H2 and SAH + H2 +
saline groups (Fig. 2).
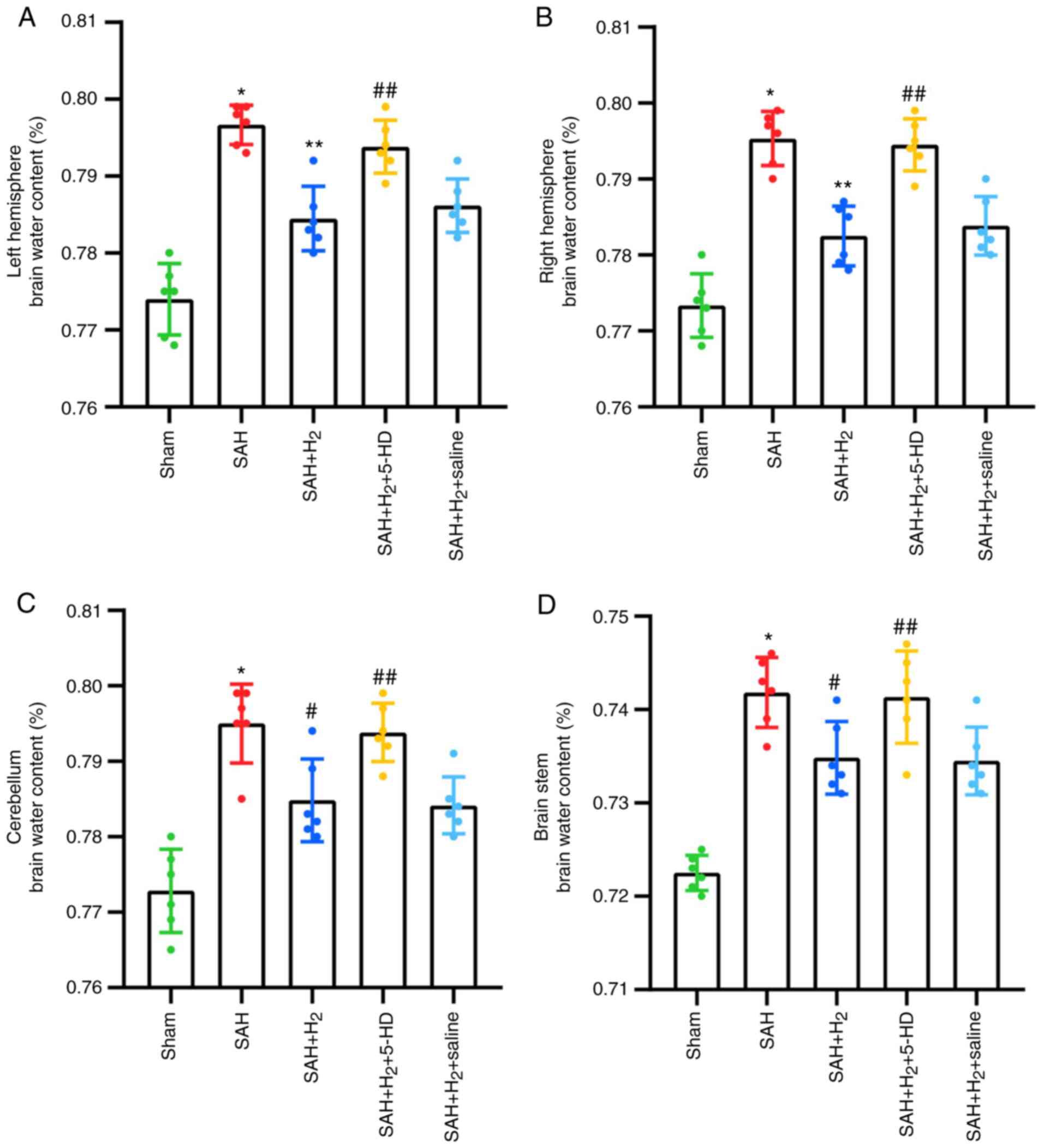
Brain water content
SAH exposure significantly increased the water
content in the bilateral hemispheres, including the right
hemisphere (P<0.001), left hemisphere (P<0.001; Fig. 3), cerebellum (P<0.001; Fig. 3) and the brain stem (P<0.001;
Fig. 3) of rats compared with that
in the rats in the sham group 24 h post-perforation. Hydrogen gas
post-conditioning significantly improved brain edema in the right
hemisphere (P<0.001; Fig. 3),
left hemisphere (P<0.001; Fig.
3), cerebellum (P<0.05; Fig.
3) and the brain stem (P<0.05; Fig. 3) compared with that in the SAH
group. It was shown that these attenuations induced by hydrogen gas
post-conditioning were partially but significantly reversed by 5-HD
administration (left hemisphere, P<0.05; right hemisphere,
P<0.05; cerebellum, P<0.05; brain stem, P<0.05; Fig. 3). Additionally, as a control for
5-HD, saline administration after SAH + H2 treatment
failed to show a significant reversion compared with that after SAH
+ H2 treatment alone (Fig.
3).
ROS production
The level of ROS production in the cortex was
significantly upregulated 24 h post-perforation in the SAH group
compared with that in the Sham group (P<0.05; Fig. 4). ROS production in cortex, however,
was significantly lower in rats exposed to hydrogen gas
post-conditioning after SAH induction compared with that after SAH
alone (P<0.05; Fig. 4). 5-HD
significantly elevated ROS production in the cortex compared with
that in the SAH + H2 group (P<0.05; Fig. 4). However, there was no significant
difference in cortex ROS production between the SAH + H2
and SAH + H2 + saline groups (Fig. 4).
Measurement of pyroptosis
Neuronal pyroptosis in the ipsilateral cortex was
assessed by immunofluorescence after labeling with cleaved
caspase-1- and NeuN-specific antibodies. The levels of cleaved
caspase-1 and NeuN co-staining were significantly elevated 24 h
post-perforation in the SAH group compared with those in the Sham
group (P<0.05; Fig. 5B).
Compared with that in the SAH group, hydrogen gas post-conditioning
significantly attenuated neuronal pyroptosis, as indicated by the
reduced number of dual cleaved caspase-1 and NeuN-positive cells in
the SAH + H2 group (P<0.05; Fig. 5B). Inhibition of mitoKATP
opening using 5-HD significantly increased neuronal pyroptosis in
the SAH + H2 + 5-HD group compared with that in the SAH
+ H2 group (P<0.05; Fig.
5B). In addition, there was no significant difference in
neuronal pyroptosis between the SAH + H2 and SAH +
H2 + saline groups (Fig.
5).
Pyroptosis-associated proteins IL-1β and IL-18, was
investigated further by western blotting. The expression levels of
IL-1β (P<0.05; Fig. 6B) and
IL-18 (P<0.05; Fig. 6C) in
protein samples collected from the ipsilateral cortex was
significantly upregulated in rats exposed to SAH compared with
those in samples from the Sham group. Hydrogen gas
post-conditioning significantly attenuated the increases in IL-1β
(P<0.05; Fig. 6B) and IL-18
(P<0.05; Fig. 6C) compared with
those in the SAH alone group. Consistent with the results from
immunofluorescence, 5-HD significantly reversed the alleviation of
hydrogen gas post-conditioning in the SAH + H2 + 5-HD
group (IL-1β, P<0.05; IL-18, P<0.05; Fig. 6B and C). No significant difference was reported
in the expression of IL-1β and IL-18 between the SAH +
H2 and SAH + H2 + saline groups (Fig. 6).
Phosphorylated ERK1/2 and p38 MAPK
levels
Increased levels of p-ERK1/2 (P<0.05; Fig. 7B) and p-p38 MAPK (P<0.05;
Fig. 7C) were observed in the SAH
group compared with those in the Sham group. Hydrogen gas
post-conditioning potentiated the levels of ERK1/2 phosphorylation
significantly further (P<0.05; Fig.
7B), whilst significantly reducing the phosphorylation of p38
MAPK (P<0.05; Fig. 7C) compared
with those in the SAH only group. A significant reduction in
p-ERK1/2 (P<0.05; Fig. 7B) and
increase in p-p38 MAPK (P<0.05; Fig.
7C) were detected in rats exposed to 5-HD treatment compared
with those in rats in the SAH + H2 group. Additionally,
there was no significant difference in the levels of p-ERK1/2 and
p-p38 MAPK between the SAH + H2 and SAH + H2
+ saline groups (Fig. 7).
Discussion
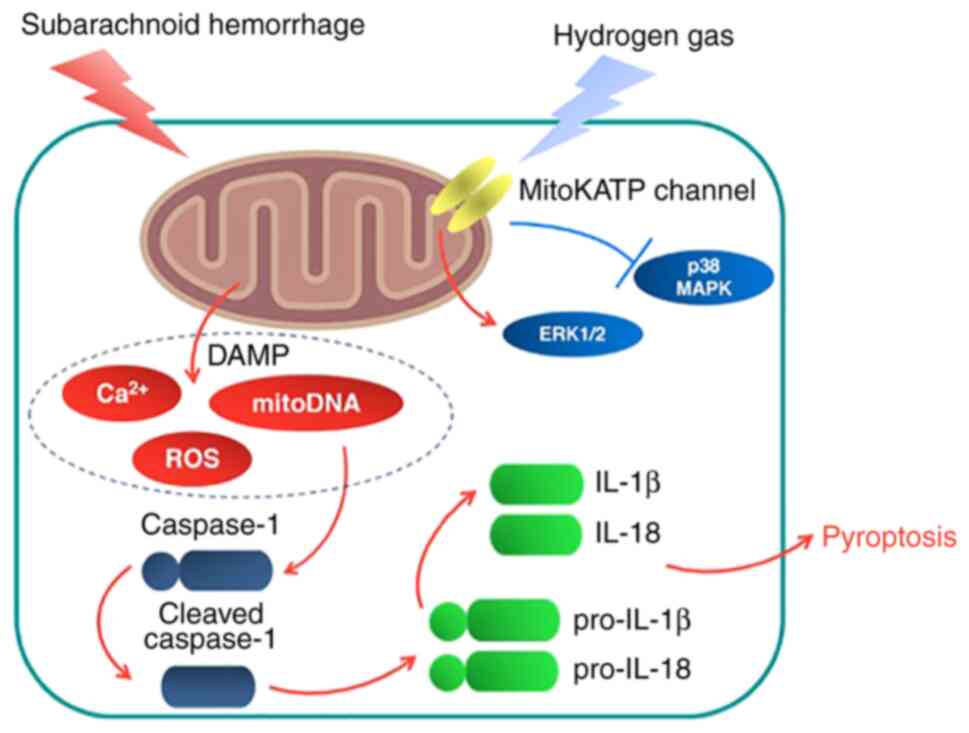
The present study investigated the potential
therapeutic effects of hydrogen gas post-conditioning against
neurological dysfunction, brain edema, ROS production and neuronal
pyroptosis in a rat model of SAH. It was found that the
neuroprotective effects mediated by hydrogen gas against
SAH-induced injury could be at least in part downstream of the
mitoKATP channels (Fig.
8).
Cerebral vasospasm after SAH could lead to
ischemia/reperfusion, which causes mitochondrial dysfunction
(23,24). Mitochondrial dysfunction after SAH
leads to the release of damage-associated molecular patterns
(DAMPs), including ROS, mitochondrial DNA and calcium ions
(25-27).
DAMPs can induce the activation of cytoplasmic inflammasome
complexes, including caspase-1, adaptor protein
apoptosis-associated speck-like protein containing a CARD and
NOD-like receptor (28).
Consequently, activated caspase-1 is activated to drive the
proteolytic cleavage and maturation of precursor cytokines,
including pro-IL-1β and pro-IL-18(29). Moreover, ROS is likely to serve a
major role in activation of caspase-1, which induces pyroptosis and
leads to cellular edema (30). A
number of previous studies have demonstrated that SAH-induced brain
injury can be alleviated by modulating ROS and caspase-dependent
neuronal death (31-33).
In agreement with a previous study, brain edema and cortical
neuronal pyroptosis was observed in a SAH model, where the
pathologic changes may be attributed to deficits in neurologic
function (34). In addition, it was
also shown that brain edema occurred on bilateral hemisphere
regardless of the perforation side (2).
It was previously reported that hydrogen gas
administration attenuated not only ischemic infarction in the
brain, but also reduced hemorrhagic transformation induced by
hyperglycemia in a model of middle cerebral artery occlusion (MCAO)
(35,36). In addition, hydrogen gas
administration exerted significant anti-inflammatory activity
against ischemia/reperfusion injury in various organ
transplantations, including the heart and liver (37,38).
Hydrogen gas can be explosive at concentrations >5%, but it is
neither explosive nor dangerous at low concentrations (39). In the present study, 2.9% hydrogen
gas post-conditioning for 2 h after SAH was used according to a
previous study (2). In addition,
2.9% hydrogen gas inhalation for 2 h ameliorated neurologic
dysfunction, alleviated brain edema and attenuated neuronal
pyroptosis, whereby suggesting that hydrogen gas post-conditioning
can confer therapeutic effects in a SAH rat model.
Neuroprotective effects of exogenous hydrogen gas
has been previously reported to be associated with mitochondrial
regulation (40). Watanabe et
al (41) reported that the
opening/activation of mitoKATP channels are involved in
delaying neuroprotection in a Mongolian gerbil model of MCAO. As a
specific inhibitor, 5-HD has been applied as an approach to study
the involvement of mitoKATP channels (42). A previous study showed that the
mitoKATP channels in heart and liver mitochondria are
the targets for 5-HD, by the inhibition of K+ flux
(43). The present study therefore
assessed the effects of mitoKATP opening on the
neuroprotective effects of hydrogen gas using 5-HD. 5-HD was found
to partially reverse the therapeutic effects of neurologic
dysfunction, attenuation of brain edema and amelioration of
neuronal pyroptosis induced by hydrogen gas post-conditioning after
SAH exposure. These results suggest that the neuroprotective
effects of hydrogen gas post-conditioning may be associated with
mitoKATP channels opening.
The downstream mechanisms underlying
mitoKATP channels in the central nervous system remained
unclear. A previous study reported that the opening of
mitoKATP channels is associated with the increased
levels of phosphorylated ERK1/2 after cerebral ischemia/reperfusion
injury (44). It has also been
suggested that the downregulation of p38 MAPK phosphorylation
contributes to the improvements in cerebral ischemia/reperfusion in
mice (45,46). Chen et al (47) demonstrated that caspase-1-related
pyroptosis is inhibited by downregulation of p38 phosphorylation
MAPK and upregulation of ERK1/2 in mice. The MC4 receptor agonist
RO27-3225 inhibited NLRP1-dependent neuronal pyroptosis via the
ASK1/JNK/p38 MAPK pathway in a mouse model of intracerebral
haemorrhage (47). The changes in
cellular potassium and mitochondrial membrane potential induced by
the opening of mitoKATP channels is likely to contribute
to changes in p38 MAPK and ERK1/2 phosphorylation (48,49).
Data from the present study showed that hydrogen gas significantly
elevated levels of ERK1/2 phosphorylation but attenuated levels of
p38 MAPK phosphorylation. However, 5-HD partially reversed this
upregulation of p-ERK1/2 and downregulation of p-p38 MAPK induced
by exogeneous hydrogen gas. Such a dichotomy of these two kinases,
including p38 MAPK and ERK1/2, was suggested to be involved in
neuronal death induced by cardiopulmonary resuscitation injury
(50). In addition, hydrogen gas
opened mitoKATP channels through direct interaction with
Cys6 and Cys26, which regulate the cellular energy charge to
activate the downstream MAPK pathways (51). Therefore, it could be hypothesized
from these aforementioned observations that activation of the
mitoKATP/ERK1/2/p38 MAPK pathway might be involved as a
potential target of exogenous hydrogen gas.
One limitation of the present study was that the
neuroprotective effects of hydrogen gas 24 h post-SAH were only
observed in this animal model. Long term changes in neurologic
function, brain edema and neuronal pyroptosis, such as 72 h and 1
week after SAH, should be investigated. In addition, only a single
application of hydrogen gas was achieved for 2 h after SAH
exposure. The potential therapeutic effects of repeated
applications of hydrogen gas post-SAH should also be explored.
In conclusion, the present study found that hydrogen
gas post-conditioning exhibited significant neuroprotective effects
against in early-stage SAH, possibly through an anti-pyroptosis
effect downstream of the mitoKATP/ERK1/2/p38 MAPK signal
pathway. Therefore, exogenous hydrogen gas has the potential to
improve early injury during the management of SAH.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Medical Science
Plan of Hebei Province of China (grant no. 20211745).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CSZ was responsible for the design of the study. QH
and CSZ were responsible for statistical analysis. CSZ, QH, ZWS,
HYJ, TPS and YPC were responsible for the experiments and data
collection. CSZ and QH confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The animal protocols included in the present study
were ratified by the Institutional Animal Care and Use Committee at
The Cangzhou Central Hospital (Cangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang C, Li T, Xue H, Wang L, Deng L, Xie
Y, Bai X, Xin D, Yuan H, Qiu J, et al: Inhibition of necroptosis
rescues SAH-induced synaptic impairments in hippocampus via
CREB-BDNF pathway. Front Neurosci. 12(990)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhan Y, Chen C, Suzuki H, Hu Q, Zhi X and
Zhang JH: Hydrogen gas ameliorates oxidative stress in early brain
injury after subarachnoid hemorrhage in rats. Crit Care Med.
40:1291–1296. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xu P, Hong Y, Xie Y, Yuan K, Li J, Sun R,
Zhang X, Shi X, Li R, Wu J, et al: TREM-1 exacerbates
neuroinflammatory injury via NLRP3 inflammasome-mediated pyroptosis
in experimental subarachnoid hemorrhage. Transl Stroke Res: Aug 30,
2020 (Epub ahead of print).
|
|
4
|
Yuan B, Zhou XM, You ZQ, Xu WD, Fan JM,
Chen SJ, Han YL, Wu Q and Zhang X: Inhibition of AIM2 inflammasome
activation alleviates GSDMD-induced pyroptosis in early brain
injury after subarachnoid haemorrhage. Cell Death Dis.
11(76)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou K, Enkhjargal B, Xie Z, Sun C, Wu L,
Malaguit J, Chen S, Tang J, Zhang J and Zhang JH: Dihydrolipoic
acid inhibits lysosomal rupture and NLRP3 through
lysosome-associated membrane protein-1/calcium/calmodulin-dependent
protein kinase II/TAK1 pathways after subarachnoid hemorrhage in
rat. Stroke. 49:175–183. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ruan W, Hu J, Zhou H, Li Y, Xu C, Luo Y,
Chen T, Xu B, Yan F and Chen G: Intranasal wnt-3a alleviates
neuronal apoptosis in early brain injury post subarachnoid
hemorrhage via the regulation of wnt target PPAN mediated by the
moonlighting role of aldolase C. Neurochem Int.
134(104656)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shao A, Wu H, Hong Y, Tu S, Sun X, Wu Q,
Zhao Q, Zhang J and Sheng J: Hydrogen-rich saline attenuated
subarachnoid hemorrhage-induced early brain injury in rats by
suppressing inflammatory response: Possible involvement of NF-κB
pathway and NLRP3 inflammasome. Mol Neurobiol. 53:3462–3476.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Xie K, Zhang Y, Wang Y, Meng X, Wang Y, Yu
Y and Chen H: Hydrogen attenuates sepsis-associated encephalopathy
by NRF2 mediated NLRP3 pathway inactivation. Inflamm Res.
69:697–710. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ono H, Nishijima Y, Ohta S, Sakamoto M,
Kinone K, Horikosi T, Tamaki M, Takeshita H, Futatuki T, Ohishi W,
et al: Hydrogen gas inhalation treatment in acute cerebral
infarction: A randomized controlled clinical study on safety and
neuroprotection. J Stroke Cerebrovasc Dis. 26:2587–2594.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang Y, Tan S, Xu J and Wang T: Hydrogen
therapy in cardiovascular and metabolic diseases: From bench to
bedside. Cell Physiol Biochem. 47:1–10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Uto K, Sakamoto S, Que W, Shimata K,
Hashimoto S, Sakisaka M, Narita Y, Yoshii D, Zhong L, Komohara Y,
et al: Hydrogen-rich solution attenuates cold ischemia-reperfusion
injury in rat liver transplantation. BMC Gastroenterol.
19(25)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Walewska A, Szewczyk A and Koprowski P:
Gas signaling molecules and mitochondrial potassium channels. Int J
Mol Sci. 19(3227)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim
HR, Jeon SB, Jeon WK, Chae HJ and Chung HT: Hydrogen sulfide
inhibits nitric oxide production and nuclear factor-kappaB via heme
oxygenase-1 expression in RAW264.7 macrophages stimulated with
lipopolysaccharide. Free Radic Biol Med. 41:106–119.
2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Carreras MC and Poderoso JJ: Mitochondrial
nitric oxide in the signaling of cell integrated responses. Am J
Physiol Cell Physiol. 292:C1569–C1580. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhai Y, Zhou X, Dai Q, Fan Y and Huang X:
Hydrogen-rich saline ameliorates lung injury associated with cecal
ligation and puncture-induced sepsis in rats. Exp Mol Pathol.
98:268–276. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
King AL and Lefer DJ: Cytoprotective
actions of hydrogen sulfide in ischaemia-reperfusion injury. Exp
Physiol. 96:840–846. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sugawara T, Ayer R, Jadhav V, Chen W,
Tsubokawa T and Zhang JH: Simvastatin attenuation of cerebral
vasospasm after subarachnoid hemorrhage in rats via increased
phosphorylation of Akt and endothelial nitric oxide synthase. J
Neurosci Res. 86:3635–3643. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sehba FA: The rat endovascular perforation
model of subarachnoid hemorrhage. Acta Neurochir Suppl.
120:321–324. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Garcia JH, Wagner S, Liu KF and Hu XJ:
Neurological deficit and extent of neuronal necrosis attributable
to middle cerebral artery occlusion in rats. Statistical
validation. Stroke. 26:627–635. 1995.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu W, Li R, Yin J, Guo S, Chen Y, Fan H,
Li G, Li Z, Li X, Zhang X, et al: Mesenchymal stem cells alleviate
the early brain injury of subarachnoid hemorrhage partly by
suppression of Notch1-dependent neuroinflammation: Involvement of
botch. J Neuroinflammation. 16(8)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang DX, Zhang LM, Zhao XC and Sun W:
Neuroprotective effects of erythropoietin against
sevoflurane-induced neuronal apoptosis in primary rat cortical
neurons involving the EPOR-Erk1/2-Nrf2/Bach1 signal pathway. Biomed
Pharmacothery. 87:332–341. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang XX, He FF, Yan GL, Li HN, Li D, Ma
YL, Wang F, Xu N and Cao F: Neuroprotective effect of cerebralcare
granule after cerebral ischemia/reperfusion injury. Neural Regen
Res. 11:623–629. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Huang S, Li H and Ge J: A cardioprotective
insight of the cystathionine γ-lyase/hydrogen sulfide pathway. Int
J Cardiol Heart Vasc. 7:51–57. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wellman GC: Ion channels and calcium
signaling in cerebral arteries following subarachnoid hemorrhage.
Neurol Res. 28:690–702. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chaudhry SR, Frede S, Seifert G, Kinfe TM,
Niemelä M, Lamprecht A and Muhammad S: Temporal profile of serum
mitochondrial DNA (mtDNA) in patients with aneurysmal subarachnoid
hemorrhage (aSAH). Mitochondrion. 47:218–226. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhou K, Shi L, Wang Z, Zhou J, Manaenko A,
Reis C, Chen S and Zhang J: RIP1-RIP3-DRP1 pathway regulates NLRP3
inflammasome activation following subarachnoid hemorrhage. Exp
Neurol. 295:116–124. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Van Opdenbosch N, Gurung P, Vande Walle L,
Fossoul A, Kanneganti TD and Lamkanfi M: Activation of the NLRP1b
inflammasome independently of ASC-mediated caspase-1
autoproteolysis and speck formation. Nat Commun.
5(3209)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Samir P, Kesavardhana S, Patmore DM,
Gingras S, Malireddi RKS, Karki R, Guy CS, Briard B, Place DE,
Bhattacharya A, et al: DDX3X acts as a live-or-die checkpoint in
stressed cells by regulating NLRP3 inflammasome. Nature.
573:590–594. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hoque R, Sohail M, Malik A, Sarwar S, Luo
Y, Shah A, Barrat F, Flavell R, Gorelick F, Husain S and Mehal W:
TLR9 and the NLRP3 inflammasome link acinar cell death with
inflammation in acute pancreatitis. Gastroenterology. 141:358–369.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang ZY, Jiang M, Fang J, Yang MF, Zhang
S, Yin YX, Li DW, Mao LL, Fu XY, Hou YJ, et al: Enhanced
therapeutic potential of nano-curcumin against subarachnoid
hemorrhage-induced blood-brain barrier disruption through
inhibition of inflammatory response and oxidative stress. Mol
Neurobiol. 54:1–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang Z, Liu J, Fan C, Mao L, Xie R, Wang
S, Yang M, Yuan H, Yang X, Sun J, et al: The GluN1/GluN2B NMDA
receptor and metabotropic glutamate receptor 1 negative allosteric
modulator has enhanced neuroprotection in a rat subarachnoid
hemorrhage model. Exp Neurol. 301:13–25. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang W, Han P, Xie R, Yang M, Zhang C, Mi
Q, Sun B and Zhang Z: TAT-mGluR1 attenuation of neuronal apoptosis
through prevention of MGluR1α truncation after experimental
subarachnoid hemorrhage. ACS Chem Neurosci. 10:746–756.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen J, Zhang C, Yan T, Yang L, Wang Y,
Shi Z, Li M and Chen Q: Atorvastatin ameliorates early brain injury
after subarachnoid hemorrhage via inhibition of pyroptosis and
neuroinflammation. J Cell Physiol: Mar 31, 2021 (Epub ahead of
print).
|
|
35
|
Chen L, Chao Y, Cheng P, Li N, Zheng H and
Yang Y: UPLC-QTOF/MS-based metabolomics reveals the protective
mechanism of hydrogen on mice with ischemic stroke. Neurochem Res.
44:1950–1963. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen CH, Manaenko A, Zhan Y, Liu WW,
Ostrowki RP, Tang J and Zhang JH: Hydrogen gas reduced acute
hyperglycemia-enhanced hemorrhagic transformation in a focal
ischemia rat model. Neuroscience. 169:402–414. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Buchholz BM, Kaczorowski DJ, Sugimoto R,
Yang R, Wang Y, Billiar TR, McCurry KR, Bauer AJ and Nakao A:
Hydrogen inhalation ameliorates oxidative stress in transplantation
induced intestinal graft injury. Am J Transplant. 8:2015–2024.
2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yao L, Chen H, Wu Q and Xie K:
Hydrogen-rich saline alleviates inflammation and apoptosis in
myocardial I/R injury via PINK-mediated autophagy. Int J Mol Med.
44:1048–1062. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ohno K and Ito M, Ichihara M and Ito M:
Molecular hydrogen as an emerging therapeutic medical gas for
neurodegenerative and other diseases. Oxid Med Cell Longev.
2012(353152)2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Noda M, Liu J and Long J: Neuroprotective
and preventative effects of molecular hydrogen. Curr Pharm Des.
27:585–591. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Watanabe M, Katsura K, Ohsawa I, Mizukoshi
G, Takahashi K, Asoh S, Ohta S and Katayama Y: Involvement of
mitoKATP channel in protective mechanisms of cerebral ischemic
tolerance. Brain Res. 1238:199–207. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Garlid KD, Paucek P, Yarov-Yarovoy V,
Murray HN, Darbenzio RB, D'Alonzo AJ, Lodge NJ, Smith MA and Grover
GJ: Cardioprotective effect of diazoxide and its interaction with
mitochondrial ATP-sensitive K+ channels. Possible mechanism of
cardioprotection. Circ Res. 81:1072–1082. 1997.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jabůrek M, Yarov-Yarovoy V, Paucek P and
Garlid KD: State-dependent inhibition of the mitochondrial KATP
channel by glyburide and 5-hydroxydecanoate. J Biol Chem.
273:13578–13582. 1998.PubMed/NCBI
|
|
44
|
Naitoh K, Ichikawa Y, Miura T, Nakamura Y,
Miki T, Ikeda Y, Kobayashi H, Nishihara M, Ohori K and Shimamoto K:
MitoKATP channel activation suppresses gap junction permeability in
the ischemic myocardium by an ERK-dependent mechanism. Cardiovasc
Res. 70:374–383. 2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bu X, Huang P, Qi Z, Zhang N, Han S, Fang
L and Li J: Cell type-specific activation of p38 MAPK in the brain
regions of hypoxic preconditioned mice. Neurochem Int. 51:459–466.
2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li J, Lang MJ, Mao XB, Tian L and Feng YB:
Antiapoptosis and mitochondrial effect of pioglitazone
preconditioning in the ischemic/reperfused heart of rat. Cardiovasc
Drugs Ther. 22:283–291. 2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen S, Zuo Y, Huang L, Sherchan P, Zhang
J, Yu Z, Peng J, Zhang J, Zhao L, Doycheva D, et al: The
MC4 receptor agonist RO27-3225 inhibits NLRP1-dependent
neuronal pyroptosis via the ASK1/JNK/p38 MAPK pathway in a mouse
model of intracerebral haemorrhage. Br J Pharmacol. 176:1341–1356.
2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Subramaniam S, Strelau J and Unsicker K:
GDNF prevents TGF-beta-induced damage of the plasma membrane in
cerebellar granule neurons by suppressing activation of p38-MAPK
via the phosphatidylinositol 3-kinase pathway. Cell Tissue Res.
331:373–383. 2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kello M, Kulikova L, Vaskova J, Nagyova A
and Mojzis J: Fruit peel polyphenolic extract-induced apoptosis in
human breast cancer cells is associated with ROS production and
modulation of p38MAPK/Erk1/2 and the akt signaling pathway. Nutr
Cancer. 69:920–931. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pan H, Yu M, Chen M, Wang X, Zhang H, Du S
and Yu S: miR-126 suppresses neuronal apoptosis in rats after
cardiopulmonary resuscitation via regulating p38MAPK. Hum Exp
Toxicol. 39:563–574. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Matei N, Camara R and Zhang JH: Emerging
mechanisms and novel applications of hydrogen gas therapy. Med Gas
Res. 8:98–102. 2018.PubMed/NCBI View Article : Google Scholar
|