Introduction
There are two types of testis-specific cell-cell
actin-based anchoring junctions (AJs) observed in the seminiferous
epithelium, i.e., apical ectoplasmic specialization (ES) and basal
ES (1). The apical ES exists
between the interface of Sertoli cells and the step 8-19 spermatids
in the adluminal compartment of the epithelium, using the
cadherin/catenin, nectin/afadin, and integrin/laminin adhesion
protein complexes as structural and functional units (2). Furthermore, the apical ES functions as
the only anchoring installation to maintain spermatid polarity,
provide the necessary nutrients and hormonal support for
spermatids, and take part in signal transduction during
spermiogenesis (3-5).
The basal ES is located between Sertoli cells, and between Sertoli
cells and spermatocytes in the basal compartment of the epithelium
(6,7); the former basal ES forms the
blood-testis barrier (BTB) combined with tight junctions (TJs) and
gap junctions, which build a specialized microenvironmental and
immune barrier for spermatogenesis and permit the timely transit of
preleptotene and leptotene spermatocytes at stage VIII. The
dynamics of AJs can be regulated by several AJ-associated signaling
molecules, including focal adhesion kinase (FAK), Src, Csk,
cytokeratin 2, P120ctn, small GTPases (e.g., RhoB, Rac1
and Cdc42) and cytokines, by affecting the phosphorylation status
of AJ-associated structural protein complexes, the organization of
the actin-based filament network, and the activation of signaling
pathways related to actin polymerization and cell adhesion
(6,8-12).
Additionally, activation of the mitogen-activated protein kinase
(MAPK) and phosphatidylinositol 3-kinase (PI3K)/Akt signaling
pathways is involved in the disruption of AJs, likely via
alterations to the organization of the actin filament-based
cytoskeleton in the testes (13,14).
As a tight functional and structural link between TJs and AJs, the
effectors of TJ dynamics are also important putative modulators of
AJ dynamics (e.g., hormones and cytokines), acting in a direct or
indirect manner.
FAK is a nonreceptor protein tyrosine kinase that is
linked to occludin/zonula occludens-1 and integrin/laminin protein
complexes, forming a functional unit at TJ and AJ sites,
respectively, in the seminiferous epithelium (14,15).
In addition, FAK is involved in the regulation of AJ and TJ
dynamics by switching its specific phosphorylated form (e.g.,
p-FAK-Tyr397, p-FAK-Tyr407 and
p-FAK-Tyr576), which shows different localizations and
functions in the seminiferous epithelium in vivo and in
vitro (5,16-19).
For example, at the TJ site in vitro,
p-FAK-Tyr397 and p-FAK-Tyr407 work as an
integrated component protein to regulate TJs, with the former
promoting assembly and the latter facilitating disassembly of TJs
by regulating the activation of actin-related protein (Arp)
2/3(19). At the apical ES in
vivo, p-FAK-Tyr407 and p-FAK-Tyr397 are
predominantly localized at the concave and convex sides of the
spermatid heads in stage VII to early stage VIII, respectively. The
former interacts with Arp 2/3, and the latter associates with
α6β1-integrin, forming a functional unit during spermiation
(8,19). In addition, p-FAK-Tyr397
is the only phosphorylated form of FAK that provides a combined
binding site for various signaling molecules, such as the Src
homology domain 2 (SH2) (20) and
PI3K (21), demonstrating
involvement in integrin-initiated signaling pathways at the plasma
membrane and various biological events.
Varicocele is the primary cause of male infertility
and can be described as excessive dilatation of the spermatic vein.
However, the mechanisms through which varicocele contributes to
male infertility are remain unclear, and to the best of our
knowledge, effective treatments have not yet been reported
(22-26).
In 1981, an experimental varicocele model was established through
partial ligation of the left kidney vein (26). In addition, damage to the BTB,
dysfunction of the neuroendocrine system, increased temperature,
hypoxia, accumulation of metabolites and toxicants, and oxidative
pressure are also involved in the pathological mechanisms of
varicocele (27,28). However, to the best of our
knowledge, the disruption of AJs and the corresponding mechanisms
induced by varicocele have not yet been reported.
Therefore, the present study evaluated the
characteristics and changes to AJs in an experimental model of
varicocele in rats. We aimed to identify new targets for the
prevention and treatment of varicocele and for the improvement of
male fertility.
Materials and methods
Reagents
The main reagents used in the present study were as
follows: Propidium iodide (PI)/RNase Staining Buffer (BD
Biosciences), horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG (cat. no. A0208; Beyotime Institute of
Biotechnology), HRP-conjugated goat anti-mouse IgG (cat. no.
IH-0031; Dingguo Changsheng Biotechnology Co., Ltd.),
radioimmunoprecipitation lysis buffer (RIPA; Beyotime Institute of
Biotechnology), phenylmethanesulfonyl fluoride (PMSF; Beyotime
Institute of Biotechnology), prestained protein ladder (Thermo
Fisher Scientific, Inc.), polyvinylidene difluoride (PVDF)
membranes (EMD Millipore), enhanced chemiluminescence reagent (ECL;
Beyotime Institute of Biotechnology), and Enhanced BCA Protein
Assay Kit (Beyotime Institute of Biotechnology), bovine serum
albumin (cat. no. ST023; Beyotime Institute of Biotechnology). The
primary antibodies and working dilutions are presented in Table I.
 | Table IPrimary antibodies used in the
western blot analysis. |
Table I
Primary antibodies used in the
western blot analysis.
| Antibody | Supplier | Cat. no. | Dilution |
|---|
| Rabbit
anti-N-cadherin | Elabscience
Biotechnology, Inc. | ENT2988 | 1:500 |
| Rabbit
anti-E-cadherin | Elabscience
Biotechnology, Inc. | ENT1454 | 1:200 |
| Rabbit
anti-α-catenin | Elabscience
Biotechnology, Inc. | ENT0669 | 1:500 |
| Rabbit
anti-β-catenin | Beijing
Biosynthesis Biotechnology Co., Ltd. | bs-1165R | 1:200 |
| Rabbit
anti-γ-catenin | Beijing
Biosynthesis Biotechnology, Co., Ltd. | bs-6990R | 1:200 |
| Rabbit
anti-FAK | Cell Signaling
Technology, Inc. | 3285 | 1:500 |
| Rabbit
anti-Phospho-FAK-Tyr397 | Cell Signaling
Technology, Inc. | 8556 | 1:500 |
| Rabbit
anti-Src | Cell Signaling
Technology, Inc. | 2108 | 1:1,000 |
| Rabbit
anti-phospho-Src-Tyr416 | Cell Signaling
Technology, Inc. | 6943 | 1:1,000 |
| Rabbit
anti-ERK1/2 | Cell Signaling
Technology, Inc. | 9102 | 1:1,000 |
| Rabbit
anti-phospho-ERK1/2 | Cell Signaling
Technology, Inc. | 9101 | 1:1,000 |
| β-actin mouse
monoclonal antibody | Beyotime Institute
of Biotechnology | AF0003 | 1:1,000 |
Animals and surgical procedure
A total of 60 male Sprague-Dawley rats (age, 6-7
weeks), weighing 200±20 g, were equally and randomly divided into
four groups: i) The sham group for 8 weeks (Sham for 8 weeks); ii)
the sham group for 12 weeks (Sham for 12 weeks); iii) the
experimental varicocele group for 8 weeks (VC for 8 weeks); iv) and
the experimental varicocele group for 12 weeks (VC for 12 weeks).
Prior to surgery, all rats were housed in normal atmosphere
(N2, 78%; O2, 21%; CO2, 0.03%) and
specific pathogen-free controlled environmental conditions, with a
12-h day/night cycle, a temperature of ~23˚C, a humidity of 40-70%
and free access to standard rat food and water. As presented in
Fig. 1, after 12 h fasting, the
left renal vein of animals in the experimental varicocele model
groups underwent partial ligation under anesthesia using 30 mg/kg
sodium pentobarbital through intraperitoneal injection to establish
the experimental left varicocele, as previously described (24), and the surgery lasted for ~10 min.
Rats in the sham group underwent the same operation without the
left vein ligation. Rats were housed alone following surgery, and
were fully recovered after ~7 days. The penicillin was used to
prevent infection via injection into the abdomen post-surgery. At 8
and 12 weeks after the operation, rats were sacrificed via cervical
dislocation after being anesthetized using 30 mg/kg sodium
pentobarbital via intraperitoneal injection to gain fresh left
testicular tissue. Animals in the experimental varicocele model
group who exhibited atrophic left kidney, or those that did not
show vascular dilation, were excluded from subsequent experiments.
A total of 30 rats that underwent surgery exhibited left vascular
dilation without left kidney atrophying and were used to perform
the subsequent experiment. The success rate of experimental
varicocele model establishment in the present study was 100%, and
no animals died during the procedures. All animals were bred and
maintained at the Laboratory Animal Center of Fujian Medical
University (Fuzhou, Fujian, China). Rats were originally obtained
from the National Seed Center of Experimental Rodent Animals
(Beijing, China). The experimental procedures were performed in
accordance with the Guidelines for the Care and Use of Laboratory
Animals approved by Fujian Medical University (Animal Approval
Committee no. #SYXK-2012-0001).
Hematoxylin and eosin (H&E)
staining
At 8 and 12 weeks after the operation, six animals
from each group were anesthetized using 30 mg/kg sodium
pentobarbital via intraperitoneal injection followed by cervical
dislocation to obtain the left testes after cardiac perfusion using
500 ml saline (0.9% sodium chloride) and 500 ml 10% formalin
sequentially. The left testes were then removed and placed in 10%
formalin solution for 24 h for post fixation. Next, the fixed left
testicular tissues were embedded in paraffin, cut into 5-µM-thick
sections, set on poly-L-lysine-coated slides, stained with H&E,
and observed using a light microscope at different magnifications
(x100, x400 and x1,000) (29). The
percentage of tubules containing deciduous immature germ cells was
calculated by counting the positive tubules out of 50 seminiferous
tubules from five to ten randomly selected fields for each
sample.
Flow cytometry analysis
Dissociation of left testicular tissues was
performed as previously described to obtain a single cell
suspension for the flow cytometry analysis (30). Briefly, ~10 mg left testicular
tissue from the six rats in each group was cut and removed in
precooled phosphate-buffered saline (PBS) to remove the tunica
albuginea and visible vessels. The tissue was then placed in 1 ml
digestion medium (1 mg/ml collagenase, 1 mg/ml hyaluronidase and 1
mg/ml DNAse I in Dulbecco's modified Eagle's medium/F12) and minced
with McPherson-Vannas scissors carefully until there were no
obvious visible tissue masses. Next, the tissue digestion
suspension was incubated at 37˚C for 30 min with gentle rotation,
followed by filtration with a 300-mesh nylon net to obtain a
single-cell suspension. The suspension was centrifuged to collect
single cells at 1,500 x g for 4 min at 4˚C. The cells were then
washed with PBS three times, followed by the addition of 1 ml 75%
alcohol and incubation for 12 h at 4˚C to fix the cells. Next, the
75% alcohol was removed via centrifugation at 1,500 x g for 5 min
at 4˚C, followed by washing the cells with PBS three times to
remove any remaining alcohol. Lastly, 500 µl PI/RNase Staining
Buffer was added to each sample, and the reaction mixture was
incubated at 37˚C for 15 min in the dark. The cell cycle was
analyzed using a flow cytometer (NovoCyte; Agilent Technologies,
Inc.) with a 488-nm excitation laser and 575/26-nm bandpass filters
for collection. NovoExpress 1.0.2 software (Agilent Technologies,
Inc.) was used to analysis the data.
Transmission electron microscopy
Testicular tissue was obtained and cut into small
pieces (2x2 mm). The tissues were then immersed in 3%
glutaraldehyde/1.5% paraformaldehyde/0.1 M PBS (pH 7.2) for at
least 2 h at 4˚C. After washing with 0.1 M PBS (pH 7.2) in
triplicate, tissues were fixed in 1% osmium/1.5% potassium
ferrocyanide for 1.5 h at 4˚C, followed by washing with 0.1 M PBS
(p 7.2) in triplicate. The tissues were then dehydrated using 50%
alcohol for 10 min, 70% alcohol saturated uranium acetate solution
for 12 h, 90% alcohol for 10 min, 90% alcohol/10% acetone for 10
min, 90% acetone/10% alcohol for 10 min and 100% acetone for 10
min. All dehydration procedures were performed at 4˚C. The tissues
were placed in 50% acetone/50% epoxy resin (618/E-51) for 1.5 h at
35˚C and 100% epoxy resin (618/E-51) for 3 h at 35˚C. Subsequently,
the tissues were embedded in epoxy resin (618/E-51) for 12 h at
35˚C, 12 h at 45˚C and 3 days for 60˚C. The sections (70-80 nm)
were stained using 2% uranium acetate for 5 min and lead citrate
for 5 min, both at room temperature, followed by washing with
double distilled water in triplicate. The ultrastructure of the
testis was observed using a transmission electron microscope (EM208
model; Philips Medical Systems, Inc.) with assistance from the
laboratory staff of Fujian Medical University (Fuzhou, Fujian,
China) trained in electron microscopy.
Western blot analysis
A total of 1 ml RIPA lysis buffer with 10 µl PMSF
and 10 µl phosphatase inhibitor cocktail was added to 100 mg left
testicular tissues to extract total protein on ice. The
concentration of total protein was measured using Enhanced BCA
Protein Assays. Proteins (40 µg per lane) were then separated via
8% SDS-PAGE. A molecular weight loading marker (2 µl) was used to
determine the relative molecular weights of the proteins. The
proteins on the gel were transferred to PVDF membranes, and the
membranes were then blocked in 5% bovine serum albumin for 2 h at
4˚C, followed by incubation with the specific primary antibodies
listed in Table I at 37˚C for at
least 12 h. The membranes were then incubated with the
corresponding horseradish peroxidase-conjugated goat anti-rabbit
IgG and goat anti-mouse IgG secondary antibodies (1:1,000) for 2 h
at 4˚C. Finally, the specific protein bands were detected using ECL
reagent, and images were captured using a biological imaging
system. ImageJ software (ImageJ 1.46r; National Institutes of
Health) was used to calculate densitometry.
Statistical analysis
All experimental images were obtained from at least
three rats in each group independently, and representative images
were selected and exhibited in this article. The experimental data
obtained from at least six rats in each group were analyzed with
SPSS software (version 21.0; SPSS, Inc.) and presented as the mean
± standard deviation (SD). One-way analysis of variance (AVOVA)
followed by the Tukey post hoc test was used to analyze the
significance of differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphology of the left testes
Representative H&E staining images are presented
in Fig. 2. The seminiferous
epithelium morphology of the testes from the experimental
varicocele group was disrupted at 8 (Fig. 2C) and 12 weeks (Fig. 2D) compared with that in the sham
group at 8 (Fig. 2A) and 12 weeks
(Fig. 2B), respectively.
Disorganization of germ cells, atrophy of seminiferous tubules,
excessive space in the testis mesenchyme, reduced numbers of germ
cells and mature sperm, and shedding of immature germ cells (black
arrowheads in Fig. 2C and D) in the cavity were observed at different
stages in the experimental varicocele group. The percentage of
tubules containing deciduous immature germ cells is presented in
Fig. 2E. The results demonstrated
that the experimental varicocele caused a loss of immature germ
cells compared with that in the sham group (P<0.05). The
percentage was higher in the experimental varicocele group at 12
weeks than at 8 weeks (P<0.05).
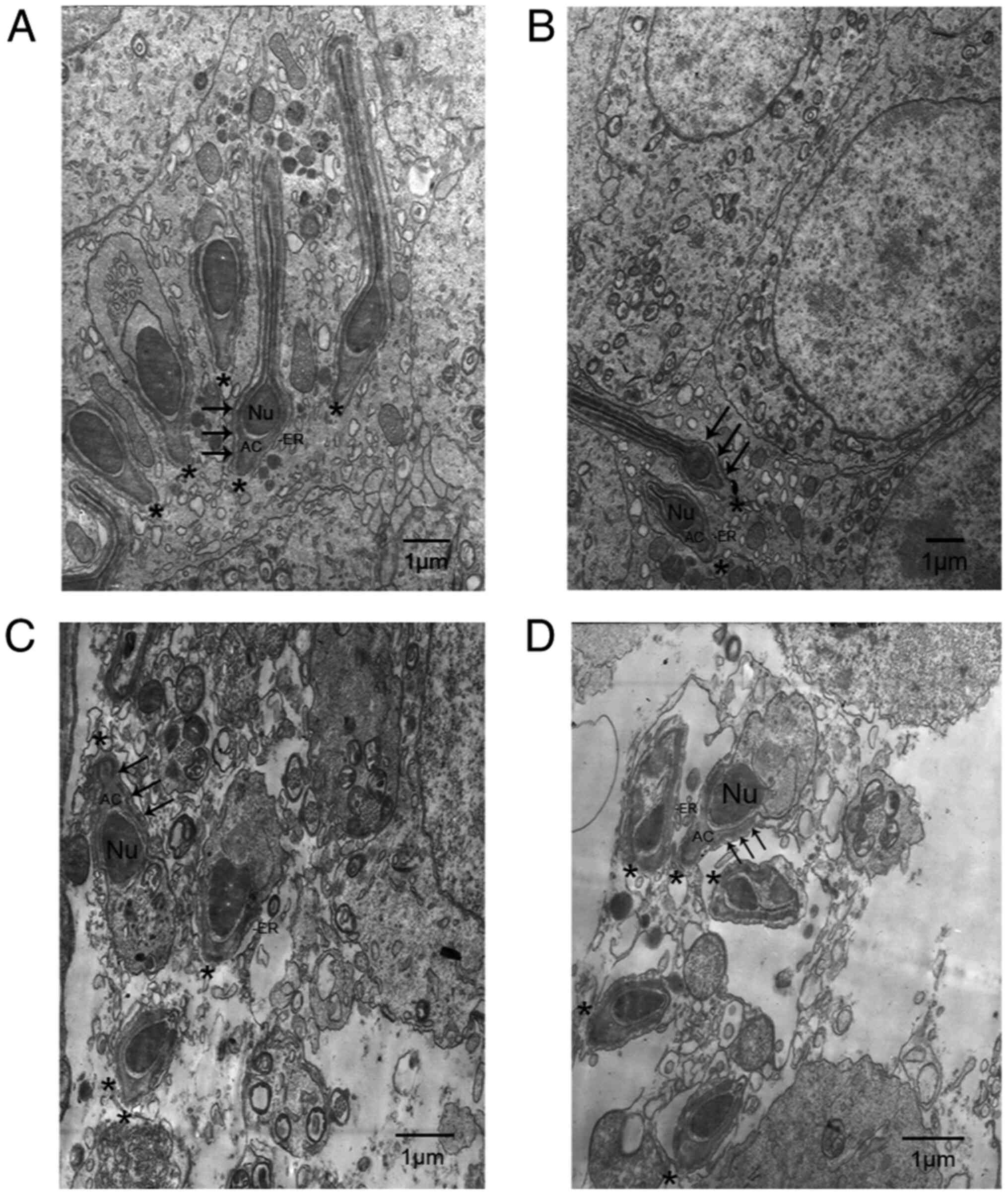
The ultrastructure of apical ES in the left testes
was observed using transmission electron microscopy (Fig. 3). In the sham group at 8 (Fig. 3A) and 12 weeks (Fig. 3B), the heads of elongating
spermatids (black asterisk in Fig.
3A and B) were oriented towards
the basement membrane, and the elongating spermatids were tightly
attached to Sertoli cells via the apical ES. The apical ES in the
normal rat testis (Fig. 3A and
B) was characterized by a
testis-specific structure, which was only observed in Sertoli
cells. The actin filament bundles (black arrowhead) were sandwiched
between the endoplasmic reticulum (ER) and the Sertoli cell
membrane. In contrast, in rat testes in the experimental varicocele
groups at 8 (Fig. 3C) and 12 weeks
(Fig. 3D) following surgery, the
structure of the apical ES was disrupted, and the elongating
spermatids showed loss of orientation toward the basement membrane
(black asterisk in Fig. 3C and
D). Furthermore, the actin filament
bundles (black arrowhead in Fig. 3C
and D) and ER, important component
structures of the apical ES, were disorganized or even absent from
their proper position.
Dysregulation of cell cycle in left
testicular cells from experimental varicocele rats
As presented in Fig.
4E, the experimental varicocele resulted in deregulation of the
cell cycle in the left testes. The percentages of cells in the
G1 and G2/M phases in the experimental
varicocele group (Fig. 4C and
D) were lower than those in the
sham group (Fig. 4A and B; P<0.05) and the percentage of cells
in the S phase in the experimental varicocele group was higher than
that in the sham group (P<0.05).
Expression of AJ component proteins in
the left testes of rats
The expression levels of AJ structural proteins,
i.e., N-cadherin, E-cadherin, α-catenin, β-catenin and γ-catenin,
were analyzed using western blotting (Fig. 5A). The results (Fig. 5B) indicated that the varicocele
resulted in a decrease in the expression of AJ structural proteins
(P<0.05).
Changes in FAK phosphorylation and ERK
signal activation
The levels of FAK, p-FAK-Tyr397, Src,
p-Src-Tyr416, ERK1/2 and p-ERK1/2 were analyzed using
western blotting (Fig. 6A and
B). The experimental varicocele
increased the levels of p-FAK-Tyr397,
p-Src-Tyr416 and p-ERK1 (P<0.05).
 | Figure 6Expression of AJ-associated signaling
molecules. (A) Immunoblot analysis of AJ-associated signaling
molecules. (B) Summary of immunoblotting results. The levels of
FAK, p-FAK-Tyr397, Src, p-Src-Tyr416, ERK1/2
and p-ERK1/2 were investigated using western blotting. The data are
presented as the mean ± SD (n=3). *P<0.05 vs. the
sham group for 8 weeks; #P<0.05 vs. the sham group
for 12 weeks; ns, not significant; AJ, anchoring junction; p,
phosphorylated; VC, varicocele; FAK, focal adhesion kinase; ERK,
extracellular signal-regulated protein kinase. |
Discussion
During spermatogenesis, germ cells differentiate
from the spermatogonium to mature spermatids and must undergo a
series of complicated biological processes (31). The AJs between Sertoli cells and
germ cells function as anchors and supply nutrients, and also
provide an important platform for signal transduction involved in
the cell cycle, seminiferous epithelium cycle, cell differentiation
and cell communication (6).
Notably, the germ cells must move through the TJ to enter into the
adluminal compartment from the basal compartment and finally
undergo spermiation (6). Therefore,
the AJs existing between Sertoli cells and germ cells are not
stable and undergo extensive and consistent assembling and
disassembling, leading to an increased sensitivity to
microenvironmental change, external stimuli and toxicants (6). The varicocele is a common disease of
the male reproductive system and the main cause of male
infertility; however, the specific underlying mechanisms have not
yet been identified (32). The
present study revealed that varicocele caused loss of premature
germ cells from the seminiferous epithelium, damage to the AJ
ultrastructure, disorientation of spermatid heads, dysregulation of
the cell cycle and downregulation of AJ structural proteins
(N-cadherin, E-cadherin, α-catenin, β-catenin and γ-catenin). Taken
together, these results supported the disruption of AJs directly or
indirectly in the left testes after induction of experimental
varicocele. These data provide insights into the pathophysiology of
varicocele and will be useful for the development of new treatments
and diagnostic techniques for the disease.
Activation of the MAPK and PI3K/AKT signaling
pathways is involved in the disruption of AJs and TJs, likely by
perturbing the bundling of actin at AJ or TJ sites. This mechanism
has been extensively studied with regard to reproductive toxicology
induced by various environmental toxicants (e.g., cadmium and
bisphenol A) and the effect of cytokines [e.g., transforming growth
factor (TGF)-β3 and tumor necrosis factor (TNF)-α] on
spermatogenesis in vivo and in vitro. The
administration of specific inhibitors targeting the MAPK and
PI3K/AKT signaling pathways could delay or block toxicant-induced
BTB disruption and immature germ cell loss from the epithelium
(13,14,33-38).
Over the last decade, studies have shown that the ERK signaling
pathway plays a crucial role in actin polymerization and cell
adhesion (39-41).
Furthermore, ERK regulates the phosphorylation of myosin
light-chain kinase and directly affects its biological activity
(42). Therefore, the dynamics of
actin-based AJs are closely associated with the activation of ERK.
The results of the present study demonstrated that varicocele
increased the phosphorylation of ERK1, indicating that the ERK
signaling pathway was activated in the left testes of rats in the
experimental varicocele model; this may be associated with the
disruption of AJs, the loss of immature germ cells and disorders of
the cell cycle. Furthermore, experimental varicocele increased the
levels of p-FAK-Tyr397 in the left testes of rats. This
is the only phosphorylated form of FAK and provides a combined site
for binding of various signaling molecules, such as the SH2 domains
of Src (20) and PI3K (21), which are involved in
integrin-initiated signaling pathways at the plasma membrane.
Varicocele also increased levels of p-Src-Tyr416, which
is the active form of Src and the most important signaling molecule
downstream of p-FAK-Tyr397, in the left testes of
experimental rats.
Due to the importance of FAK in the regulation of AJ
dynamics and its various biological activities in other epithelial
tissues or cell migration, the results of the present study
suggested that activation of the ERK signaling pathway may be
stimulated, at least in part, by increased phosphorylation of FAK
at Tyr397 and through three putative pathways involving
integrins. Specifically, following autophosphorylation of
FAK-Tyr397 at apical ES sites, p-FAK-Tyr397
recruits Src (20) to form the
activated FAK-Src complex, and then recruits and activates the
p130Cas (Crk-associated substrate)/Crk complex. The latter utilizes
Ras to activate the Raf-1/MEK/ERK signaling pathway (43). Alternatively, the activated FAK/Src
complex phosphorylates FAK at Tyr925, and
p-FAK-Tyr925 then recruits Grb2, partly contributing to
the activation of the Raf-1/MEK/ERK signaling pathway (39). Finally, p-FAK-Tyr397 at
the apical ES sequentially interacts with PI3K p85α and PI3K p110;
the complex then recruits and activates Akt, resulting in the
activation of the PI3K/Akt signaling cascade (44). In addition, Akt can phosphorylate
p21-activated kinase (PAK). Activation of PAK results in the
phosphorylation of Raf-1 and activation of the Raf-1/MEK/ERK
signaling pathway (45). In
addition, our previous study showed that experimental varicocele
increased TGF-β3 and TNF-α levels in the left testes of rats, which
may also contribute to the activation of the MAPK signaling pathway
(46).
FAK is a non-receptor protein tyrosine kinase that
is highly expressed at the apical ES and BTB in the testes and is
an important regulator of TJ and AJ dynamics (7). Distribution of different forms of
phosphorylated FAK (e.g., p-FAK-Tyr397 and
p-FAK-Tyr407) is spatial and temporal at particular
sites in specific stages of the epithelium cycle, suggesting an
intimate connection with cell junction dynamics. Notably,
p-FAK-Tyr397 is the only autophosphorylated form of FAK.
In a number of other epithelial tissues, FAK is the main component
of focal adhesion complexes, which act as anchoring devices between
the cells and matrix. After being activated by integrin or growth
factor receptors, Tyr397 is autophosphorylated and
mediates signal transduction to the actin-based cytoskeleton or
activates signaling pathways by interacting with downstream
signaling molecules, such as related kinases, adaptor proteins,
guanine nucleotide exchanging factors and small GTPases (e.g., Ras,
Rac and Rho) (47). In the present
study, increased phosphorylation of FAK at Tyr397 in the
testes of rats with experimental varicocele may have disrupted AJs
through mechanisms other than mediating the activation of ERK1
signaling, as aforementioned. For example, FAK regulates the
polymerization of actin mediated by Arp 2/3, which is realized by
preventing the nuclear translocation of Arp 2/3 activator [namely,
the neuronal Wiskott-Aldrich syndrome protein (N-WASP)] by
increasing FAK-mediated phosphorylation and activating Arp 2/3
through direct interaction between the FAK 4.1/ezrin/radixin/moesin
domain and Arp 3(19). In addition,
the direct interaction between FAK and Arp3 is negatively regulated
by p-FAK-Tyr397 (19),
leading to disorganization of actin polymerization, followed by
disruption of AJs and TJs (48-50).
In contrast, the apical ES and BTB may work as a functional unit,
namely, the apical ES-BTB functional axis, due to the opening of
TJs, which permits preleptotene and leptotene spermatocytes to
cross the BTB, and the degeneration of apical ES during spermiation
both occur during stage VIII of the seminiferous epithelium cycle
(51). In addition, the association
between apical ES and BTB may occur as a result of the recycling of
p-FAK-Tyr397. After the release of mature spermatids at
stage VIII, p-FAK-Tyr397 at the apical ES is transferred
to the cytoplasm, and high levels of p-FAK-Tyr397 in the
cytoplasm may induce the opening of the BTB (19). In the present study, increased
levels of p-FAK-Tyr397 in the left testes of rats with
experimental varicocele may have altered the balance between the
apical ES-BTB functional axis, resulting in disruption of the
AJ.
Regulation of cell junction (e.g., TJ, apical ES and
basal ES) dynamics in the testis is a complicated biological
process that involves the transduction of multiple signaling
molecules and is not yet fully understood. Disruption of AJs in the
left testes of rats with experimental varicocele may be the primary
or secondary damage induced by varicocele. Further studies are
required in order to assess the other inducers and elucidate the
specific mechanisms. In addition, the exact mechanisms inducing
FAK-Tyr397 phosphorylation, ERK activation and AJ
structural protein downregulation are unclear. Nevertheless, the
loss of premature germ cells and enhancement of
p-FAK-Tyr397 levels in the model used in the present
study suggested that protein kinase inhibitors of
FAK-Tyr397 or inhibitors of the ERK signaling pathway
could be used in the treatment of varicocele to prevent the loss of
premature spermatids and improve fertility. Furthermore, the
results of the present study also implied that
FAK-Tyr397 phosphorylation could be used to prevent the
formation of mature spermatids for male contraception.
In summary, varicocele resulted in disruption of the
structure and function of AJs in the left testes of rats. To a
certain extent, the increased p-FAK-Tyr397 levels may
have contributed to AJ damage, potentially by activating the ERK1
signaling pathway, disturbing the actin-based filament network and
disrupting the balance of the apical ES-BTB functional axis, which
is crucial for understanding the pathological mechanisms of
varicocele that contribute to male infertility and for identifying
new therapeutic targets for varicocele. Nevertheless, due to the
sophisticated structure and function of AJ, the complicated
pathological mechanisms of varicocele, as well as the limitation
that all the results were gained from the in vivo study, the
upregulated p-FAK-Tyr397 levels and the activation of
ERK1 signaling pathway were perhaps not directly and necessarily
associated with the disruption of AJs in experimental rat testes,
and at least were not the only inducements. Future studies will be
designed and performed that include both in vitro and in
vivo experiments in order to verify the exact mechanism and
investigate these hypotheses. For example, utilizing the Sertoli
cells and germ cells co-culture system, then increasing or
decreasing FAK phosphorylation at Tyr397, or activating
or inhibiting the ERK1 signaling pathway to evaluate the structure
and function of AJs.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Training Programme
Foundation for the Talents of Health and Family Planning Commission
in Fujian Province (grant no. 2018-1-74), the Start-up Foundation
of Fujian Medical University (grant no. 2017XQ1005), the Research
Foundation for High-level Talents of Fujian Medical University
(grant no. XRCZX2017033), the Natural Science Foundation of Fujian
Province (grant no. 2017J01819), and the Co-construction Science
Foundation of National Health and Family Planning Commission-Joint
Program for Tackling Key Problems of Health and Education in Fujian
Province (grant no. WKJ2016-2-35).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
LZ performed the experiments, conducted the
statistical analysis and drafted the manuscript. WW conceived the
study, performed the experiments and helped draft the manuscript.
XZ participated in the design and coordination of the study and
helped draft the manuscript. LZ and WW confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The experimental procedures were performed in
accordance with the Guidelines for the Care and Use of Laboratory
Animals approved by Fujian Medical University (Animal Approval
Committee no. #SYXK-2012-0001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Russell L: Observations on rat Sertoli
ectoplasmic (‘junctional’) specializations in their association
with germ cells of the rat testis. Tissue Cell. 9:475–498.
1977.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siu MK and Cheng CY: Dynamic cross-talk
between cells and the extracellular matrix in the testis.
BioEssays. 26:978–992. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vogl AW, Pfeiffer DC, Mulholland D, Kimel
G and Guttman J: Unique and multifunctional adhesion junctions in
the testis: Ectoplasmic specializations. Arch Histol Cytol.
63:1–15. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wong EW, Mruk DD and Cheng CY: Biology and
regulation of ectoplasmic specialization, an atypical adherens
junction type, in the testis. Biochim Biophys Acta. 1778:692–708.
2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Siu MK, Wong CH, Xia W, Mruk DD, Lee WM
and Cheng CY: The β1-integrin-p-FAK-p130Cas-DOCK180-RhoA-vinculin
is a novel regulatory protein complex at the apical ectoplasmic
specialization in adult rat testes. Spermatogenesis. 1:73–86.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cheng CY and Mruk DD: Cell junction
dynamics in the testis: Sertoli-germ cell interactions and male
contraceptive development. Physiol Rev. 82:825–874. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Siu ER, Wong EW, Mruk DD, Porto CS and
Cheng CY: Focal adhesion kinase is a blood-testis barrier
regulator. Proc Natl Acad Sci USA. 106:9298–9303. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gungor-Ordueri NE, Mruk DD, Wan HT, Wong
EW, Celik-Ozenci C, Lie PP and Cheng CY: New insights into FAK
function and regulation during spermatogenesis. Histol Histopathol.
29:977–989. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Daniel JM and Reynolds AB: Tyrosine
phosphorylation and cadherin/catenin function. BioEssays.
19:883–891. 1997.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wine RN and Chapin RE: Adhesion and
signaling proteins spatiotemporally associated with spermiation in
the rat. J Androl. 20:198–213. 1999.PubMed/NCBI
|
|
11
|
Fashena SJ and Thomas SM: Signalling by
adhesion receptors. Nat Cell Biol. 2:E225–E229. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Hamaguchi M, Matsuyoshi N, Ohnishi Y,
Gotoh B, Takeichi M and Nagai Y: p60v-src causes tyrosine
phosphorylation and inactivation of the N-cadherin-catenin cell
adhesion system. EMBO J. 12:307–314. 1993.PubMed/NCBI
|
|
13
|
Cheng CY, Wong EW, Lie PP, Li MW, Su L,
Siu ER, Yan HH, Mannu J, Mathur PP, Bonanomi M, et al:
Environmental toxicants and male reproductive function.
Spermatogenesis. 1:2–13. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Siu MK, Wong CH, Lee WM and Cheng CY:
Sertoli-germ cell anchoring junction dynamics in the testis are
regulated by an interplay of lipid and protein kinases. J Biol
Chem. 280:25029–25047. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Siu ER, Wong EW, Mruk DD, Sze KL, Porto CS
and Cheng CY: An occludin-focal adhesion kinase protein complex at
the blood-testis barrier: A study using the cadmium model.
Endocrinology. 150:3336–3344. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Quadri SK: Cross talk between focal
adhesion kinase and cadherins: Role in regulating endothelial
barrier function. Microvasc Res. 83:3–11. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Su L, Mruk DD, Lui WY, Lee WM and Cheng
CY: P-glycoprotein regulates blood-testis barrier dynamics via its
effects on the occludin/zonula occludens 1 (ZO-1) protein complex
mediated by focal adhesion kinase (FAK). Proc Natl Acad Sci USA.
108:19623–19628. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wan HT, Mruk DD, Wong CK and Cheng CY:
Perfluorooctanesulfonate (PFOS) perturbs male rat Sertoli cell
blood-testis barrier function by affecting F-actin organization via
p-FAK-Tyr(407): An in vitro study. Endocrinology. 155:249–262.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lie PP, Mruk DD, Mok KW, Su L, Lee WM and
Cheng CY: Focal adhesion kinase-Tyr407 and
-Tyr397 exhibit antagonistic effects on blood-testis
barrier dynamics in the rat. Proc Natl Acad Sci USA.
109:12562–12567. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Eide BL, Turck CW and Escobedo JA:
Identification of Tyr-397 as the primary site of tyrosine
phosphorylation and pp60src association in the focal adhesion
kinase, pp125FAK. Mol Cell Biol. 15:2819–2827. 1995.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Parsons JT: Focal adhesion kinase: The
first ten years. J Cell Sci. 116:1409–1416. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fretz PC and Sandlow JI: Varicocele:
Current concepts in pathophysiology, diagnosis, and treatment. Urol
Clin North Am. 29:921–937. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bach PV, Najari BB and Goldstein M:
Varicocele - a case for early intervention. F1000 Res.
5(5)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Luo DY, Yang G, Liu JJ, Yang YR and Dong
Q: Effects of varicocele on testosterone, apoptosis and expression
of StAR mRNA in rat Leydig cells. Asian J Androl. 13:287–291.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chiba K, Ramasamy R, Lamb DJ and Lipshultz
LI: The varicocele: Diagnostic dilemmas, therapeutic challenges and
future perspectives. Asian J Androl. 18:276–281. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Saypol DC, Howards SS, Turner TT and
Miller ED Jr: Influence of surgically induced varicocele on
testicular blood flow, temperature, and histology in adult rats and
dogs. J Clin Invest. 68:39–45. 1981.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pastuszak AW and Wang R: Varicocele and
testicular function. Asian J Androl. 17:659–667. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ozbek E, Yurekli M, Soylu A, Davarci M and
Balbay MD: The role of adrenomedullin in varicocele and impotence.
BJU Int. 86:694–698. 2000.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vyas A, Ram H, Purohit A and Jatwa R:
Adverse effects of subchronic dose of aspirin on reproductive
profile of male rats. J Pharm (Cairo). 2016(6585430)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rotgers E, Cisneros-Montalvo S,
Jahnukainen K, Sandholm J, Toppari J and Nurmio M: A detailed
protocol for a rapid analysis of testicular cell populations using
flow cytometry. Andrology. 3:947–955. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Neto FT, Bach PV, Najari BB, Li PS and
Goldstein M: Spermatogenesis in humans and its affecting factors.
Semin Cell Dev Biol. 59:10–26. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Khera M and Lipshultz LI: Evolving
approach to the varicocele. Urol Clin North Am. 35:183–189.
2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lui WY, Wong CH, Mruk DD and Cheng CY:
TGF-beta3 regulates the blood-testis barrier dynamics via the p38
mitogen activated protein (MAP) kinase pathway: An in vivo study.
Endocrinology. 144:1139–1142. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li MW, Xia W, Mruk DD, Wang CQ, Yan HH,
Siu MK, Lui WY, Lee WM and Cheng CY: Tumor necrosis factor {alpha}
reversibly disrupts the blood-testis barrier and impairs
Sertoli-germ cell adhesion in the seminiferous epithelium of adult
rat testes. J Endocrinol. 190:313–329. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li MW, Mruk DD, Lee WM and Cheng CY:
Disruption of the blood-testis barrier integrity by bisphenol A in
vitro: Is this a suitable model for studying blood-testis barrier
dynamics? Int J Biochem Cell Biol. 41:2302–2314. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Thuillier R, Manku G, Wang Y and Culty M:
Changes in MAPK pathway in neonatal and adult testis following
fetal estrogen exposure and effects on rat testicular cells.
Microsc Res Tech. 72:773–786. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wong CH, Mruk DD, Siu MK and Cheng CY:
Blood-testis barrier dynamics are regulated by
{alpha}2-macroglobulin via the c-Jun N-terminal protein kinase
pathway. Endocrinology. 146:1893–1908. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wong CH, Mruk DD, Lui WY and Cheng CY:
Regulation of blood-testis barrier dynamics: An in vivo study. J
Cell Sci. 117:783–798. 2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hirata H, Gupta M, Vedula SR, Lim CT,
Ladoux B and Sokabe M: Quantifying tensile force and ERK
phosphorylation on actin stress fibers. Methods Mol Biol.
1487:223–234. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mendoza MC, Vilela M, Juarez JE, Blenis J
and Danuser G: ERK reinforces actin polymerization to power
persistent edge protrusion during motility. Sci Signal.
8(ra47)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Logue JS, Cartagena-Rivera AX, Baird MA,
Davidson MW, Chadwick RS and Waterman CM: Erk regulation of actin
capping and bundling by Eps8 promotes cortex tension and leader
bleb-based migration. eLife. 4(e08314)2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Klemke RL, Cai S, Giannini AL, Gallagher
PJ, de Lanerolle P and Cheresh DA: Regulation of cell motility by
mitogen-activated protein kinase. J Cell Biol. 137:481–492.
1997.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cary LA and Guan JL: Focal adhesion kinase
in integrin mediated signaling. Frontiers in bioscience. Front
Biosci. 4:D102–D113. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
44
|
Foster FM, Traer CJ, Abraham SM and Fry
MJ: The phosphoinositide (PI) 3-kinase family. J Cell Sci.
116:3037–3040. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kumar R and Vadlamudi RK: Emerging
functions of p21-activated kinases in human cancer cells. J Cell
Physiol. 193:133–144. 2002.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tomar A and Schlaepfer DD: Focal adhesion
kinase: Switching between GAPs and GEFs in the regulation of cell
motility. Curr Opin Cell Biol. 21:676–683. 2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Schaller MD: Cellular functions of FAK
kinases: Insight into molecular mechanisms and novel functions. J
Cell Sci. 123:1007–1013. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Goley ED and Welch MD: The ARP2/3 complex:
An actin nucleator comes of age. Nat Rev Mol Cell Biol. 7:713–726.
2006.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wu X, Suetsugu S, Cooper LA, Takenawa T
and Guan JL: Focal adhesion kinase regulation of N-WASP subcellular
localization and function. J Biol Chem. 279:9565–9576.
2004.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Serrels B, Serrels A, Brunton VG, Holt M,
McLean GW, Gray CH, Jones GE and Frame MC: Focal adhesion kinase
controls actin assembly via a FERM-mediated interaction with the
Arp2/3 complex. Nat Cell Biol. 9:1046–1056. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yan HH, Mruk DD, Wong EW, Lee WM and Cheng
CY: An autocrine axis in the testis that coordinates spermiation
and blood-testis barrier restructuring during spermatogenesis. Proc
Natl Acad Sci USA. 105:8950–8955. 2008.PubMed/NCBI View Article : Google Scholar
|