Introduction
Vascular anomalies describe two pathological
entities: Congenital vascular malformations (CVMs) and vascular
tumors (1). Depending on the
presence of arterial flow, these can be described as fast-flow
[vascular malformations-arteriovenous malformation, arteriovenous
fistula, and complex malformations such as Parkes-Weber syndrome
and vascular tumors (hemangiomas or neoplasms such as Kaposiform
hemangioendothelioma (KHE), hemangiopericytoma, angiosarcoma)] and
low-flow, benign lesions [venous malformations, lymphatic
malformations, capillary malformations such as diffuse capillary
malformation with overgrowth (DCMO, combined/complex malformations,
such as Klippel-Trenaunay syndrome (KTS), Proteus syndrome, CLOVES
syndrome, Sturge-Weber syndrome] (2-4).
CVMs result from a failure in angiogenesis during
the development of the vascular system; they are present at birth
and usually grow with the infant (3). If arterial flow is observed through
imaging studies, differential diagnosis includes arterio-venous
malformations, arteriovenous fistula or a complex vascular
malformation (2).
Capillary malformations are clinically diagnosed and
present as port-wine stains and telangiectasia. Most of the times
they are isolated anomalies but can be part of a complex vascular
malformation (1,3-5).
Venous malformations and complex malformations with
a venous component have a variable presentation depending on their
depth and associated complications. Complications associated with
venous malformations are venous stasis, thrombosis and localized
intravascular coagulopathy (6).
Despite being congenital, venous malformations usually manifest
later in childhood because they grow with the patient. Upon
physical examination, they appear as compressible bluish colored,
sponge-like masses (3,6).
Lymphatic malformations appear following aberrant
morphogenesis of primordial lymphatic structures and can be
classified as microcystic or macrocystic (7-9).
Lymphatic malformations or complex vascular malformations with a
lymphatic component can lead to disfigurement caused by tissue
hypertrophy and skeletal overgrowth; depending on the organs
involved, they can lead to chylous effusions with organ compromise.
Large lesions can be complicated by fluid loss, hypoproteinemia,
bleeding, and infection (10).
Vascular tumors develop from the endothelium; they
are fully formed at birth and are divided into congenital
hemangiomas that can either be rapidly involuting or non-involuting
and more complex tumors such as KHE or tufted angioma (2). Complex vascular neoplasms are
infiltrative lesions and can cause Kasabach-Merritt phenomenon
(KMP). KMP is a consumptive coagulopathy syndrome consisting of
platelet trapping with profound thrombocytopenia, enlargement of
the lesion and significant hypofibrinogenemia (10-12).
Currently, there is no established standard of care
for the treatment of vascular anomalies. Therapy is guided based on
symptoms and association of complications. Management is comprised
of surgical excision, sclerotherapy or embolization (2,3,13).
Medical management includes steroids, vincristine and interferon
with potential significant side effects in infants, especially
neurotoxicity (10,14). Propranolol represents the first line
of treatment for patients with vascular tumors such as hepatic
hemangiomas (14-16).
Ideally, therapies for vascular anomalies would
target specific cellular pathways involved in abnormal cellular
proliferation and growth.
In the last few years, multiple cases have been
reported for the safety of sirolimus as a therapeutic option for
various congenital vascular anomalies (9,10,12,17-23).
The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)
signaling pathway is pivotal for cellular functions such as growth
and survival and has been demonstrated to be involved in normal
vascular development and angiogenesis (24,25).
Extracellular signals activating the PI3K/AKT pathway transfer
signals to mammalian target of rapamycin receptor (mTOR) which, in
return, increases expression of vascular endothelial growth factors
(VEGF)-A and VEGF-C. VEGF-A and VEGF-C are regulators of both
angiogenesis and lymphangiogenesis, promoting protein synthesis,
cellular growth and proliferation (9,10,24,25).
Over time, inhibitors that target this signaling pathway have been
shown to reduce VEGF secretion and angiogenesis, thus making them a
suitable option for the treatment of CMVs (24,25).
Sirolimus is a direct antagonist of mTOR that acts by blocking the
downstream synthesis of the PI3K/AKT/mTOR pathway resulting in
antitumoral and antiangiogenic effects by impairing VEGF production
(10,26). mTOR receptors have been shown to be
overexpressed in vascular tumors, which makes sirolimus suitable as
a treatment option for vascular malformations (24). Similar to sirolimus, propranolol, a
nonselective β-adrenergic receptor antagonist that causes
vasoconstriction and used as first line of treatment for congenital
hemangiomas, inhibits angiogenesis and promotes apoptosis (14) by acting on the PI3K/AKT signaling
pathway (27). Studies have shown
that protein levels and mRNA of both PI3K and AKT were decreased
after propranolol treatment, thus suggesting that a reduction in
hemangiomas is related to the inhibition of PI3K and AKT (27-29).
Case reports
We analyzed 4 patients admitted to the Neonatal
Intensive Care Unit of ‘Marie Curie’ Emergency Clinical Hospital
for Children, Bucharest, Romania between August 2019 and September
2020. All patients were diagnosed with CVMs and treated with
combined antiproliferative therapy with propranolol and sirolimus.
We analyzed the medical records and summarized the date available
into a case series. Our aim was to monitor the patient clinical and
radiological responses and possible adverse reactions to the
therapy and demonstrate the treatment safety in comparison with
other cases reported in the literature. All patients were started
on oral propranolol at 1 mg/kg/day with doses adjusted up to 3
mg/kg/day according to the tolerance and sirolimus at an initial
dose of 0.4 mg/m2 with doses tired according to
plasmatic levels. Blood tests were drawn every other week and then
monthly to check for possible side effects.
All of our patients were females, admitted to our
unit between 1 and 21 days of life. One patient was diagnosed
prenatally through fetal-magnetic resonance imaging while 3
newborns were diagnosed in our unit. All patients had distinct
congenital vascular anomalies: 1 patient was diagnosed with
congenital lymphangioma of the left hemithorax and left upper arm;
1 patient with CHH complicated with liver failure; 1 patient with
DCMO; and 1 patient with KHE of the left lower limb. Surgical
intervention was intended for 2 patients and later postponed
because of the high risk of negative outcomes. Treatment was
initiated between 4 days of age and 7 weeks. Response to treatment
was noted beginning with the first days of therapy in all patients
through changes in the overall aspect and size of the mass, changes
in the overlaying skin, and improved mobility. Plasmatic levels of
sirolimus were monitored at 7 days of treatment and every other
week for the duration of stay in our unit. After a stable level was
achieved, plasmatic levels were monitored monthly. Plasmatic levels
of sirolimus were difficult to maintain in the recommended ranges.
All patients had initially a higher plasmatic level that later
decreased. Hypertriglyceridemia was the only side effect observed
in all four patients. Duration of treatment varied between 27 weeks
and 20 months. Three patients still undergo the treatment while for
one patient the treatment was stopped after 48 weeks after complete
calcification of the liver mass.
Case 1
A female baby was diagnosed prenatally through fetal
MRI with lymphatic malformation on the left hemythorax and left
upper arm. The newborn was transferred to our unit at 21 h of life,
hemodynamically stable, breathing unaided. On inspection, she
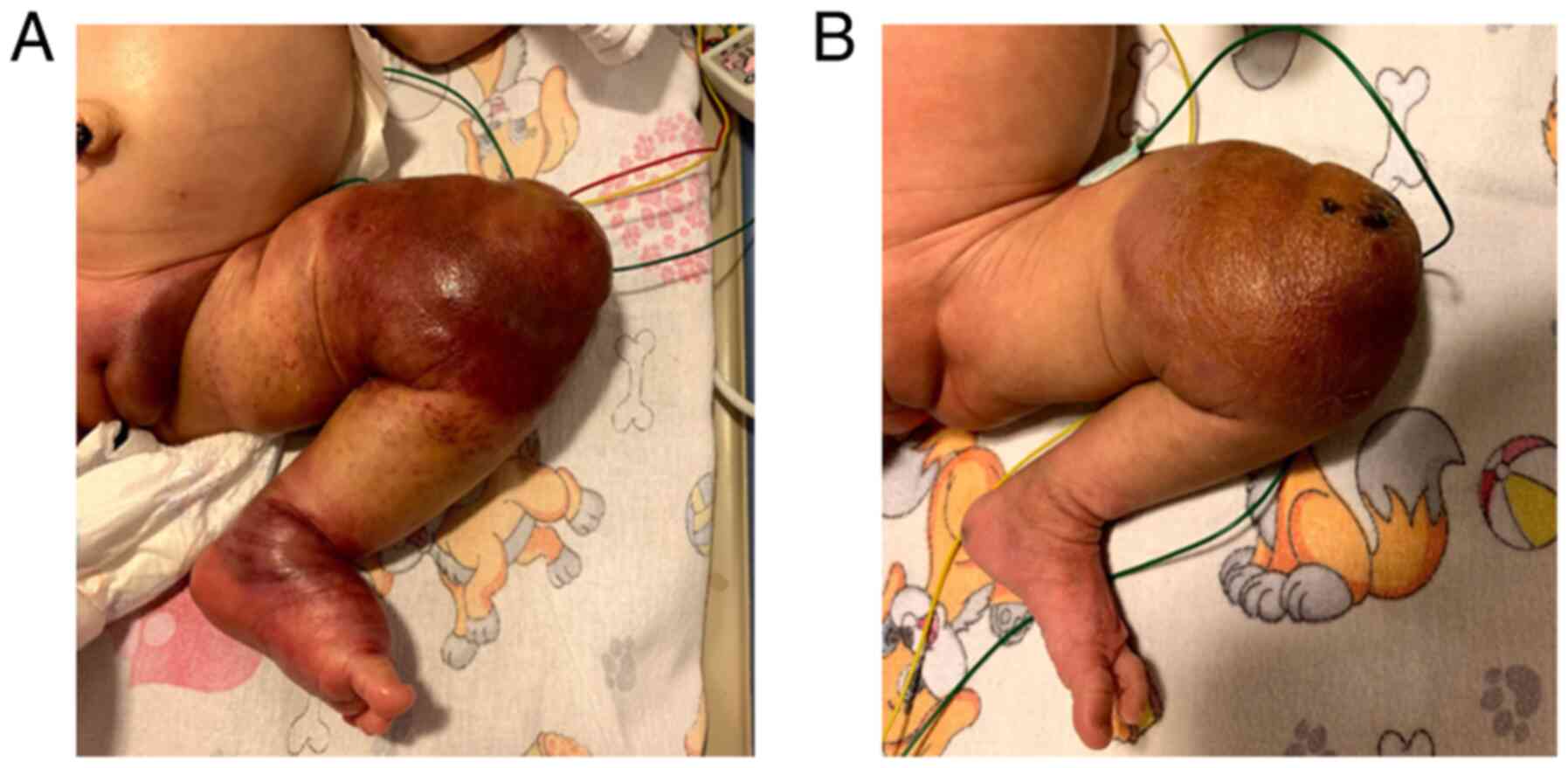
presented with a giant lesion on the left hemithorax (~30/25 cm)
extending to the left upper limb; overlaying skin was purplish and
there was a central area of telangiectasis (Fig. 1A). There were no differences in
color or temperature between the two upper limbs; grasping reflex
and spontaneous movements were present.
Chest angio-computed tomography (CT) at 4 days of
life (Fig. 1B) showed a massive
[12/8.5 cm (axial), 13.8 cm (longitudinal)] soft tissue, cystic
lesion with fine septal and intralesional hemorrhage. The lesion
infiltrated the interscapulo-costal space without damaging the
scapulo-humeral articulation. The CT scan revealed only one fine
vascular branch inside the mass.
Surgical removal of the lesion was attempted and
later postponed following significant hemorrhage after a fine
needle aspiration was performed in the operating room. Monotherapy
with propranolol was started on admittance but after 4 weeks of
treatment there were no visible effects. On the 20th day of life,
we began combination therapy with sirolimus (0.4 mg/m2
per dose, twice daily) and propranolol. After the first week of
combined therapy, the overall size of the mass shrunk, the
overlying skin changed to a natural color and the arm movement
improved.
Plasmatic levels of sirolimus were monitored and
varied between 2.7-29 µg/l with doses adjusted between 0.32 and
0.66 mg/m2.
The only side effects noted were a slight tendency
to prolonged dyslipidemia with hypertriglyceridemia and decreased
levels of high-density lipoprotein (HDL). Massive reduction of the
lesion size was noted at 3 months of age (Fig. 1C). Control angio-CT performed at
that time (11 weeks of combined therapy) showed a reduction in mass
size predominantly through reduction of the cystic component
(maximum axial size, 10/5.7 cm) (Fig.
1D). The patient was discharged at home at 3½ months of age,
continuing the combined antiproliferative therapy. Currently, she
is still undergoing treatment going on 20 months of combined
therapy and is on clinical, pharmacological and radiological
follow-up.
Case 2
A female baby at clinical examination presented with
abdominal distention and a palpable mass in the right
hypochondrium. She was diagnosed at the local maternity department
with CHH. The diagnosis was based on the angio-CT that showed
characteristic findings suggestive of CHH: A well-defined solitary
lesion of ~66.5/61.5/66 mm in the central hilar portion of the
liver with centripetal enhancement and central sparing (Fig. 2A). Her evolution was complicated by
moderate anemia, thrombocytopenia, progressive hepatic cytolysis,
cholestatic and inflammatory syndromes. She was transferred to our
clinic at 21 days of life.
Upon admittance, the patient was breathing smoothly,
had a stable heart rate and was hemodynamically stable; she was
moderately jaundiced, had a distended abdomen and a palpable mass
of about 7/6 cm was found on clinical examination.
Abdominal ultrasound (US) on admittance showed a
central hilar lesion occupying the Vth and VIth liver segments and
partially the IVth, VIIth and VIIIth segments; the tumor had
well-defined margins, was heterogenous and had a central
inhomogeneous necrotic zone surrounded by a peripheral, well
vascularized area (Fig. 2C).
Echocardiographic findings were normal.
Upon arrival, she was started on oral propranolol
with an initial dose of 1 mg/kg/day which was increased slowly
until a maintenance dose of 3 mg/kg/day was reached.
Ursodeoxycholic acid was initiated for the cholestatic syndrome at
a dose of 10 mg/kg/day. In evolution, the patient exhibited an
associated feeding intolerance with frequent postprandial
regurgitation and vomiting because of the mass effect of the
lesion.
Abdominal US studies showed no visible changes after
4 weeks of treatment. Liver function tests remained elevated over
the normal reference ranges and she presented with persistent
hypoalbuminemia, for which albumin infusion was needed. Cholestasis
persisted over the course of the treatment with propranolol.
Giving the large size and central localization of
the lesion, after a multi-disciplinary hearing composed of the
neonatology, pediatric surgery and radiology teams, a decision was
made to postpone the surgical treatment, given the high risk for a
negative outcome.
At the age of 1 month and 3 weeks, the patient was
started on combined antiproliferative therapy with oral sirolimus
(0,4 mg/m2 per dose, twice daily) and propranolol (3
mg/kg/day). The dose of sirolimus ranged between 0.35 and 0.8
mg/m2, adjusted according to plasmatic levels (4.4-16.73
µg/l).
Abdominal US after 1 week of combined
antiproliferative treatment showed diminished intratumoral
vascularization and new necrotic areas inside the tumoral tissue.
After 2 weeks of treatment, a reduction in size was noticeable
(63/56/57 mm compared to ~66.5/61.5/66 mm) (Fig. 2D). Side effects noted were
persistent dyslipidemia with hypertriglyceridemia and low
HDL-cholesterol levels. Control angio-CT scan after 4 weeks of
treatment showed moderate reduction of mass size (63/54/51 mm) and
multiple intratumoral calcifications (Fig. 2B). The patient was discharged at
home at 3 months and 1 week of age on oral propranolol (3.5
mg/kg/day) and sirolimus (~0.55 mg/m2). At present the
patient is well at home.
After 30 weeks of treatment, abdominal US showed
that the mass shrunk to half of its original size (current size
32/29 mm vs. ~66.5/61.5/66 mm at birth) and is fully calcified.
Combined antiproliferative therapy was stopped after
48 weeks of treatment. Currently she is under clinical and
radiological follow-up.
Case 3
A female newborn presented with a giant mass
encompassing the left thigh, knee and calf. In evolution, the mass
extended upwards to the inguinal region and downwards to the distal
1/3 of the calf and foot; the limb became infiltrated with purplish
overlaying skin and tender to touch. She was transferred to our
unit at 4 days of age, breathing unaided and hemodynamically
stable. Upon inspection, the left lower limb was swollen. A giant
mass was noted with ill-defined margins, purplish in color with
overlaying petechia and telangiectasis. The limb was tender to
touch and warmer than the right lower limb; the left thigh girth
upon arrival was ~25 cm compared to 11 cm around the right thigh,
and active movement of the left limb was limited (Fig. 3A). Peripheral pulses were
present.
Laboratory tests showed profound thrombocytopenia
(PLT 5,000/mm3) and coagulation disturbances requiring
administration of FFP and platelet transfusions. Upon arrival, she
was started on combined antiproliferative therapy with propranolol
(1 mg/kg/day) and sirolimus (starting dose 0.4 mg/m2) in
association with methylprednisolone (2 mg/kg/day).
Angio-CT scan performed on admission showed a
massive hypervascular, relatively well-defined lesion encompassing
the left thigh and knee that receives arterial flow from the
inferior segment of the left femoral artery and left popliteal
artery with early enhancement of the femoral vein; soft tissue
edema of the left labia and thigh was present (Fig. 3B).
Response to treatment was noted starting the first
days of therapy. Upon inspection, the foot and calf were slowly
normalized in regards to color, swelling of the limb was diminished
as well as the petechiae and telangiectasis, margins of the lesion
became well defined, encompassing 2/3 distal thigh and 1/3 proximal
calf (Fig. 4A and B). Platelet count increased gradually in
the first 3 weeks of treatment and normalized after 4 weeks of
combined therapy when the PLT count was elevated >200,000/µl.
Corticosteroid therapy was tapered over the course of 4 weeks and
stopped when the platelets reached a normal value.
After 11 weeks of combined antiproliferative
therapy, during which the patient showed good response to therapy,
her status was complicated with swelling of the whole left lower
limb including the left major labia. The diameter of the thigh
increased from 24 to 31 cm in a week. Mobility of the limb was
good, overlaying skin color was normal and there was local warmth
compared to the contralateral limb. Laboratory tests were normal.
Doppler-US of the leg showed no arterial or venous obstruction;
control angio-CT showed diffused edema of the leg without changes
in tumor size.
Propranolol and sirolimus doses were doubled and
methylprednisolon for 4 weeks was reintroduced with no visible
effect.
At 13 weeks of combined therapy, the patient was
started on vincristine at 0.05 mg/m2/week. After 8
rounds of vincristine therapy associated with sirolimus and
propranolol, we observed only mild changes in the tumor aspect.
Initially, there was a reduction in thigh girth with resolution of
labial edema but after 5 rounds of vincristine the thigh girth
increased to 32 cm. Because of severe hypertriglyceridemia, we were
forced to reduce the dose of sirolimus to 1 mg/m2.
Vincristine therapy was discontinued after 8 courses.
Throughout the course of the treatment, sirolimus
doses ranged between 0.5 and 2.0 mg/m2 with plasmatic
levels of 5.2-14 µg/l.
The patient is a social case and was transferred to
a chronic care facility still undergoing combined therapy, being on
week 30 of treatment.
Case 4
A female newborn presented in the delivery room with
cyanosis predominantly on the inferior limbs, hypertrophy on the
left side of the body with port-wine stains on the inferior limbs
and trunk. She was transferred to our NICU at 7 days of life with
suspicions of DCMO vs. KTS. Upon admission, she was breathing
unaided and was hemodynamically stable. Port-wine stains were
observed on inspection, predominantly on the left lower limb with
extension on the truncal skin, while the left side of the body was
hypertrophied apparently through soft tissue overgrowth (Fig. 5A). There was a 2 cm difference in
girth between the left and right inferior limbs.
Laboratory tests found elevated D-Dimers with normal
fibrinogen and coagulation studies. She was started on sirolimus
(0.5 mg/m2) upon admission and was administered combined
antiproliferative therapy starting on day of life 10. The initial
dose of propranolol was 0.5 mg/kg/day and it was slowly increased
up to 3 mg/kg/day. Whole exome sequencing was performed and came
back negative for KTS.
Angio-CT scan performed at 3 weeks of life showed
soft tissue asymmetry on the limbs, thorax and abdominal wall and
diffusely delimitated dense areas of fluid/parafluid consistency in
the soft tissue, without any signs of associated vascular anomalies
(arterial or venous) (Fig. 5B-D).
CT scans incidentally found incomplete transposition of the
inferior vena cava to the left of the aorta in the infrarenal
segment (suprarenal segment of the IVC at the right of the
aorta).
After 7 days of treatment, port-wine stains started
to become paler and the diameter of both left limbs decreased
mildly. The sirolimus dose was tapered according to the plasmatic
levels. The initial dose of 0.4 mg/m2 was decreased to
0.3 and then 0.2 mg/m2 based on plasmatic levels that
ranged from 16.8 to 26 µg/l. The only side effect noted was
inconsistent hypertriglyceridemia.
The patient was discharged at home at 7 weeks of
life. Currently, she is on week 27 of combined therapy and is on
clinical and pharmacological follow-up.
Discussion
Congenital vascular anomalies are a heterogenous
group of pathologies comprised of vascular tumors and CVMs.
Reported incidence of CVMs is 0.3-1.5% while vascular tumors such
as hemangiomas appear in 2-3% of newborns (1).
We reported a case series of four patients admitted
to our NICU Unit between August 2019 and September 2020, diagnosed
with distinct vascular anomalies [CLM, CHH, KHE and DCMO]
complicated with intralesional bleeding, liver failure or
consumptive coagulopathy.
Currently there is no consensus regarding the
standard therapy for CVMs, as literature dealing with vascular
anomalies consists mainly of case reports. Treatment options are
individualized based on the type of malformation, localization,
associated symptoms and complications.
Sirolimus formerly known as rapamycin is a mTOR
inhibitor with antiproliferative, immunosuppressive and antitumoral
effects acting on the mTOR/PI3K/AKT pathway (9). The mTOR/PI3K/AKT pathway is a cell
signaling pathway implicated in both angiogenesis and
lymphangiogenesis resulting in cellular growth and vascular
proliferation (19,24,25,30,31).
Sirolimus inhibits aberrant vascular proliferation by blocking the
mTOR/PI3K/AKT pathway and decreasing VEGF production (9,26,31).
A recent paper published by Lin et al
evaluated the effect of propranolol on infantile hemangiomas and
demonstrated regression of the vascular lesions that was related to
inhibition of the PI3K/AKT pathway (27).
Cases 1 and 2 diagnosed with CLM of the left
hemithorax (case 1) and CHH (case 2) were both initially started on
propranolol without a visible effect after 4 weeks of
monotherapy.
Propranolol was initiated with starting doses of
0.5-1 mg/kg/day which was increased gradually up to a dose of 3
mg/kg/day for a better tolerance. Sirolimus was started on the
lower recommended dose of 0.4-0.5 mg/m2 and was adjusted
according to plasmatic levels. Our protocol for monitoring
plasmatic levels of sirolimus was to draw the first test 7 days
after the beginning of the treatment and then every other week for
the duration of the patient admission in our unit. After a steady
plasmatic level was reached, tests were drawn monthly.
Recommended plasmatic levels of sirolimus vary
between reports ranging from 7.5-10, 10-15 and 12-20 ng/ml
(9,10,19,22,30)
while reported doses for newborns are 0.4-0.8 mg/m2
(9,10,17-19,32,33).
Our aim was to use the lowest therapeutic dose of sirolimus to
avoid potential side effects associated with the drug.
Despite suboptimal plasmatic levels, cases 1, 2 and
3 showed impressive results starting in the first days of therapy.
On the other hand, case 4 diagnosed with DCMO had higher than
recommended plasmatic levels despite receiving lower doses of
sirolimus (maximum plasmatic level of 26 ng/ml at a dose of 0.2
mg/m2). We could not explain this finding compared with
the other patients in our report.
Studies on adult renal transplant patients on
immunosuppressive therapy with sirolimus showed variations of
plasmatic concentrations of sirolimus among patients receiving the
same dose and an intrapatient variability in blood concentration.
These variations are dependent on varying contents of enterocyte
P-glycoprotein and CYP3A4 between individuals; both enterocyte
P-glycoprotein and CYP3A4 being implicated in the metabolism of
sirolimus (29).
Case 3 diagnosed with KHE of the left lower limb
complicated with KMP required associative therapy with propranolol,
sirolimus and methylprednisolone for 4 weeks until platelet levels
reached a normal value.
Currently there is no consensus regarding first line
of treatment for patients diagnosed with KHE with or without KMP.
Medical therapy for patients with KHE and KMP includes
corticosteroids, vincristine, propranolol, interferon and in recent
years sirolimus (30). Regimens
reported in the literature as first line therapies for patients
with KHE are corticosteroid plus vincristine or corticosteroid plus
sirolimus (11,12,30,34).
Despite showing good initial response to combined
antiproliferative therapy with sirolimus and propranolol, the
patient developed lymphedema of the ipsilateral limb after 12 weeks
of therapy. Giving the fast increase in thigh girth (8 cm in 1
week) we decided to increase the dose of sirolimus to 2
mg/m2 and reintroduced methylprednisolone. We based our
decision on a reported case by Chinello et al, where
sirolimus was administered in doses up to 3 mg/m2 to
maintain plasmatic levels between 7.5-10 ng/ml (30).
Vincristine was introduced during the 13th week of
combined antiproliferative therapy. After 8 rounds of vincristine,
the patient showed diminished leg swelling (thigh girth decreased
by 2 cm), subcutaneous tissue became softer, less indurated and
mobility of the limb improved.
A retrospective multicenter analysis conducted in
2018 by Ji et al concluded that lymphedema is a frequent
sequalae found in patients diagnosed with KHE and that associated
KMP or sirolimus treatment has no prediction in its development
(35). KHE can predispose the
patient secondary lymphedema through infiltration of the tumor in
the lymphatic vessels or to primary lymphedema because of aberrant
formation of the lymphatic vasculature with insufficient vessels
(35,36). Giving the reported evolution of
patients with KHE with long term follow-up it is difficult to
conclude whether vincristine or sirolimus may have any effect on
lymphedema.
Response to treatment for case 4 was noted during
the first days of therapy by changes in the color of the capillary
lesions which became paler. Over time, no major changes were
observed on the overall size of the limbs.
Prior to starting the treatment, all patients had
blood tests drawn to check for baseline levels of complete blood
count, acute inflammatory markers, liver and renal function tests,
lipid profile and plasmatic electrolytes based on the most common
adverse effects of sirolimus.
Adverse effects observed in our patients were
concordant with reported cases in the literature (9,10,14,17,19-22).
Sirolimus is a new immunosuppressant drug originally approved for
use in kidney transplant recipients, but no patient showed signs of
infection/sepsis or hematologic changes throughout the course of
treatment. Long-term administration at these dosages seems to be
safe. All patients showed increased triglycerides. Cases 1 and 2
showed persistent hypertriglyceridemia that resolved after ~10
weeks of therapy while cases 3 and 4 showed inconsistent high
triglyceride levels. Studies conducted on adult patients
demonstrated that sirolimus increases liver synthesis of
triglycerides by increasing lipase activity in the adipose tissue
and/or decreasing lipoprotein lipase activity (37).
Before initiation of treatment, case 2 with CHH had
elevated liver enzymes that remained over the normal reference
range over the course of the first 20 weeks of therapy but we could
not distinguish if this was an adverse effect of sirolimus or if
the liver enzymes were elevated because of tissue destruction by
the liver mass.
In conclusion, combined therapy with propranolol and
sirolimus is a safe therapeutic choice for patients with congenital
vascular anomalies with good outcomes and without life threatening
adverse effects. More studies must be conducted to confirm our
findings.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
More information concerning the 4 cases can be
obtained from the corresponding author upon reasonable request.
Authors' contributions
CC conceived the presented idea and the study
design, and provided final approval of the version to be published.
AMB investigated the aspects of the case series. CM developed the
theory and contributed to the computations of the data. SM and AIB
collected the data of the patients for this study and verified the
analytical methods. DS and VM helped supervise the project in view
of the data collected. DAI and RIS investigated the aspects and
supervised the findings of this work. All authors have read and
approved the final manuscript for publication.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
‘Marie S. Curie’ Emergency Clinical Hospital for Children. Patients
who participated in this study had complete clinical data. Written
informed consent to participate was signed by the patient's
guardian/next of kin.
Patient consent for publication
Written informed consent was obtained from the
minors' guardian/next of kin for publication of any identifiable
images or data included in this article. The corresponding author
is in possession of these documents.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rendón-Elíasa FG, Hernández-Sánchez M,
Albores-Figueroa R, Montes-Tapia FF and Gómez-Danés LH: Congenital
vascular malformations update. Med Univer. 16:184–198. 2014.
|
|
2
|
Behr GG and Johnson C: Vascular anomalies:
Hemangiomas and beyond-part I, Fast-flow lesions. AJR Am J
Roentgenol. 200:414–422. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Behr GG and Johnson C: Vascular anomalies:
Hemangiomas and beyond-part 2, Slow flow lesions. AJR Am J
Roentgenol. 200:423–436. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
White CL, Olivieri B, Restrepo R, McKeon
B, Karakas SP and Lee EY: Low-flow vascular malformation pitfalls:
From clinical examination to practical imaging evaluation-part 1,
lymphatic malformation mimickers. AJR Am J Roentgenol. 206:940–951.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cox JA, Bartlett E and Lee EI: Vascular
malformations: A review. Semin Plast Surg. 28:58–63.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Behravesh S, Yakes W, Gupta N, Naidu S,
Chong BW, Khademhosseini A and Oklu R: Venous malformations:
Clinical diagnosis and treatment. Cardiovasc Diagn Ther. 6:557–569.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zheng W, Aspelund A and Alitalo K:
Lymphangiogenic factors, mechanisms and applications. J Clin
Invest. 124:878–887. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Colbert SD, Seager L, Haider F, Evans BT,
Anand R and Brennan PA: Lymphatic malformations of the head and
neck-current concepts in management. Br J Oral Maxilofac Surg.
51:98–102. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Amodeo I, Colnaghi M, Raffaeli G,
Cavallaro G, Ciralli F, Gangi S, Leva E, Pignataro L, Borzani I,
Pugni L and Mosca F: The use of sirolimus in the treatment of giant
cystic lymphangioma: Four case reports and update of medical
therapy. Medicine (Baltimore). 96(e8871)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hammill AM, Wentzel MS, Gupta A, Nelson S,
Lucky A, Elluru R, Dasgupta R, Azizkhan RG and Adams DM: Sirolimus
for the treatment of complicated vascular anomalies in children.
Clin Med Insights Blood Disord. 57:1018–1024. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mahajan P, Margolin J and Iacobas I:
Kasabach-merritt phenomenon: Classic presentation and management
options. Clin Med Insights Blood Disord.
10(1179545X17699849)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ji Y, Chen S, Yang K, Xia C and Li L:
Kaposiform hemangioendothelioma: Current knowledge and future
perspectives. Orphaneet J Rare Dis. 15(39)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang B and Ma L: Updated classification
and therapy of vascular malformations in pediatric patients.
Pediatr Invest. 2:119–123. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Raphael MF, Breur JM, Vlasveld FA, Elbert
NJ, Liem YT, Kon M, Breugem CC and Pasmans SG: Treatment of
infantile hemangiomas: Therapeutic options in regard to side
effects and adverse events-a review of the literature. Expert Opin
Drug Saf. 15:199–214. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Droitcourt C, Kerbrat S, Rault C, Botrel
MA, Happe A, Garlantezec R, Guillot B, Schleich JM, Oger E and
Dupuy A: Safety of oral propranolol for infantile hemangioma.
Pediatrics. 141(e20173783)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Léauté-Labrèze C, Hoeger P,
Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, Phillips RJ,
Caceres H, Lopez Gutierrez JC, Ballona R, et al: A randomized,
controlled trial of oral propranolol in infantile hemangioma. N
Engl J Med. 372:735–746. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Triana P, Miguel M, Díaz M, Cabrera M and
López Gutiérrez JC: Oral sirolimus: An option in the management of
neonates with life-threatening upper airway lymphatic
malformations. Lymphat Res Biol. 17:504–511. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang Z, Yao W, Sun H, Dong K, Ma Y, Chen
L, Zheng S and Li K: Sirolimus therapy for kaposiform
hemangioendothelioma with long-term follow-up. J Dermatol.
46:956–961. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Adams DM, Trenor CC III, Hammill AM, Vinks
AA, Patel MN, Chaudry G, Wentzel MS, Mobberley-Schuman PS, Campbell
LM, Brookbank C, et al: Efficacy and safety of sirolimus in the
treatment of complicated vascular anomalies. Pediatrics.
137(e20153257)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Freixo C, Ferreira V, Martins J, Almeida
R, Caldeira D, Rosa M, Costa J and Ferreira J: Efficacy and safety
of sirolimus in the treatment of vascular anomalies: A systematic
review. J Vasc Surg. 71:318–327. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sandbank S, Molho-Pessach V, Farkas A,
Barzilai A and Greenberger S: Oral and topical sirolimus for
vascular anomalies: A multicentre study and review. Acta Derm
Venereol. 99:990–996. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Honnorat M, Viremouneix L, Ayari S,
Guibaud L, Coste K, Claris O and Butin M: Early adjuvant medication
with the mTOR inhibitor sirolimus in a preterm neonate with
compressive cystic lymphatic malformation. Front Pediatr.
8(418)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cirstoveanu C, Bizubac M, Mustea C,
Barascu I, Manolache S, Nine L, Istrate-Barzan A, Spataru R and
Marcu V: Combined antiproliferative therapy with rapamycin and
propranolol for giant congenital lymphangioma. Chest.
157(A317)2020.
|
|
24
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4(51)2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Laplante M and Sabatini DM: mTOR signaling
at a glance. J Cell Sci. 122:3589–3594. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Greenberger S, Yuan S, Walsh LA, Boscolo
E, Kang KT, Matthews B, Mulliken JB and Bischoff J: Rapamycin
suppresses self-renewal and vasculogenic potential of stem cells
isolated from infantile hemangioma. J Invest Dermatol.
131:2467–2476. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lin Z, Wang L, Huang G, Wang W and Lin H:
Propranolol inhibits the activity of PI3K, AKT, and HIF-1α in
infantile hemangiomas. Pediatr Surg Int. 34:1233–1238.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pan WK, Li P, Guo ZT, Huang Q and Gao Y:
Propranolol induces regression of hemangioma cells via the
down-regulation of the PI3K/Akt/eNOS/VEGF pathway. Pediatr Blood
Cancer. 62:1414–1420. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mahalati K and Kahan BD: Clinical
pharmacokinetics of sirolimus. Clin Pharmacokinet. 40:573–585.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chinello M, Di Carlo D, Olivieri F, Balter
R, De Bortoli M, Vitale V, Zaccaron A, Bonetti E, Parisi A and
Cesaro S: Successful management of kaposiform hemangioendothelioma
with long-term sirolimus treatment: A case report and review of the
literature. Mediterr J Hematol Infect Dis.
10(e2018043)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mukhopadhyay S, Frias MA, Chatterjee A,
Yellen P and Foster DA: The enigma of rapamycin dosage. Mol Cancer
Ther. 15:347–353. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Alaqeel AM, Alfurayh NA, Alhedyani AA and
Alajlan SM: Sirolimus for treatment of kaposiform
hemangioendothelioma associated with Kasabach-Merritt phenomenon.
JAAD Case Rep. 2:457–461. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Warren D, Diaz L and Levy M: Diffuse
hepatic hemangiomas successfully treated using sirolimus and
high-dose propranolol. Pediatr Dermatol. 34:e286–e287.
2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Drolet BA, Trenor CC III, Brandão LR, Chiu
YE, Chun RH, Dasgupta R, Garzon MC, Hammill AM, Johnson CM, Tlougan
B, et al: Consensus-derived practice standards plan for complicated
Kaposiform hemangioendothelioma. J Pediatr. 163:285–291.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ji Y, Chen S, Xia C, Zhou J, Jiang X, Xu
X, Yang K, Zhang X, Kong F, Lu G and Zhang Y: Chronic lymphedema in
patients with kaposiform hemangioendothelioma: Incidence, clinical
features, risk factors and management. Orphanet J Rare Dis.
15(313)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Konczyk DJ, Goss JA, Maclellan RA and
Greene AK: Association between extremity kaposiform
hemangioendothelioma and lymphedema. Pediatr Dermatol. 35:e92–e93.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Morrisett JD, Abdel-Fattah G and Kahan BD:
Sirolimus changes lipid concentrations and lipoprotein metabolism
in kidney transplant recipients. Transplant Proc. 35(3
Suppl):143S–150S. 2003.PubMed/NCBI View Article : Google Scholar
|