Introduction
Ischemic heart disease (IHD) is a major cause of
disability and mortality worldwide (1). According to the World Health
Organization, the number of acute myocardial infarction cases is
~32.4 million per year worldwide as of 2020(2). At present, timely revascularization
is the most effective approach for reducing cardiomyocyte cell
death (3). Nevertheless,
reperfusion treatment can cause myocardial ischemia/reperfusion
(I/R) injury (MIRI), which is a complex pathophysiological process
that can cause additional myocardial damage (4). In recent years, evidence has shown
that inflammation serves a crucial role in the pathogenesis of MIRI
(5). Therefore, identification of
effective approaches for reducing inflammation to attenuate MIRI
remain in demand.
As a fat-derived plasma protein, C1q/TNF-related
protein 9 (CTRP9) has reported beneficial effects on glucose
metabolism and vascular function (6,7).
Previous studies have indicated that CTRP9 can regulate the
inflammatory response in the setting of various cardiovascular
diseases, including atrial fibrillation (8), myocardial infarction (9), atherosclerosis (10) and heart failure (11). It was also demonstrated that CTRP9
is a potential protective factor against MIRI (12,13).
Kambara et al (12) found
that CTRP9 can protect the myocardium from I/R injury through an
AMP-activated protein kinase (AMPK)-dependent mechanism. In another
study, Zhao et al (13)
revealed that cardiac-derived CTRP9 can protect against MIRI
through calreticulin-dependent inhibition of apoptosis. However, to
the best of our knowledge, the function of CTRP9 in MIRI remains
unclear due to the complex molecular mechanism involved.
Previous studies have documented that I/R injury can
induce the release of proinflammatory cytokines in cardiomyocytes
by triggering toll-like receptor 4 (TLR4)/myeloid differentiation
primary response 88 (MyD88)/NF-κB signaling (14,15).
Inhibition of TLR4/MyD88/NF-κB-related signaling has been shown to
decrease I/R injury-induced proinflammatory cytokine release and
ameliorate cardiac dysfunction (16,17).
Of note, TLR4/MyD88/NF-κB-related signaling transduction has also
been found to be suppressed by CTRP9 during IHD (8,9).
However, the effects of CTRP9 on inflammation and TLR4/MyD88/NF-κB
activation during MIRI remain unclear.
In this experiment, adenoviral vectors containing
CTRP9 were used to increase the expression of CTRP9 in neonatal rat
cardiomyocytes (NRCMs), and then a hypoxia/reoxygenation (H/R)
injury model was established to explore the role and mechanism of
CTRP9 during MIRI.
Materials and methods
Chemicals and reagents
Adenoviruses encoding CTRP9 (Ad-CTRP9) or green
fluorescent protein (GFP; Ad-GFP) were constructed and amplified by
Gene Company, Ltd.. Lactate dehydrogenase (LDH; cat. no. A020-1-2),
creatine kinase (CK; cat. no. A032-1-1) and CK-myocardial band
(CK-MB; cat. no. E006-1-1) biochemical kits were obtained from
Nanjing Jiancheng Bioengineering Institute. The following primary
antibodies were purchased from Abcam: TLR4 (cat. no. ab13867;
1:1,000 dilution), MyD88 (cat. no. ab219413; 1:800 dilution), NF-κB
(cat. no. ab220803; 1:1,000 dilution) and GAPDH (cat. no. ab8245;
1:1,000 dilution). HRP-conjugated anti-rabbit (cat. no. bs-0295G;
1:2,000 dilution) or anti-mouse secondary antibodies (cat. no.
bs-0296G; 1:2,000 dilution) were obtained from BIOSS. A LightShift
Chemiluminescent electrophoretic mobility shift assay (EMSA) kit
(cat. no. SIDET201) was purchased from Viagene Biotech, Inc..
NRCM culture
The NRCMs were isolated from ~2-day old Sprague
Dawley rats (n=5; weight, 8±2 g; sex, unknown), which were provided
by the Experimental Animal Center of Southern Medical University
(Shenzhen, China). The Sprague Dawley rats (housed at 23±2˚C with
50% relative humidity, 12-h light/dark cycles and free access to
water) were anesthetized with 3% sodium pentobarbital (30 mg/kg)
and euthanized via decapitation (18). Their hearts were then quickly
removed and their large blood vessels carefully excised. The
obtained heart tissues were rinsed in ice-cold PBS to remove any
residual blood. Next, 0.08% collagenase type II and 0.125% trypsin
were used to digest the tissues at 37˚C for 7 min. Finally, the
NRCMs (fusiform or polygonal, determined with spontaneous
pulsation) were centrifuged (1,000 x g at 4˚C for 10 min) and
re-suspended in DMEM (Gibco; Thermo Fisher Scientific, Inc.) with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin at 37˚C with 5% CO2 and 95%
O2. All experimental procedures were approved by the
Ethics Committee of Experimental Animals of the Southern Medical
University (approval no. 2020-01-03A).
Adenoviral transfection and
establishment of the H/R injury model
NRCMs were transfected with Ad-CTRP9 or Ad-GFP (both
containing GFP) at a MOI of 50, and the H/R injury model was
established 2 days after transfection. Cells were observed using
fluorescence microscopy after 48 h to visualize GFP expression.
Cells no older than passage 5 were used for these experiments.
Briefly, the cultured NRCMs were preserved in serum-free DMEM at
37˚C for 12 h. Next, NRCMs were incubated in an anerobic chamber
(95% N2-5% CO2) at 37˚C for 2 h. NRCMs were
then moved into a normal incubator (37˚C) for an additional 4 h to
induce reoxygenation. The primary cardiomyocytes were randomly
separated into the following four groups: Control, H/R, Ad-CTRP9
and Ad-GFP groups (both of which also underwent H/R). Each
experiment was repeated ≥5 times.
Cell viability assay
Cell viability assay was performed to assess the
cytotoxicity of adenovirus on NRCMs. Briefly, after the NRCMs
(5x105/ml) were transfected with adenoviruses at various
multiplicities of infection (MOI=5, 20, 50, 100 and 200) at 37˚C
for 48 h, they were stained with 0.4% trypan blue 37˚C for 3 min
and observed under a light microscope (magnification, x200). The
ratio of unstained cells to total cell number was calculated to
estimate cell viability.
Determination of markers of myocardial
injury
In the present study, the supernatant of cultured
NRCMs was collected and an automatic biochemical analyzer (Jinan
Tianheng Technology Co., Ltd.) was used with the aforementioned
biochemical kits, according to the manufacturers' protocols, to
determine the LDH, CK and CK-MB levels.
Measurement of TNF-α, IL-6 and IL-10
levels
TNF-α (cat. no. SRTA00), IL-6 (cat. no. SR6000B) and
IL-10 (cat. no. SR1000) ELISA kits (R&D Systems, Inc.) were
used according to the manufacturer's protocol to determine the
TNF-α, IL-6 and IL-10 levels in supernatants. Samples was
centrifuged 500 x g at 4˚C for 10 min to obtain supernatants.
Western blotting
Western blotting was performed to analyze protein
expression (19). Briefly, NRCMs
were first homogenized and lysed in RIPA buffer (cat. no. R0278;
MilliporeSigma). Next, the protein was extracted and the
concentration was determined using a BCA assay (Beyotime Institute
of Biotechnology). Subsequently, 10% SDS-PAGE was used to separate
the extracted proteins (40 µg), which were then electrophoretically
transferred onto PVDF membranes. The membranes were then blocked
with 5% non-fat dry milk in PBS with 0.05% Tween-20 for 2 h at room
temperature. Next, the membranes were incubated with antibodies
against TLR4, MyD88 and NF-κB overnight at 4˚C. The next day, the
membranes were incubated with HRP-conjugated anti-rabbit or
anti-mouse secondary antibodies for another 2 h at room
temperature. Finally, a Pierce™ ECL Western Blotting
Substrate kit (cat. no. 32109; Pierce; Thermo Fisher Scientific,
Inc.) was used to detect protein expression. An Odyssey Infrared
Imaging system (model 9120; LI-COR Biosciences) was used to capture
images of the membranes and Quantity One 1-D software (version
4.6.9; Bio-Rad Laboratories, Inc.) was used to quantify the protein
bands.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed to detect mRNA levels.
Briefly, TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from NRCMs.
Obtained RNA (~4.0 µg) was then reverse transcribed into cDNA using
SuperScript IV Reverse Transcriptase (Thermo Fisher Scientific,
Inc.) at 37˚C for 60 min. Next, qPCR was performed using a SYBR
green Master Mix kit (Thermo Fisher Scientific, Inc.) on a 7500 ABI
PRISM system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The qPCR thermocycling conditions were as follows: 45˚C for 2 min;
95˚C for 10 min, immediately followed by 45 cycles of 95˚C for 30
sec and 60˚C for 30 sec. The mRNA expression of CTRP9, TLR4, MyD88
and NF-κB were normalized to that of GAPDH. The 2-ΔΔCq
method was used to calculate changes in mRNA expression (20). The following primers were used:
CTRP9 forward, 5'-GGCTTCTACTGGTTATGGACGC-3' and reverse,
5'-GGAGCCTGGATCACCTTTGAT-3'; TLR4 forward,
5'-TGCTCAGACATGGCAGTTTC-3' and reverse, 5'-CTGGATTCAAGGCTTTTCCA-3';
MyD88 forward, 5'-GAGATCCGCGAGTTTGAGAC-3' and reverse,
5'-CTGTTTCTGCTGGTTGCGTA-3'; NF-κB forward,
5'-GGCAGCACTCCTTATCAACC-3' and reverse, 5'-GAGGTGTCGTCCCATCGTAG-3'
and GAPDH forward, 5'-CGCTAACATCAAATGGGGTG-3' and reverse,
5'-TTGCTGACAATCTTGAGGGAG-3'.
EMSA
The binding activity of NF-κB was detected using an
electrophoretic mobility shift assay. Briefly, nuclear extracts
were prepared from the NRCMs and stored at -80˚C for the EMSA
assay. The protein concentration was measured using a Bio-Rad
protein assay reagent (Bio-Rad Laboratories, Inc.). Equal amounts
(5 µg) of nuclear protein were incubated with poly
(2'-deoxyinosinic-2'-deoxycytidylic acid) and synthesized NF-κB
binding consensus oligonucleotides (sense,
5'-AGTTGAGGGGACTTTCCCAGGC-3'; antisense,
5'-GCCTGGGAAAGTCCCCTCAACT-3') for 20 min at room temperature using
a LightShift EMSA Optimization and Control kit. Subsequently,
protein-DNA complexes were separated via electrophoresis on a 6.5%
non-denaturing polyacrylamide gel, transferred to a nylon membrane
and cross-linked by UV light. The membrane was incubated with
streptavidin-horseradish peroxidase and detected via enhanced
chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.).
Statistical analysis
SPSS 22.0 software (IBM Corp.) was used for data
analysis. Data are presented as the mean ± SD (n=5). Student's
unpaired t-test and one-way ANOVA were used for comparisons between
groups. If interactions were significant, a Tukey post hoc test was
used for multiple comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
NRCM viability
As shown in Fig.
1A, Ad-CTRP9 exerted no toxic effects on NRCM viability at MOI
of <100. However, cell viability was 86.8% at an MOI of 200.
After 48 h of transfection, GFP expression was assessed using a
fluorescence microscope at an MOI of 50 (Fig. 1B). In the present study, the
transfection efficiency at MOI of 20 was only ~84.7%, whereas that
at MOI of 50 and 100 were ~92.6 and ~93.8% respectively, with no
clear differences between MOI of 50 and 100 (data not shown).
Considering these aforementioned results, MOI of 50 was used due to
its higher transfection efficiency but minimal effects on NRCM
viability.
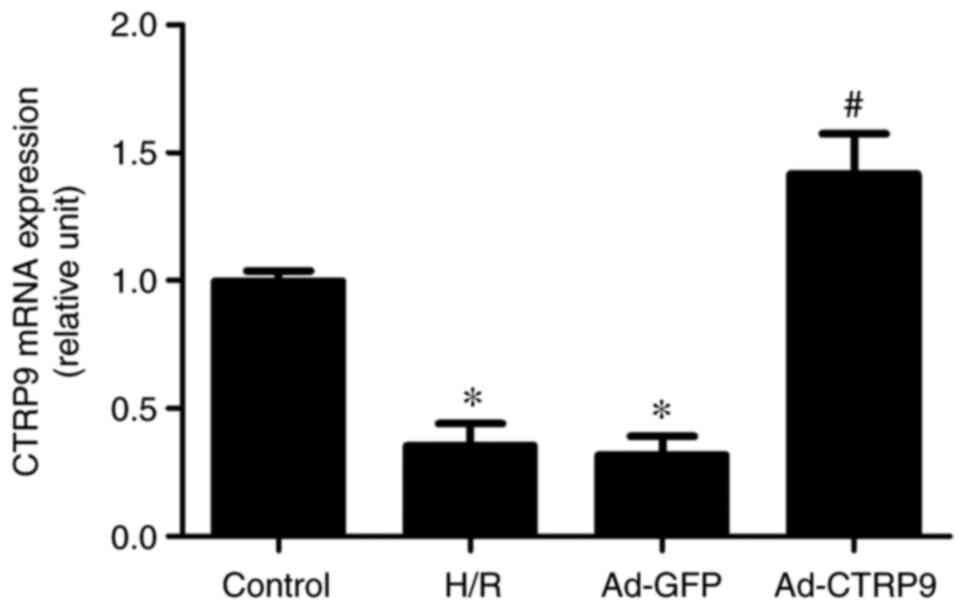
Ad-CTRP9 induces CTRP9 upregulation in
cardiomyocytes after H/R
Following adenoviral infection and the establishment
of the H/R injury model, CTRP9 expression was examined by RT-qPCR.
As shown in Fig. 2, the mRNA
expression levels of CTRP9 were significantly reduced in the H/R
and Ad-GFP groups compared with those in the control group.
However, the mRNA expression of CTRP9 was significantly increased
in the Ad-CTRP9 group compared with that in the Ad-GFP or H/R
groups.
CTRP9 attenuates H/R-induced NRCM
injury
LDH, CK and CK-MB are enzymes that are released by
cardiomyocytes following severe injury, and their levels are used
to estimate the severity of myocardial damage (17). To assess the effects of CTRP9 on
H/R-induced cellular damage, cell viability, as well as LDH, CK and
CK-MB activity in the cell culture supernatant, were evaluated.
Cell viability was significantly suppressed by H/R compared with
that in cells that underwent normoxic treatment in all three of the
transfection groups (Fig. 3A).
However, after H/R, cell viability was significantly increased in
the Ad-CTRP9 group compared with that in the control group
(Fig. 3A). LDH (Fig. 3B), CK (Fig. 3C) and CK-MB (Fig. 3D) activities were also
significantly increased in the H/R group compared with those in the
control group. However, the H/R-induced enzyme release was
significantly reversed by CTRP9 overexpression (Fig. 3B-D).
CTRP9 alleviates inflammation after
H/R injury
MIRI is closely associated with an excessive
inflammatory response (5). In
addition, CTRP9 has been shown to be involved in the progression of
inflammation in the heart (9).
ELISA was therefore used to measure TNF-α, IL-6 and IL-10
expression in cell culture supernatant. The levels of TNF-α and
IL-6 were found to be significantly increased following H/R
compared with those in the control group, but the levels of the
anti-inflammatory cytokine IL-10 was significantly downregulated
(Fig. 4). However, CTRP9
overexpression significantly decreased the levels of TNF-α and IL-6
whilst increasing the levels of IL-10 compared with those in the
Ad-GFP or H/R groups (Fig. 4).
CTRP9 inhibits TLR4/MyD88/NF-κB
signaling
To further understand the possible mechanism
underlying the CTRP9-mediated mitigation of H/R damage, the
expression of components of TLR4/MyD88/NF-κB signaling was
determined by western blotting and RT-qPCR. As shown in Fig. 5, H/R significantly upregulated the
protein (Fig. 5A) and mRNA
(Fig. 5B) expression levels of
TLR4, MyD88 and NF-κB compared with those in the control group.
However, CTRP9 overexpression after the onset of H/R significantly
reversed these aforementioned increases. Similarly, the binding
activity of NF-κB to DNA was markedly increased after H/R, but
decreased following Ad-CTRP9 transfection (Fig. 5B). Therefore, this suggests that
H/R injury may be ameliorated by CTRP9 overexpression, possibly
through suppression of TLR4/MyD88/NF-κB signaling.
Discussion
MIRI leads to a range of pathological changes,
including activation of the inflammatory response, which can lead
to cell injury (21). Effectively
reducing inflammation can improve the outcomes of MIRI in animal
and cell models (22). Previous
studies have found that CTRP9 exerts protective effects against
ischemic heart injury through a variety of signaling pathways, such
as the protein kinase A and ERK1/2 signaling pathways (23-25).
In particular, cardiac-derived CTRP9 has been found to protect
against MIRI through the inhibition of apoptosis and endoplasmic
reticulum stress (13,26). However, to the best of our
knowledge, the role of CTRP9 and possible underlying mechanism in
MIRI and has not been completely elucidated. In the present study,
a H/R model was established to simulate MIRI and CTRP9 was found to
alleviate MIRI by significantly reducing myocardial inflammation,
which was characterized by the upregulation of cell viability and
reducing the release of CK, CK-MB and LDH. In addition, it was
observed that CTRP9 overexpression markedly inhibited the
TLR4/MyD88/NF-κB signaling pathway. These aforementioned results
suggest that CTRP9 may possess the ability to ameliorate
H/R-induced inflammation by regulating the TLR4/MyD88/NF-κB
signaling pathway.
Previous studies have found that CTRP9 can regulate
the inflammatory response in various pathological processes, such
as myocardial infarction and endothelial dysfunction (27-29).
In db/db mice, Li et al (30) found that overexpression of CTRP9
may reduce retinal inflammation and protect the blood-retinal
barrier. Liu et al (9)
revealed that overexpression of CTRP9 restored cardiac function
following myocardial infarction by regulating TLR4/MD2/MyD88 and
AMPK/NF-κB signaling in a rat model of myocardial infarction. In
another study, Zhang et al (31) demonstrated that overexpression of
CTRP9 attenuated a mouse model of atherosclerosis by inhibiting
AMPK/NLR family pyrin domain containing 3 signaling. Qian et
al (32) showed that
overexpression of CTRP9 alleviated airway inflammation in a mouse
model of asthma. Zhao et al (33) demonstrated that Ad-CTRP9
transfection weakened neuro-inflammation by activating adiponectin
receptor 1 following intracerebral hemorrhage in mice. Therefore
these previously reported biological activities of CTRP9
aforementioned attracted the interest of the research community.
The present study demonstrated that CTRP9 overexpression may
reverse the H/R-induced upregulated expression of TNF-α and IL-6 in
addition to reversing the H/R-induced reduction in the levels of
the anti-inflammatory cytokine IL-10.
H/R can directly decrease cardiomyocyte
contractility and induce inflammation in cardiomyocytes by
activating the TLR4/MyD88-dependent signaling pathway (16). The TLR4 signaling pathway has been
extensively studied, where exerts its effects through the
MyD88-mediated activation of NF-κB (34,35).
As a transcription factor, NF-κB has been confirmed to be closely
associated with inflammation activation (36). TLR4 signaling-related inflammatory
activation is closely linked to myocardial injury during MIRI
(37). A number of endogenous
factors that can negatively regulate TLR4 signal transduction
directly have been found (9,38).
Among them, CTRP9 has particularly garnered interest (9). MyD88 and NF-κB are downstream
molecules of TLR4, and the activation of MyD88 and NF-κB are partly
dependent on TLR4; Liu et al (9) found that CTRP9 could directly bind to
TLR4 to regulate the downstream molecules of MyD88 and NF-κB. In
addition, ample evidence suggests that CTRP9 exerts pleiotropic
effects, such as anti-inflammation, on a variety of pathological
conditions by suppressing NF-κB, such as cardiac hypertrophy
(39) and osteoarthritis (40). In terms of the possible involvement
of CTRP9 in the suppression of TLR4/MyD88/NF-κB-related
inflammatory signaling, the present study suggest that CTRP9 may
exert protective effects on cardiomyocytes following H/R insult by
conferring anti-inflammatory effects through suppressing
TLR4/MyD88/NF-κB signaling downstream. However, it should be noted
that the cause-effect relationship between TLR4 signaling and
inflammation after overexpression of CTRP9 require further study.
TLR4-knockout mice need to be established for further study, which
will contribute to the understanding of the relationship between
TLR4 signaling and inflammation after overexpression of CTRP9.
In conclusion, the present study indicated that the
overexpression of CTRP9 could alleviate H/R by attenuating
inflammation in a TLR4/MyD88/NF-κB-dependent manner. However, the
pathophysiological process of MIRI is complex, such that the
possibility of other signaling pathways being involved in the
protective effects of CTRP9 in MIRI cannot be ruled out.
Nevertheless, results from the present study suggest that CTRP9 can
represent a novel therapeutic target for MIRI.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Health and
Family Planning Commission of Wuhan Municipality (grant no.
WX18A07).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZXZ, FA and ZYH were responsible for the conception
and design of the study. DZ, FA and YJW provided administrative
support. XLL, JH and JQL provided the study materials. ZYH, DZ and
YJW conducted NRCM culture, adenoviral transfection and
establishment of the H/R injury model; XLL and ZXZ conducted
RT-qPCR and western blotting assays; JH and LSJ performed ELISA;
and JQL and FA performed ELISA and cell viability assays. ZXZ and
DZ were responsible for the analysis and interpretation of data.
ZYH, DZ, FA and ZXZ confirmed the authenticity of all the raw data.
All authors contributed to the writing of the manuscript and read
and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Ethics Committee of Experimental Animals of the Southern Medical
University (approval no. 2020-01-03A; Shenzhen, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peng H and Abdel-Latif A: Cellular therapy
for ischemic heart disease: An update. Adv Exp Med Biol.
1201:195–213. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Anderson JL and Morrow DA: Acute
myocardial infarction. N Engl J Med. 376:2053–2064. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Godoy LC, Lawler PR, Farkouh ME, Hersen B,
Nicolau JC and Rao V: Urgent revascularization strategies in
patients with diabetes mellitus and acute coronary syndrome. Can J
Cardiol. 35:993–1001. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Guan BF, Dai XF, Huang QB, Zhao D, Shi JL,
Chen C, Zhu Y and Ai F: Icariside II ameliorates myocardial
ischemia and reperfusion injury by attenuating inflammation and
apoptosis through the regulation of the PI3K/AKT signaling pathway.
Mol Med Rep. 22:3151–3160. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Qian X, Zhu M, Qian W and Song J: Vitamin
D attenuates myocardial ischemia-reperfusion injury by inhibiting
inflammation via suppressing the RhoA/ROCK/NF-ĸB pathway.
Biotechnol Appl Biochem. 66:850–857. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Fujita T, Watanabe H, Murata Y, Hemmi S,
Yabuki M, Fuke Y, Satomura A and Soma M: Plasma C1q/TNF-related
protein 9: A promising biomarker for diabetic renal vascular
injury. Minerva Urol Nefrol. 69:195–200. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu Q, Zhang H, Lin J, Zhang R, Chen S,
Liu W, Sun M, Du W, Hou J and Yu B: C1q/TNF-related protein 9
inhibits the cholesterol-induced Vascular smooth muscle cell
phenotype switch and cell dysfunction by activating AMP-dependent
kinase. J Cell Mol Med. 21:2823–2836. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu M, Li W, Wang H, Yin L, Ye B, Tang Y
and Huang C: CTRP9 ameliorates atrial inflammation, fibrosis, and
vulnerability to atrial fibrillation in post-myocardial infarction
rats. J Am Heart Assoc. 8(e013133)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu M, Yin L, Li W, Hu J, Wang H, Ye B,
Tang Y and Huang C: C1q/TNF-related protein-9 promotes macrophage
polarization and improves cardiac dysfunction after myocardial
infarction. J Cell Physiol. 234:18731–18747. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang J, Hang T, Cheng XM, Li DM, Zhang QG,
Wang LJ, Peng YP and Gong JB: Associations of C1q/TNF-related
protein-9 levels in serum and epicardial adipose tissue with
coronary atherosclerosis in humans. Biomed Res Int.
2015(971683)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gao C, Zhao S, Lian K, Mi B, Si R, Tan Z,
Fu F, Wang S, Wang R, Ma X and Tao L: C1q/TNF-related protein 3
(CTRP3) and 9 (CTRP9) concentrations are decreased in patients with
heart failure and are associated with increased morbidity and
mortality. BMC Cardiovasc Disord. 19(139)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kambara T, Ohashi K, Shibata R, Ogura Y,
Maruyama S, Enomoto T, Uemura Y, Shimizu Y, Yuasa D, Matsuo K, et
al: CTRP9 protein protects against myocardial injury following
ischemia-reperfusion through AMP-activated protein kinase
(AMPK)-dependent mechanism. J Biol Chem. 287:18965–18973.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhao D, Feng P, Sun Y, Qin Z, Zhang Z, Tan
Y, Gao E, Lau WB, Ma X, Yang J, et al: Cardiac-derived CTRP9
protects against myocardial ischemia/reperfusion injury via
calreticulin-dependent inhibition of apoptosis. Cell Death Dis.
9(723)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang J, Zhang J, Yu P, Chen M, Peng Q,
Wang Z and Dong N: Remote ischaemic preconditioning and sevoflurane
postconditioning synergistically protect rats from myocardial
injury induced by ischemia and reperfusion partly via inhibition
TLR4/MyD88/NF-κB signaling pathway. Cell Physiol Biochem. 41:22–32.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ye B, Chen X, Dai S, Han J, Liang X, Lin
S, Cai X, Huang Z and Huang W: Emodin alleviates myocardial
ischemia/reperfusion injury by inhibiting gasdermin D-mediated
pyroptosis in cardiomyocytes. Drug Des Devel Ther. 13:975–990.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xue J, Ge H, Lin Z, Wang H, Lin W, Liu Y,
Wu G, Xia J and Zhao Q: The role of dendritic cells regulated by
HMGB1/TLR4 signalling pathway in myocardial ischaemia reperfusion
injury. J Cell Mol Med. 23:2849–2862. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li X, Yang J, Yang J, Dong W, Li S, Wu H
and Li L: RP105 protects against myocardial ischemia-reperfusion
injury via suppressing TLR4 signaling pathways in rat model. Exp
Mol Pathol. 100:281–286. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Simpson P, McGrath A and Savion S: Myocyte
hypertrophy in neonatal rat heart cultures and its regulation by
serum and by catecholamines. Circ Res. 51:787–801. 1982.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hnasko TS and Hnasko RM: The western blot.
Methods Mol Biol. 1318:87–96. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xu W, Zhang K, Zhang Y, Ma S and Jin D:
Downregulation of DEC1 by RNA interference attenuates
ischemia/reperfusion-induced myocardial inflammation by inhibiting
the TLR4/NF-κB signaling pathway. Exp Ther Med. 20:343–350.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang R, Xu L, Zhang D, Hu B, Luo Q, Han
D, Li J and Shen C: Cardioprotection of ginkgolide B on myocardial
ischemia/reperfusion-induced inflammatory injury via regulation of
A20-NF-κB pathway. Front Immunol. 9(2844)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yan W, Guo Y, Tao L, Lau WB, Gan L, Yan Z,
Guo R, Gao E, Wong GW, Koch WL, et al: C1q/tumor necrosis
factor-related protein-9 regulates the fate of implanted
mesenchymal stem cells and mobilizes their protective effects
against ischemic heart injury via multiple novel signaling
pathways. Circulation. 136:2162–2177. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sun Y, Yi W, Yuan Y, Lau WB, Yi D, Wang X,
Wang Y, Su H, Wang X, Gao E, et al: C1q/tumor necrosis
factor-related protein-9, a novel adipocyte-derived cytokine,
attenuates adverse remodeling in the ischemic mouse heart via
protein kinase A activation. Circulation. 128 (11 Suppl
1):S113–S120. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Weng CF, Wu CF, Kao SH, Chen JC and Lin
HH: Down-regulation of miR-34a-5p potentiates protective effect of
adipose-derived mesenchymal stem cells against ischemic myocardial
infarction by stimulating the expression of C1q/tumor necrosis
factor-related protein-9. Front Physiol. 10(1445)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bai S, Cheng L, Yang Y, Fan C, Zhao D, Qin
Z, Feng X, Zhao L, Ma J, Wang X, et al: C1q/TNF-related protein 9
protects diabetic rat heart against ischemia reperfusion injury:
Role of endoplasmic reticulum stress. Oxid Med Cell Longev.
2016(1902025)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li Y, Geng X, Wang H, Cheng G and Xu S:
CTRP9 ameliorates pulmonary arterial hypertension through
attenuating inflammation and improving endothelial cell survival
and function. J Cardiovasc Pharmacol. 67:394–401. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu T, Xu B and Liu Z: CTRP9 alleviates
inflammation to ameliorate myocardial infarction in rats by
activating Nrf2. Minerva Endocrinol. 45:268–270. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jung CH, Lee MJ, Kang YM, Lee YL, Seol SM,
Yoon HK, Kang SW, Lee WJ and Park JY: C1q/TNF-related protein-9
inhibits cytokine-induced vascular inflammation and leukocyte
adhesiveness via AMP-activated protein kinase activation in
endothelial cells. Mol Cell Endocrinol. 419:235–243.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li W, Ma N, Liu MX, Ye BJ, Li YJ, Hu HY
and Tang YH: C1q/TNF-related protein-9 attenuates retinal
inflammation and protects blood-retinal barrier in db/db mice. Eur
J Pharmacol. 853:289–298. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang H, Gong X, Ni S, Wang Y, Zhu L and
Ji N: C1q/TNF-related protein-9 attenuates atherosclerosis through
AMPK-NLRP3 inflammasome singling pathway. Int Immunopharmacol.
77(105934)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Qian M, Yang Q, Li J, Zhao B, Zhang Y and
Zhao Y: C1q/TNF-related protein-9 alleviates airway inflammation in
asthma. Int Immunopharmacol. 81(106238)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhao L, Chen S, Sherchan P, Ding Y, Zhao
W, Guo Z, Yu J, Tang J and Zhang JH: Recombinant CTRP9
administration attenuates neuroinflammation via activating
adiponectin receptor 1 after intracerebral hemorrhage in mice. J
Neuroinflammation. 15(215)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Azam S, Jakaria M, Kim IS, Kim J, Haque ME
and Choi DK: Regulation of toll-like receptor (TLR) signaling
pathway by polyphenols in the treatment of age-linked
neurodegenerative diseases: Focus on TLR4 signaling. Front Immunol.
10(1000)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ju M, Liu B, He H, Gu Z, Liu Y, Su Y, Zhu
D, Cang J and Luo Z: MicroRNA-27a alleviates LPS-induced acute lung
injury in mice via inhibiting inflammation and apoptosis through
modulating TLR4/MyD88/NF-κB pathway. Cell Cycle. 17:2001–2018.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zheng Z, Zeng YZ, Ren K, Zhu X, Tan Y, Li
Y, Li Q and Yi GH: S1P promotes inflammation-induced tube formation
by HLECs via the S1PR1/NF-κB pathway. Int Immunopharmacol.
66:224–235. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yuan X, Juan Z, Zhang R, Sun X, Yan R, Yue
F, Huang Y, Yu J and Xia X: Clemastine fumarate protects against
myocardial ischemia reperfusion injury by activating the
TLR4/PI3K/Akt signaling pathway. Front Pharmacol.
11(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Guo X, Jiang H, Yang J, Chen J, Yang J,
Ding JW, Li S, Wu H and Ding HS: Radioprotective 105 kDa protein
attenuates ischemia/reperfusion-induced myocardial apoptosis and
autophagy by inhibiting the activation of the TLR4/NF-κB signaling
pathway in rats. Int J Mol Med. 38:885–893. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Appari M, Breitbart A, Brandes F,
Szaroszyk M, Froese N, Korf-Klingebiel M, Mohammadi MM, Grund A,
Scharf GM, Wang H, et al: C1q-TNF-related protein-9 promotes
cardiac hypertrophy and failure. Circ Res. 120:66–77.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zheng S, Ren J, Gong S, Qiao F and He J:
CTRP9 protects against MIA-induced inflammation and knee cartilage
damage by deactivating the MAPK/NF-κB pathway in rats with
osteoarthritis. Open Life Sci. 15:971–980. 2020.PubMed/NCBI View Article : Google Scholar
|