Introduction
Prostate cancer is the second most commonly
diagnosed cancer and the sixth leading cause of cancer-associated
mortality among men worldwide, with an estimated 1,276,000 new
prostate cancer cases and 359,000 deaths in 2018(1). As PCa is an androgen-dependent cancer,
where endocrine therapy (castration and antihormone therapy) is one
of the main methods used to treat the disease (2). The majority of patients with PCa are
initially responsive to androgen-deprivation therapy; however, this
treatment not only increases the risk of dementia, but it may also
induce PCa-related androgen receptor mutations (3,4), which
renders PCa difficult to treat. At present, to the best of our
knowledge, the pathogenesis of PCa remains poorly understood. An
enhanced understanding of the mechanisms involved in the occurrence
and development of PCa will be of significant value to the early
diagnosis, treatment and monitoring of the disease (5).
MicroRNAs (miRNAs/miRs) are a class of small
non-coding RNAs that target specific mRNA sequences to inhibit
protein translation (6). It has
been reported that miRNAs are involved throughout the entire
tumorigenesis process, where they have been closely associated with
tumor cell proliferation, differentiation, invasion and metastasis
(7). It has also been extensively
reported that the expression levels of miRNAs in human cancer
tissues are dysregulated (8). In
fact, miRNAs have been proposed as alternative biomarkers and
therapeutic tools for PCa prognosis and diagnosis (9). For example, miR-15a and miR-16 have
been reported to function as tumor suppressors in PCa by inhibiting
cell proliferation and invasion (10). In another study, miR-449a induced
PCa cell cycle arrest by targeting histone deacetylase 1(11). Furthermore, inhibitors of miR-346,
miR-361-3p and miR-197 were found to significantly inhibit PCa
migration and invasion (12).
Previously, Fuse et al (13) identified 56 downregulated miRNAs in
PCa tissues in comparison to that in adjacent non-PCa tissues,
including miR-28-3p. To the best of our knowledge, the role of
miR-28-3p in PCa has not been reported. A previous study
demonstrated that the knockdown of ADP-ribosylation factor 6 (ARF6)
may exert an inhibitory effect on the hormone-independent PCa cell
line, PC-3, and the molecular mechanisms associated with these
changes were suggested to be due to the downregulation of
phosphorylated (p)-Erk1/2 and Rac1 expression (14). In addition, the stable
overexpression of the androgen driver tetraspanin 1 markedly
promoted cell migration and upregulated the expression of
ARF6(15). However, it remains
unclear whether miRNAs can target ARF6 in PCa.
The present study aims to explore the role of
miR-28-3p and its mechanism in the development of PCa. miR-28-3p
and ARF6 expression was quantified in both PCa tissues and cell
lines. Based on these previous findings, the role of miR-28-3p and
ARF6 in relation to the biological behaviors of PCa cells was
investigated.
Materials and methods
Cell lines and culture
Four human PCa cell lines (LNCaP, 22Rv-1, PC-3 and
DU145) and normal prostate cells (RWPE-1) were purchased from The
Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. PCa cell lines were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.). RWPE-1 cells were cultured in
serum-free RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
containing bovine pituitary extract (50 µg/ml; Thermo Fisher
Scientific, Inc.) and epidermal growth factor (5 ng/ml, Invitrogen;
Thermo Fisher Scientific, Inc.). All cells were maintained in a
humidified atmosphere with 5% CO2 at 37˚C.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using 0.5 ml NucleoZOL
reagent (Gene Company, Ltd.) from LNCaP, 22Rv-1, PC-3, DU145 and
RWPE-1 cells, according to the manufacturer's protocol. Total RNA
was reverse transcribed into cDNA using a Prime Script™ RT reagent
kit (cat. no. DRR037A; Takara Bio, Inc.). The reverse transcription
temperature protocol was as follows: 37˚C for 15 min and 85˚C for 5
sec. qPCR was subsequently performed using a SYBR Green Real-Time
PCR Master mix (Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for qPCR: 50˚C for 2 min,
initial denaturation at 95˚C for 10 min, followed by 40 cycles of
95˚C for 30 sec, 55˚C for 1 min and 72˚C for 30 sec. The following
primer sequences were used for qPCR: miR-28-3p forward,
5'-CGCGCACTAGATTGTGAGCT-3' and reverse, 5'-AGTGCAGGGTCCGAGGTATT-3';
U6 forward, 5'-CTCGCTTCGGCAGCACATATACT-3' and reverse,
5'-ACGCTTCACGAATTTGCGTGTC-3'; ARF6 forward,
5'-GCGGCATTACTACACTGGGA-3' and reverse, 5'-CCTGGATCTCGTGGGGTTTC-3';
and GAPDH forward, 5'-GTCAAGGCTGAGAACGGGAA-3' and reverse,
5'-AAATGAGCCCCAGCCTTCTC-3' miR-28-3p expression was normalized to
U6 expression levels, whereas ARF6 expression was normalized to
GAPDH expression levels. Relative gene expression was calculated
using the 2-ΔΔCq method (16).
Cell transfection
The miR-28-3p mimic (5'-CACUAGAUUGUGAGCUCCUGGA-3'),
mimic negative control (NC; 5'-UCUACUCUUUCUAGGAGGUUGUGA-3'),
miR-28-3p inhibitor (5'-UCCAGGAGCUCACAAUCUAGUG-3') and inhibitor NC
(5'-UCUACUCUUUCUAGGAGGUUGUGA-3') were obtained from Shanghai
GenePharma Co., Ltd. The ARF6 gene was synthesized and then cloned
into the pcDNA3.1 vector (Thermo Fisher Scientific, Inc.). To
construct the pcDNA-ARF6 recombinant plasmid. Transfection of the
empty vector pcDNA3.1 was used as negative control (pcDNA). DU145
cells were seeded at a density of 1x105 cells/well in
24-well plates. Transfection of each oligonucleotide or plasmid
into DU145 cells was performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Briefly, 5 µl Lipofectamine 2000 was
incubated in 250 µl serum-free RPMI-1640 medium for 5 min at 37˚C.
An appropriate amount of miR-28-3p mimic (25 nM), miR-28-3p
inhibitor (50 nM), mimic NC (25 nM), inhibitor NC (50 nM), pcDNA (2
µg) or pcDNA-ARF6 (2 µg) was added and incubated for a further 20
min at room temperature. Following the incubation, the final
mixture was added to each well and incubated at 37˚C with cells for
48 h. Subsequently, cells were used for the follow-up
experiments.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was analyzed using CCK-8 reagent
(Beyotime Institute of Biotechnology). Briefly, cells were seeded
into 96-well plates at a density of 1x103 cells/well and
cultured for 0, 24, 48, 72 or 96 h. Following the incubation, 10 µl
CCK-8 solution was added to each well and further incubated for 2 h
at 37˚C. The absorbance was measured at a wavelength of 450 nm
using a microplate reader.
Colony formation assay
Cell proliferation was also analyzed using a colony
forming assay. Briefly, transfected cells in the logarithmic growth
phase were collected, and 800 cells/well were plated into a 6-well
plate and incubated at 37˚C. The state of the cells was observed
until the number of cells in the majority of the individual
colonies in the well was ≥50. Following the incubation, the cells
were fixed with 4% paraformaldehyde at 4˚C for 1 h and stained with
clean, impurity-free 0.1% crystal violet dye solution for a further
2 min at room temperature. Images of colonies were captured with a
digital camera and counted.
Flow cytometric analysis of
apoptosis
Flow cytometry was performed to detect cell
apoptosis using an Annexin V-fluorescein isothiocyanate apoptosis
measurement kit (BD Biosciences). Briefly, following transfection,
the cells (1x106/m) were washed twice with cold PBS, and
stained with 5 µl Annexin V and 10 µl PI for 15 min in the dark at
room temperature. Apoptotic cells were analyzed using a BD
FACSVerse™ flow cytometer (BD Biosciences) and data were analyzed
with the FlowJo software Version 10 (FlowJo LLC). The percentage of
early and late apoptotic cells was calculated.
Transwell assays
The migratory and invasive abilities of cells were
analyzed using Transwell assays. For the migration assays, cells
were seeded into 24-well plates, before 1x105 cells
suspended in serum-free RPMI-1640 medium were added to the upper
chambers of Transwell plates (Corning, Inc.), whilst RPMI-1640
medium supplemented with 20% FBS was added to the lower chamber.
After 24 h of incubation at 37˚C, the cells remaining in the upper
chamber were removed with a cotton swab, while cells that had
migrated to the lower chamber were fixed with 4% paraformaldehyde
for 0.5 h at room temperature and stained with 0.5% crystal violet
for 15 min at room temperature. Migratory cells from each group
were visualized and counted in three randomly selected fields of
view using a light microscope (magnification, x400; Olympus
Corporation). The percentage migrated area was calculated with
Image Pro Plus 6.0 (Media Cybernetics, Inc.).
For the cell invasion assays, the upper chambers of
the 24-well Transwell plates were precoated with standard Matrigel
(BD Biosciences) diluted 1:8 in serum-free medium, and were
air-dried at 4˚C and thoroughly solidified at 37˚C for 30 min.
Subsequently, 100 µl cells from each group suspended in serum-free
medium were plated at a density of 1x105 cells/ml into
the upper chambers of. The remaining steps are consistent with
those aforementioned for the migration assay.
Vector construction and dual
luciferase reporter assay
To determine potential target genes of miR-28-3p,
TargetScan 7.2 (http://www.targetscan.org/vert_72/) was used.
According to the sequence of the ARF6 3'-untranslated region (UTR)
found in GenBank (accession no. NM_001663.4), primers were designed
and ARF6 was amplified (The template cDNA was obtained from 293T
cells) using PrimerSTAR® Max DNA Polymerase (cat. no.
R045A; Takara Biotechnology Co., Ltd.) and cloned into the
psiCHECK™-2 dual luciferase reporter vector (Promega Corporation)
to construct a wild-type (WT) dual-luciferase recombinant plasmid
(ARF6-WT). The following primer sequences were used for amplified:
forward, 5'-CCTCGAGTGACTTCCAGCAGATGGGATG-3' and reverse,
5'-ATTTGCGGCCGCACATACTGAGGTGCAACTGGA-3'. The following
thermocycling conditions were used for qPCR: Initial denaturation
at 95˚C for 5 min, denaturation at 95˚C for 30 sec, followed by 32
cycles of 55˚C for 30 sec, 72˚C for 45 s and 72˚C for 10 sec.
ARF6-Mut were synthesized by Universal biological systems (Anhui)
Co., Ltd. (https://www.generalbiol.com/) by mutating the specific
binding sequence for miR-28-3p in the 3'-UTR of ARF6. 293T cells
purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences and were cultured in DMEM (Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) at 37˚C under 5% CO2.
293T cells (1x106) were cultured in 12-well plates for
24 h at 37˚C. Subsequently, 20 µM miR-28-3p mimic or miR-28-3p
inhibitor and 50 ng ARF6-WT or -Mut reporter plasmids were
transfected into the cells using Lipofectamine® 2000 at
37˚C. Cells transfected with 20 µM mimic NC or inhibitor NC and 50
ng ARF6-WT or -Mut reporter plasmids were used as control groups. A
total of 48 h post-transfection, a Dual Luciferase Reporter assay
system (Promega Corporation) was used to measure the relative
luciferase activity. Firefly luciferase activity was normalized to
Renilla luciferase activity.
Western blotting
Total protein was extracted from collected cells in
RIPA buffer (Sigma-Aldrich; Merck KGaA) containing 1 mM PMSF
(Sigma-Aldrich; Merck KGaA) and total protein concentration was
quantified using a BCA assay (Beyotime Institute of Biotechnology).
In total, 20 µg proteins were separated by 10% SDS-PAGE and
subsequently transferred onto a PVDF membrane (EMD Millipore),
which was blocked with 5% bovine serum albumin (Sigma-Aldrich;
Merck KGaA) diluted in TBS-0.1% Tween-20 buffer at room temperature
for 2 h. The membranes were then incubated with the following
primary antibodies (all 1:1,000) at 4˚C overnight: Anti-ARF6
(Abcam; cat. no. ab226389), anti-Rac1 (Abcam; cat. no. ab155938),
anti-Bcl-2 (Abcam; cat. no. ab194583), anti-Bax (Abcam; cat. no.
ab263897), anti-Erk1/2 (Abcam; cat. no. ab17942), anti-p-ERK1/2
(Abcam; cat. no. ab214362) and anti-β-actin (Abcam; cat. no.
ab213262). Following the primary antibody incubation, the membranes
were washed with TBST and incubated with a HRP-conjugated secondary
antibody (Abcam; cat. no. ab6721; 1:5,000) at room temperature for
2 h. Protein bands were visualized using SuperSignal™ West Pico
PLUS Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.;
cat. no. 34080) on a VersaDoc™ gel imaging system (Bio-Rad
Laboratories, Inc.). Densitometric analysis was performed using the
Image J software (version 1.51; National Institutes of Health).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software version 5.0 (GraphPad Software, Inc.) and data are
presented as the mean ± SD. Statistical differences between groups
were analyzed using a one-way ANOVA followed by a Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference. All experiments were repeated three
times.
Results
Expression levels of miR-28-3p and
ARF6 in human PCa cell lines
To investigate the role of miR-28-3p and ARF6 in
human PCa cell lines, miR-28-3p and ARF6 expression levels were
analyzed in four PCa cell lines (LNCaP, 22Rv-1, PC-3 and DU145).
Compared with those detected in RWPE-1 cells, the expression levels
of miR-28-3p were significantly downregulated in the four PCa cell
lines (Fig. 1A), and DU145 cells
exhibited the greatest decrease in miR-28-3p expression.
Conversely, the mRNA expression levels of ARF6 were significantly
upregulated in the four PCa cell lines compared with those in
RWPE-1 cells (Fig. 1B), and DU145
cells had the highest expression levels of ARF6. Therefore, DU145
cells were selected for use in subsequent experiments, including
the establishment of miR-28-3p mimic-/inhibitor-transfected
cells.
Transfection efficiency of miR-28-3p
inhibitor and mimic in DU145 cells
To determine the role of miR-28-3p in PCa cells, the
human PCa cell line, DU145, was transfected with the miR-28-3p
mimic, which is a chemically synthesized double-stranded
oligonucleotide that mimics the endogenous mature function of
miR-28-3p, or a miR-28-3p inhibitor, which is a modified antisense
oligonucleotide that inhibits miR-28-3p function. Cells transfected
with the mimic NC or inhibitor NC were used as controls. A total of
48 h post-transfection, the expression levels of miR-28-3p were
determined using RT-qPCR. The results revealed that transfection
with the miR-28-3p mimic upregulated the expression levels of
miR-28-3p by ~30-fold, whereas transfection with the miR-28-3p
inhibitor downregulated the expression of miR-28-3p in DU145 cells
compared with those in cells transfected with the respective NCs
(Fig. 2). These results indicated
that the miR-28-3p mimic and inhibitor were able to effectively
regulate miR-28-3p expression levels in DU145 cells.
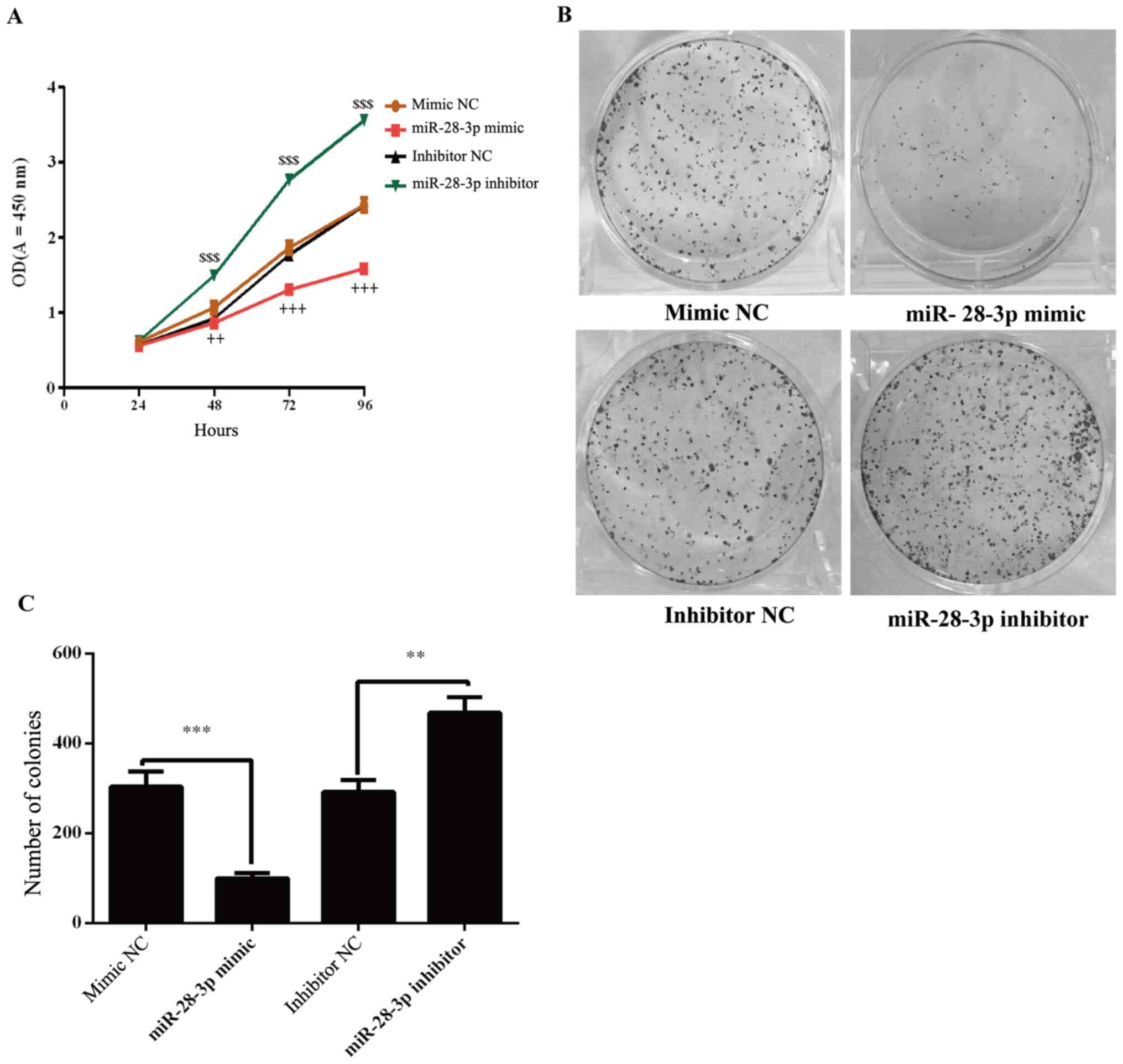
Effect of miR-28-3p on the
proliferation of DU145 cells
The effects of miR-28-3p on the proliferation of
DU145 cells were determined using a CCK-8 assay. The results
demonstrated that the proliferation of miR-28-3p mimic-transfected
cells was significantly decreased compared with the mimic
NC-transfected cells (Fig. 3A).
Conversely, the knockdown of miR-28-3p expression in cells
significantly increased the proliferation compared with the
inhibitor NC-transfected cells (Fig.
3A). To further analyze cell proliferation, colony formation
experiments were performed. The results revealed that the number of
colonies was significantly decreased in the miR-28-3p mimic group
compared with that in the mimic NC group (Fig. 3B and C). Compared with the inhibitor NC group,
the number of colonies was significantly increased in the miR-28-3p
inhibitor group.
Effect of miR-28-3p on the apoptosis
of DU145 cells
A total of 48 h post-transfection with the miR-28-3p
mimic or inhibitor, cell apoptosis was determined by flow
cytometry. The results revealed that the apoptotic rate was
significantly increased in the miR-28-3p mimic group compared with
that in the mimic NC group (Fig.
4A). Compared with the inhibitor NC group, the apoptotic rate
was significantly decreased in the miR-28-3p inhibitor group. In
addition, the ratio of Bcl-2/Bax was detected by western blotting.
The results revealed that miR-28-3p overexpression reduced the
Bcl-2/Bax ratio, which may aggravate apoptosis, whereas inhibition
of miR-28-3p expression upregulated the Bcl-2/Bax ratio, which
could inhibit apoptosis (Fig.
4B).
Effect of miR-28-3p on the migration
and invasion of DU145 cells
Whether miR-28-3p regulated human PCa cell migration
and invasion was investigated in the PCa cell line, DU145,
following transfection with the miR-28-3p mimic, miR-28-3p
inhibitor, mimic NC or inhibitor NC. The results revealed that the
migratory and invasive abilities of DU145 cells were significantly
decreased in the miR-28-3p mimic group compared with those in the
mimic NC group (Fig. 5). By
contrast, the migration and invasion were significantly increased
in the miR-28-3p inhibitor group compared with those in the
inhibitor NC group (Fig. 5). These
results indicated that miR-28-3p may be a negative regulator of PCa
migration and invasion.
ARF6 is a direct target gene of
miR-28-3p
TargetScan was used to predict the potential gene
target of miR-28-3p. The 3'-UTR of ARF6 was identified to contain a
complementary site for the seed region of miR-28-3p (Fig. 6A). To further validate whether
miR-28-3p targeted ARF6, a dual luciferase reporter assay was used
to determine the relationship between miR-28-3p and ARF6 in the
293T cell line. As illustrated in Fig.
6A, transfection with the miR-28-3p mimic significantly
decreased the relative luciferase activity of the ARF6-WT 3'-UTR
compared with that in cells following the transfection of mimic NC.
Transfection with the miR-28-3p inhibitor significantly increased
the relative luciferase activity of the ARF6-WT 3'-UTR compared
with that in cells after the transfection of inhibitor NC.
Furthermore, these effect was abolished when the nucleotides in the
seed binding site of the ARF6 3'-UTR were mutated. Moreover, the
effect of miR-28-3p on the expression levels of ARF6 was analyzed
using RT-qPCR and western blotting. The results revealed that
overexpression of miR-28-3p significantly downregulated the mRNA
expression levels of ARF6 in DU145 cells compared with those in the
mimic NC-transfected cells (Fig.
6B). Conversely, knockdown of miR-28-3p significantly
upregulated the mRNA expression levels of ARF6 compared with those
in the inhibitor NC-transfected cells (Fig. 6B). In addition, overexpression of
miR-28-3p significantly downregulated the protein expression levels
of ARF6 in DU145 cells compared with those in the mimic
NC-transfected cells, whereas knockdown of miR-28-3p significantly
upregulated the protein expression levels of ARF6 compared with
those in the inhibitor NC-transfected cells (Fig. 6C). These results suggested that ARF6
may be a direct target gene of miR-28-3p.
Effect of ARF6 and miR-28-3p
overexpression on DU145 cells
The transfection efficiency of pcDNA-ARF6 was
validated using RT-qPCR (Fig. 7A).
As expected, the expression levels of ARF6 in cells transfected
with pcDNA-ARF6 were significantly upregulated compared with those
in the pcDNA-transfected cells. To investigate the effect of ARF6
on the miR-28-3p mimic-transfected DU145 cells, pcDNA-ARF6 was
transfected into miR-28-3p mimic-transfected DU145 cells. The
results of the CCK-8 assay demonstrated that the overexpression of
ARF6 significantly attenuated the miR-28-3p mimic-induced
inhibitory effect on cell proliferation (Fig. 7B), which was consistent with the
results of the cell colony formation assay (P<0.05; Fig. 7C and D).
Apoptosis was further determined by flow cytometry
and western blotting after co-transfecting cells with miR-28-3p
mimic and pcDNA-ARF6 for 48 h. The results revealed that the
apoptotic rate was significantly decreased (Fig. 8A) and the ratio of Bcl-2/Bax was
markedly elevated (Fig. 8B)
following co-transfection compared with that in the miR-28-3p
mimic-transfected group; however, the apoptotic rate was not
completely recovered to the same levels as the control. Finally,
migratory and invasive abilities were determined using Transwell
assays. The results showed that the overexpression of ARF6 in
miR-28-3p mimic-transfected cells promoted both migration
(P<0.01) and invasion (P<0.01) compared with those in the
miR-28-3p mimic group (Fig. 9).
These findings suggested that AFR6 may attenuate miR-28-3p
mimic-induced inhibitory effects.
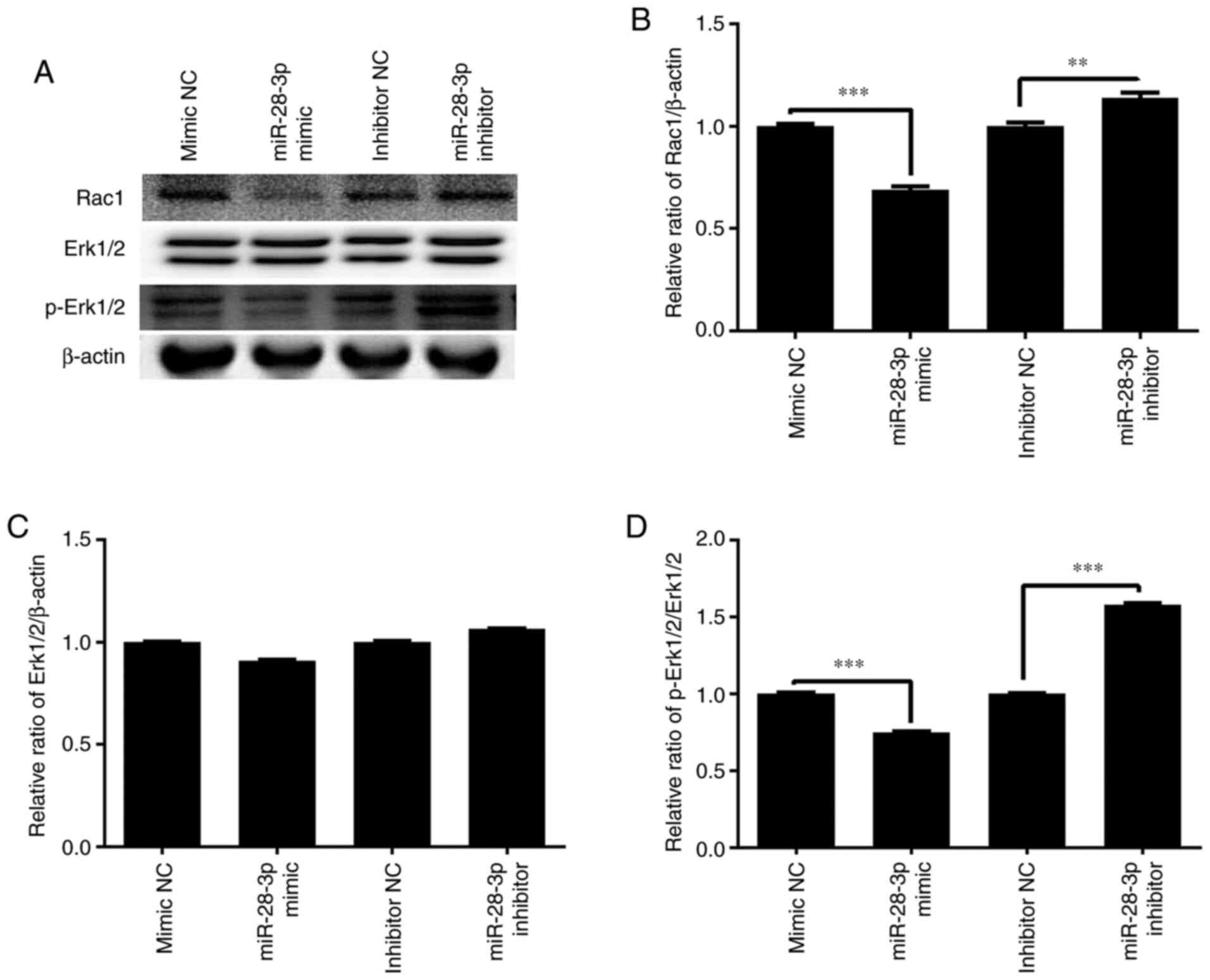
Effect of miR-28-3p overexpression and
knockdown on p-Erk1/2 and Rac1 expression
To further determine the molecular mechanism of the
miR-28-3p-mediated biological functions, the effect of miR-28-3p
overexpression and knockdown on p-Erk1/2 and Rac1 expression was
analyzed. The results demonstrated that the overexpression of
miR-28-3p significantly downregulated the protein expression levels
of p-Erk1/2/Erk1/2 and Rac1 in DU145 cells compared with those in
the mimic NC-transfected cells, but exerted no effect on Erk1/2
expression (Fig. 10). Meanwhile,
the knockdown of miR-28-3p significantly upregulated the protein
expression levels of p-Erk1/2/Erk1/2 and Rac1 compared with those
in the inhibitor NC-transfected cells, but had no effect on Erk1/2
expression.
Effect of ARF6 and miR-28-3p
overexpression on p-Erk1/2 and Rac1
To further determine the mechanism by which
miR-28-3p and ARF6 affected the biological functions of PCa cells,
the effect of the overexpression of these two factors on p-Erk1/2
and Rac1 expression was investigated. As shown in Fig. 11, the results revealed that
transfection with the miR-28-3p mimic significantly downregulated
the protein expression levels of Rac1 and p-Erk1/2/Erk1/2, without
altering total Erk1/2 expression. Notably, the co-overexpression of
ARF6 partially reversed the effects of the miR-28-3p mimic on Rac
and p-Erk1/2 expression. Thus, miR-28-3p and ARF6 may mediate the
Erk signaling pathway.
 | Figure 11Effect of miR-28-3p and ARF6
overexpression on p-Erk1/2 and Rac1 expression. (A) Western
blotting was performed to analyze the effect of miR-28-3p and ARF6
overexpression on p-Erk1/2 and Rac1 expression. Protein expression
levels of (B) Rac1, (C) Erk1/2 (molecular weight at 44 and 42 kDa,
respectively) and (D) p-Erk1/2 (molecular weight at 44 and 42 kDa,
respectively) in DU145 cells after transfection with miR-28-3p
mimic and pcDNA-ARF6. *P<0.05,
**P<0.01, ***P<0.001. miR, microRNA;
NC, negative control; pcDNA, pcDNA3.1; ARF6, ADP-ribosylation
factor 6; p-, phosphorylated. |
Discussion
PCa is a malignant epithelial tumor that occurs in
the prostate (17). Numerous
miRNAs, such as miR-1, miR-21, miR-106b, miR-141, miR-145, miR-205,
miR-221 and miR-375(18), have been
reported to be implicated in PCa progression. Among them, miR-28-3p
was discovered to play a major role in several tumor types. For
example, the overexpression of miR-28-3p increased the migration
and invasion of colorectal cancer cells in vitro (19). Another previous study found that the
expression levels of miR-28-3p were significantly upregulated in
esophageal squamous cell carcinoma (ESCC) tissues and it was
therefore suggested to be a potential novel serum miRNA marker to
screen for ESCC (20). In addition,
some miRNAs in urine supernatants, including miR-28-3p, were found
to be potential noninvasive markers for bladder cancer diagnosis
(21). Fuse et al (13) screened 56 downregulated miRNAs in
PCa tissues in comparison to non-PCa tissues and discovered that
miR-222 and miR-31 inhibited the proliferation, invasion and
migration of the hormone-independent PCa cell lines, PC3 and DU145.
Nevertheless, to the best of our knowledge, the role of miR-28-3p
in PCa remains unclear.
In the present study, the expression levels of
miR-28-3p were found to be downregulated in PCa cells compared with
those in RWPE-1 cells; thus, it was hypothesized that miR-28-3p may
play a role in the development of PCa. To confirm this hypothesis,
miR-28-3p-overexpressing and -knockdown models were established in
PCa cells, and miR-28-3p overexpression was discovered to inhibit
the cell proliferation, migration and invasion, and induce the
apoptosis of PCa cells. miR-28-3p knockdown was found to promote
the cell proliferation, migration and invasion, whilst inhibiting
the apoptosis of PCa cells. These results indicated that miR-28-3p
may serve as a tumor suppressor in PCa and could represent a target
for PCa treatment.
To further investigate the mechanism of miR-28-3p in
PCa, potential targets of miR-28-3p were predicted using
bioinformatics software and ARF6 was identified as a candidate
target gene of miR-28-3p. Subsequently, ARF6 was confirmed as a
direct target of miR-28-3p using a dual luciferase reporter assay,
RT-qPCR and western blotting. ARF6 is a small GTPase that mainly
regulates membrane trafficking and actin remodeling (22). Previous studies have shown that the
upregulated expression of ARF6 may be closely associated with the
invasion, migration and metastasis of multiple malignant tumor
types (23), such as glioma
(24), melanoma (25), breast cancer (26), lung cancer (27) and PCa (28). Shan et al (14) also reported that the knockdown of
ARF6 effectively inhibited the proliferation, migration and
invasion of PC-3 cells. Thus, miR-28-3p was hypothesized to affect
PCa cell behavior via targeting ARF6 expression. In the present
study, rescue experiments revealed that ARF6 overexpression could
reverse the effect of the miR-28-3p mimic on proliferation,
apoptosis, migration and invasion. These data indicated that ARF6
may be involved in miR-28-3p-mediated PCa progression.
Erk1/2 is a member of the mitogen-activated protein
kinase signaling pathway, and Erk1/2 phosphorylation has been found
to promote the proliferation, migration and invasion of PCa cells
(29). Rac1, one of the
best-characterized members of the small GTPase family, has been
reported to be involved in the organization of the actin
cytoskeleton, cell proliferation, tumorigenesis and metastasis
(30). ARF6 has also been
identified as a potent modulator of Erk and Rac1 activity. Hu et
al (31) reported that ARF6
regulated glioma cell migration and invasion via a Rac1-dependent
pathway. Tague et al (32)
revealed that ARF6 promoted melanoma cell invasion by enhancing
Erk1/2 phosphorylation. In hepatocellular carcinoma cells, the
knockdown of ARF6 inhibited cell migration and invasion by
decreasing Erk1/2 phosphorylation levels and Rac1 activity
(33). In PC-3 PCa cells, the
knockdown of ARF6 inhibited proliferation, migration and invasion
by downregulating p-Erk1/2 and Rac1 expression (14). Thus, the present study sought to
determine whether miR-28-3p regulated p-Erk1/2 and Rac1 expression
by targeting ARF6 in PCa. Western blotting showed that transfection
with the miR-28-3p mimic downregulated p-Erk1/2 and Rac1 protein
expression levels, indicating that miR-28-3p may suppress p-Erk1/2
and Rac1 in PCa. Furthermore, rescue experiments revealed that the
overexpression of ARF6 attenuated the inhibitory effects of the
miR-28-3p mimic on p-Erk1/2 and Rac1. Taken together, these results
suggested that miR-28-3p may suppress p-Erk1/2 and Rac1 expression,
at least partly, by targeting ARF6 in PCa.
In conclusion, the present results suggested that
miR-28-3p may act as a tumor suppressor gene in PCa and may be
involved in PCa by targeting ARF6 to downregulate the expression of
p-Erk1/2 and Rac1. Therefore, miR-28-3p may represent a novel
target for the treatment of PCa. However, this study has its own
limitations. For example, the effect of miR-28-3p in PCa was only
explored in PCa DU145 cells and will need to be investigated in
different cell lines in the future to verify the current
findings.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grant from the
Natural Science Foundation of Fujian Province (grant no.
2018J01219).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and YY conceived and designed the experiments. HL
and SY performed the experiments and analyzed the data. JZ and YY
wrote and revised the manuscript. All authors read and approved the
final manuscript. JZ and YY confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nagaya N and Horie S: Endocrine therapy
for prostate cancer. Clin Calcium. 28:1527–1533. 2018.PubMed/NCBI(In Japanese).
|
|
3
|
Nead KT, Sinha S and Nguyen PL: Androgen
deprivation therapy for prostate cancer and dementia risk: A
systematic review and meta-analysis. Prostate Cancer Prostatic Dis.
20:259–264. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fujita K and Nonomura N: Role of androgen
receptor in prostate cancer: A review. World J Mens Health.
37:288–295. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Carruba G: Estrogens in prostate cancer.
In: Prostate Cancer: A Comprehensive Perspective. Tewari, AK (ed).
Springer, London, pp369-381, 2013.
|
|
6
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tutar Y: miRNA and cancer; computational
and experimental approaches. Curr Pharm Biotechnol.
15(429)2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Vanacore D, Boccellino M, Rossetti S,
Cavaliere C, D'Aniello C, Di Franco R, Romano FJ, Montanari M, La
Mantia E, Piscitelli R, et al: Micrornas in prostate cancer: An
overview. Oncotarget. 8:50240–50251. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bonci D, Coppola V, Musumeci M, Addario A,
Giuffrida R, Memeo L, D'Urso L, Pagliuca A, Biffoni M, Labbaye C,
et al: The miR-15a-miR-16-1 cluster controls prostate cancer by
targeting multiple oncogenic activities. Nat Med. 14:1271–177.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Noonan EJ, Place RF, Pookot D, Basak S,
Whitson JM, Hirata H, Giardina C and Dahiya R: miR-449a targets
HDAC-1 and induces growth arrest in prostate cancer. Oncogene.
28:1714–1724. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fletcher CE, Sulpice E, Combe S, Shibakawa
A, Leach DA, Hamilton MP, Chrysostomou SL, Sharp A, Welti J, Yuan
W, et al: Androgen receptor-modulatory microRNAs provide insight
into therapy resistance and therapeutic targets in advanced
prostate cancer. Oncogene. 38:5700–5724. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fuse M, Kojima S, Enokida H, Chiyomaru T,
Yoshino H, Nohata N, Kinoshita T, Sakamoto S, Naya Y, Nakagawa M,
et al: Tumor suppressive microRNAs (miR-222 and miR-31) regulate
molecular pathways based on microRNA expression signature in
prostate cancer. J Hum Genet. 57:691–699. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shan XW, Lv SD, Yu XM, Hu ZF, Zhang JJ,
Wang GF and Wei Q: Small RNA interference-mediated ADP-ribosylation
factor 6 silencing inhibits proliferation, migration and invasion
of human prostate cancer PC-3 cells. Nan Fang Yi Ke Da Xue Xue Bao.
36:735–743. 2016.PubMed/NCBI(In Chinese).
|
|
15
|
Hongu T, Yamauchi Y, Funakoshi Y, Katagiri
N, Ohbayashi N and Kanaho Y: Pathological functions of the small
GTPase Arf6 in cancer progression: Tumor angiogenesis and
metastasis. Small GTPases. 7:47–53. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Han W and Li J: Structure-activity
relationship analysis of 3-phenylpyrazole derivatives as androgen
receptor antagonists. J Biomol Struct Dyn. 38:2582–2591.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Luu HN, Lin HY, Sørensen KD, Ogunwobi OO,
Kumar N, Chornokur G, Phelan C, Jones D, Kidd L, Batra J, et al:
miRNAs associated with prostate cancer risk and progression. BMC
Urol. 17(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Almeida MI, Nicoloso MS, Zeng L, Ivan C,
Spizzo R, Gafà R, Xiao L, Zhang X, Vannini I, Fanini F, et al:
Strand-specific miR-28-5p and miR-28-3p have distinct effects in
colorectal cancer cells. Gastroenterology. 142:886–896.e9.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang Z, Zhang L, Zhu D, Shan X, Zhou X,
Qi LW, Wu L, Zhu J, Cheng W, Zhang H, et al: A novel serum microRNA
signature to screen esophageal squamous cell carcinoma. Cancer Med.
6:109–119. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Pospisilova S, Pazourkova E, Horinek A,
Brisuda A, Svobodova I, Soukup V, Hrbacek J, Capoun O, Hanus T,
Mares J, et al: MicroRNAs in urine supernatant as potential
non-invasive markers for bladder cancer detection. Neoplasma.
63:799–808. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hongu T and Kanaho Y: Versatile in vivo
functions of the small GTPase Arf6. Seikagaku. 88:78–85.
2016.PubMed/NCBI(In Japanese).
|
|
23
|
Li R, Peng C, Zhang X, Wu Y, Pan S and
Xiao Y: Roles of Arf6 in cancer cell invasion, metastasis and
proliferation. Life Sci. 182:80–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li M, Wang J, Ng SS, Chan CY, He ML, Yu F,
Lai L, Shi C, Chen Y, Yew DT, et al: Adenosine
diphosphate-ribosylation factor 6 is required for epidermal growth
factor-induced glioblastoma cell proliferation. Cancer.
115:4959–4972. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Grossmann AH, Yoo JH, Clancy J, Sorensen
LK, Sedgwick A, Tong Z, Ostanin K, Rogers A, Grossmann KF, Tripp
SR, et al: The small GTPase ARF6 stimulates β-catenin
transcriptional activity during WNT5A-mediated melanoma invasion
and metastasis. Sci Signal. 6(ra14)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hashimoto S, Onodera Y, Hashimoto A,
Tanaka M, Hamaguchi M, Yamada A and Sabe H: Requirement for Arf6 in
breast cancer invasive activities. Proc Natl Acad Sci USA.
101:6647–6652. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Menju T, Hashimoto S, Hashimoto A, Otsuka
Y, Handa H, Ogawa E, Toda Y, Wada H, Date H, Sabe H, et al:
Engagement of overexpressed Her2 with GEP100 induces autonomous
invasive activities and provides a biomarker for metastases of lung
adenocarcinoma. PLoS One. 6(e25301)2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liang C, Qin Y, Zhang B, Ji S, Shi S, Xu
W, Liu J, Xiang J, Liang D, Hu Q, et al: ARF6, induced by mutant
Kras, promotes proliferation and Warburg effect in pancreatic
cancer. Cancer Lett. 388:303–311. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Klemke RL, Cai S, Giannini AL, Gallagher
PJ, de Lanerolle P and Cheresh DA: Regulation of cell motility by
mitogen-activated protein kinase. J Cell Biol. 137:481–492.
1997.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Burridge K and Wennerberg K: Rho and Rac
take center stage. Cell. 116:167–179. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hu B, Shi B, Jarzynka MJ, Yiin JJ,
D'Souza-Schorey C and Cheng SY: ADP-ribosylation factor 6 regulates
glioma cell invasion through the IQ-domain GTPase-activating
protein 1-Racl-mediated pathway. Cancer Res. 69:794–801.
2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tague SE, Muralidharan V and
D'Souza-Schorey C: ADP-ribosylation factor 6 regulates tumor cell
invasion through the activation of the MEK/ERK signaling Pathway.
Proc Natl Acad Sci USA. 101:967I–9676. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hu Z, Du J, Yang L, Zhu Y, Yang Y, Zheng
D, Someya A, Gu L and Lu X: GEPl00/Arf6 is required for epidermal
growth factor-induced ERK/Rac 1 signaling and cell migration in
human hepatoma HepG2 cells. PLoS One. 7(e38777)2012.PubMed/NCBI View Article : Google Scholar
|