Introduction
Acute myocardial infarction (AMI) is one of the
leading causes of mortality in patients with cardiovascular disease
worldwide (1), accounting for 80%
of cardiogenic shock (2). AMI is
caused by coronary artery blockage and myocardial ischemic
necrosis, which eventually leads to heart failure (3). Therefore, early reperfusion therapy
can save the dying myocardium, which is essential for blood supply
and nutritional support of ischemic myocardium, as well as
improving the prognosis of patients with AMI (1,3).
However, there is evidence that traditional reperfusion therapy can
cause additional damage to myocardial structure and function,
namely myocardial ischemia-reperfusion injury (IRI) (3,4). IRI
is a pathological phenomenon in which the reintroduction of oxygen
and other nutrients into the myocardium further aggravates
pathological damage to the myocardium, resulting in decreased
cardiac function, arrhythmias and irreversible death of
cardiomyocytes (1,3,4).
Therefore, elucidating the mechanism of IRI and improving
myocardial damage caused by IRI is important for the clinical
treatment of patients with AMI to restore coronary blood flow.
MicroRNAs (miRNAs/miRs) are RNA regulators
consisting of 18-22 nucleotides, which can bind to mRNAs and
decrease protein translation (5).
In recent years, it was reported that miRNAs, as a small regulator,
are closely associated with numerous diseases, including myocardial
infarction (MI), heart failure and acute coronary syndrome
(5). Several miRNAs have been
identified as potential biomarkers for cardiovascular disease, and
their expression levels are closely associated with the occurrence
and prognosis of cardiovascular disease (5). Previous studies have revealed that
miR-24 is essential in myocardial infarction. For example, bone
marrow mesenchymal stem cell exosomes inhibited myocardial cell
apoptosis in AMI rats via the upregulation of miR-24 under hypoxic
conditions (6). miR-24 inhibited
the proliferation of vascular smooth muscle cells and regulated
vascular remodeling in the diabetic rat model by inhibiting the
platelet-derived growth factor/BB pathway (7). Furthermore, miR-24 is abnormally
expressed in myocardial fibrosis after infarction, which is
regulated by TGF-β1 signaling (8,9).
Compared with wild-type mice, miR-24 transgenic mice with MI showed
decreased myocardial cell apoptosis and improved cardiac function
after MI (10). Following induction
of MI, inhibiting the expression of miR-24 has significant
biological effects on cardiomyocytes and fibroblasts, which can
reduce the infarct size and markedly improve cardiac function
(11).
It has been previously reported that the occurrence
of IRI involves a variety of factors, and the damage caused by
inflammatory response to cells is an important pathological
mechanism (12). NF-κB, as an
inflammation marker, is involved in the occurrence of various
diseases, such as chronic inflammatory diseases, heart disease and
cancer (13,14). NF-κB signaling regulates the immune
response, hypoxia response, apoptosis and embryonic development, as
well as tumorigenesis and development (12-14).
TNF-α serves an important role as a proinflammatory cytokine in
NF-κB-mediated inflammation (15).
However, whether miR-24 can alleviate IRI-induced MI via the
NF-κB/TNF-α pathway remains to be elucidated. The present study
aimed to investigate the role and mechanism of miR-24 in
IRI-induced myocardial damage via in vitro and in
vivo studies.
Materials and methods
Cell culture
Cardiomyocytes (H9C2 cell line; American Type
Culture Collection) were cultured in high-glucose DMEM containing
10% FBS and 1% penicillin-streptomycin (all from Thermo Fisher
Scientific, Inc.). Cells were cultured in a 5% CO2
incubator with 95% saturation humidity at 37˚C. Subsequent
experiments were performed with cells in the logarithmic growth
phase.
IRI model
Cardiomyocytes in the logarithmic phase were
randomly divided into three groups: i) Cells in the negative
control (NC) group were cultured in normal DMEM; ii) cells in the
model group were cultured in glucose-free DMEM (Thermo Fisher
Scientific, Inc.) with a mixture of 5% CO2 and 95%
N2 for 10 h at 37˚C under ischemia, followed by addition
of high-glucose (4,500 mg/l) DMEM for 2 h of routine culture and
iii) cells in the miRNA group were incubated in glucose-free DMEM
with a mixture of 5% CO2 and 95% N2 gas for
10 h at 37˚C under ischemia, followed by addition of normal DMEM
for 2 h of routine culture after transfection of miR-24(1). Briefly, H9C2 cells were seeded in a
6-well plate at a density of 1x105 cells/well. Once cell
fusion reached 70%, Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to transfect cells in the
NC and miR-24 (Sangon Biotech Co., Ltd.) groups according to the
kit instructions. miRNA NC sequence, 5'-CCTTGGATGGCCTAGGAGATAG-3';
and miR-24 mimic sequence, 5'-TGGCTCAGTTCAGCAGGAACAG-3'. The cells
were incubated in an incubator at 37˚C and 5%
CO2 for 48 h before subsequent experiments.
Cell apoptosis via flow cytometry
The apoptosis of H9C2 was analyzed via flow
cytometry (Beckman Coulter, Inc.). Cells were collected and washed
twice with PBS. Cells were resuspended in 300 µl binding buffer and
incubated in 5 µl Annexin V-FITC and 5 µl PI at room temperature in
the dark for 15 min. Finally, 200 µl binding buffer was added to
resuspend the cells, which were then filtered. Flow cytometry was
performed within 1 h.
Western blotting
Total protein in H9C2 cells and tissues were
extracted with RIPA buffer (Thermo Fisher Scientific, Inc.), and
proteins were quantified using a BCA assay protein kit. Loading
buffer (Thermo Fisher Scientific, Inc.) was added to the lysate
solution, boiled at 100˚C for 10 min and then stored at -20˚C.
Equal amounts of protein samples (30 µg) were separated via 10%
SDS-PAGE. Separated proteins were transferred to nitrocellulose
filter membranes. Membranes were blocked with 5% skimmed milk
powder [prepared with TBS-0.1% Tween-20 (TBS-T)] at room
temperature for 1 h. Subsequently, membranes were incubated with
the following primary antibodies: NF-κB (cat. no. 8242S), caspase-3
(cat. no. 9662S), Bax (cat. no. 2772S), Bcl-2 (cat. no. 3498S),
TNF-α (cat. no. 3707S), vascular cell adhesion molecule 1 (VCAM-1;
cat. nos. 13662S and 39036S), intercellular adhesion molecule 1
(ICAM-1; cat. no. 4915S) and monocyte chemoattractant protein-1
(MCP-1; cat. nos. 81559S and 41987S) (all from Cell Signaling
Technology, Inc.) and incubated overnight at 4˚C. Membranes were
washed three times with TBS-T (5 min each time) and then incubated
at room temperature for 1 h with the corresponding secondary
antibody (anti-rabbit IgG HRP-linked antibody; 1:3,000; cat. no.
7074; Cell Signaling Technology, Inc.) under gentle rotation. An
ECL kit (Thermo Fisher Scientific, Inc.) was used to detect protein
bands. GAPDH was used as an internal reference.
Animal experiments
Healthy male Sprague-Dawley rats (weight, 200±20 g;
age, 8 weeks) were purchased from Shanghai SLAC Laboratory Animal
Co., Ltd. Under normal conditions, rats were fed under a normal
circadian rhythm of 12/12 h light/dark cycles. Establishment of an
IRI model was as follows: Rats were anesthetized by intraperitoneal
injection of 60 mg/kg pentobarbital sodium. Rats were placed in the
right decubitus position, and microscopic scissors were used to
open the chest cavity between the three and four intercostals of
the left forelimb to fully expose the heart. Then, microscopic
straight forceps were used to gently pick up a small amount of
pericardium and slightly tear the pericardium under the left atrial
appendage to fully expose the left anterior descending coronary
artery (LAD) or the area. A 7-0 needle suture with a needle holder
was utilized, and the needle was inserted 2-mm from the lower edge
of the left atrial appendage. The suture was passed through the LAD
to completely block the blood flow of LAD. Changes in the
electrocardiogram and myocardial color were observed, and the
ligation was removed after 30 min and reperfusion was conducted for
120 min. The experimental rats were randomly divided into three
groups (15 rats in total; five rats in each group): i) Sham group,
threaded but with no ligated artery; ii) Model group, where animals
underwent IRI modeling after ischemia for 30 min and reperfusion
was performed for 120 min; and iii) miRNA group, where animals
underwent IRI modeling, ischemia was first performed for 30 min and
then rats underwent reperfusion for 120 min. After 7 days, miR-24
was injected into the myocardium. The animal experiments involved
in the present study were approved by the Ethics Committee of
Zhoupu Hospital affiliated to Shanghai Medical University (approval
no. 2017-C-040-E01).
MI area determination
After reperfusion in each group, the animals were
euthanized by cervical dislocation. The rat hearts were removed,
and 1 ml 3% Evans blue (Sigma-Aldrich; Merck KGaA) was injected
into the left ventricle. Perpendicular to the long axis direction
of the heart, the heart tissue was cut into thin slices with a
thickness of 5 µm and 1% 2,3,5-triphenyltetrazolium chloride
solution was incubated with the heart tissue section at 37˚C for 15
min. Image Pro Plus 6.0 analysis software (Media Cybernetics, Inc.)
was used to determine the infarct size.
Hematoxylin and eosin (H&E)
staining
Heart tissues were fixed with 4% paraformaldehyde
for 30 min at room temperature, and paraffin sections (5 µm) were
sliced, followed by H&E staining. The heart tissue sections
were immersed in hematoxylin solution for 5-20 min at room
temperature and differentiated with 1% hydrochloric acid alcohol
for 1-5 sec, and then stained with 1% eosin solution for 1 min at
room temperature. Myocardial injury was observed under an optical
microscope (magnification, x200).
TUNEL assay
Cardiomyocyte apoptosis in heart tissues were
detected using the TUNEL method (cat. no. 11684817910; Roche
Diagnostics). Tissue sections were washed twice with xylene for
dewaxing, washed once with gradient ethanol (100, 95, 90, 80 and
70%) for rehydration and then treated with cell permeate for 8 min.
Subsequently, 50 µl TUNEL reaction mixture was added to the
specimens and incubated for 1 h at 37˚C in the dark in a wet box. A
total of 50 µl converter-POD was added and sections were incubated
for 30 min. Finally, 50-100 µl diaminobenzidine (DAB) substrate was
added to the tissues and incubated at 15-25˚C for 10 min. Sections
were observed and imaged using an optical microscope
(magnification, x200) and Image-Pro Plus software (Media
Cybernetics, Inc.).
Immunohistochemical staining
Slices were placed in an oven for 30 min for
dewaxing and were then hydrated with gradient alcohol. A total of 1
ml 30% H2O2 and 9 ml methanol were used to
remove endogenous catalase. Subsequently, 5% BSA blocking solution
(cat. no. A1933-25g; Sigma-Aldrich; Merck KGaA) was added and
slides were incubated in a 37˚C incubator for 30 min. Heart tissues
were incubated with primary antibodies (NF-κB and TNF-α) (as
aforementioned; dilution, 1:500) at 4˚C overnight, and then
incubated with corresponding secondary antibody
[SignalStain® Boost IHC Detection Reagent (HRP, Rabbit);
cat. no. 8114; Cell Signaling Technology, Inc.] at room temperature
for 1 h. DAB was added immediately after the liquid around the
tissue dried, slides were incubated at room temperature for 5 min
and the reaction was terminated with water washing. The slices were
dehydrated and made transparent after counter-staining with
hematoxylin for 5 min at room temperature. The results were
observed using an inverted microscope (Nikon Corporation).
Statistical analysis
Statistical analysis was performed with GraphPad
Prism 5 software (GraphPad Software, Inc.). Data are presented as
the mean ± SD. Comparison between two groups was analyzed using
Student's t-test, and one-way ANOVA followed by Tukey's post hoc
test was used for comparison between ≥3 groups. All experiments
were repeated ≥3 times. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-24 inhibits cardiomyocyte
apoptosis
After the cells were transfected with miR-24 mimic,
transfection efficiency analysis by RT-qPCR indicated that the
expression of miR-24 was significantly higher in the transfection
group compared with the NC group (Fig.
1A). The apoptotic rate of H9C2 cells in each group was
detected via flow cytometry (Fig.
1B). The results demonstrated that cardiomyocyte apoptosis was
significantly induced following IRI in the model group compared
with the miRNA group (Fig. 1B).
Compared with the model group, the apoptotic rate of H9C2 cells in
the miRNA group was significantly inhibited (Fig. 1B).
miR-24 decreases H9C2 cell apoptosis
via NF-κB/TNF-α signaling in vitro
To determine whether miR-24-induced apoptosis in
H9C2 cells was associated with NF-κB/TNF-α signaling, the
expression of proteins associated with the NF-κB/TNF-α pathway were
detected using western blotting (Fig.
2A and B). The results
identified that compared with the NC group, the protein expression
levels of NF-κB, TNF-α, caspase-3, Bax, Bcl-2, VCAM-1, ICAM-1 and
MCP-1 were significantly increased in the model group (Fig. 2A and B). However, in the miRNA group
significantly inhibited protein expression levels of NF-κB, TNF-α,
caspase-3, Bax, Bcl-2, VCAM-1, ICAM-1 and MCP-1 were recorded
compared with the model group (Fig.
2A and B).
miR-24 pretreatment improves
myocardial function in IRI
To evaluate the role of miR-24 in rat heart
function, an IRI animal model was established and an
electrocardiogram was performed before and after animal experiments
(Fig. 3A). MI in rats was detected
using Evans blue staining (Fig.
3B). The area of MI in the model group was significantly larger
compared with the sham group, but the area of MI in the miRNA group
was significantly reduced compared with the model group (Fig. 3B). The pathological results of
H&E staining indicated that the myocardial fibers in the sham
group were neatly arranged and were regular in structure (Fig. 3C). However, myocardial cell
disorder, inflammatory cell infiltration and myocardial fibrosis
were observed in the model group (Fig.
3C). The pathological changes in cardiomyocytes in the miRNA
group were markedly improved compared with the model group
(Fig. 3C).
Myocardial tissue apoptosis was analyzed using the
TUNEL method (Fig. 3D). The number
of TUNEL-positive cells in the model group was significantly higher
compared with the sham group (Fig.
3D). Compared with the model group, following miR-24
overexpression, the apoptosis rate in myocardial tissue was
significantly decreased (Fig.
3D).
miR-24 protects MIRI via the
NF-κB/TNF-α pathway
In order to examine the mechanism underlying miR-24
influencing myocardial function in rats, the expression levels of
related proteins were measured in vivo using
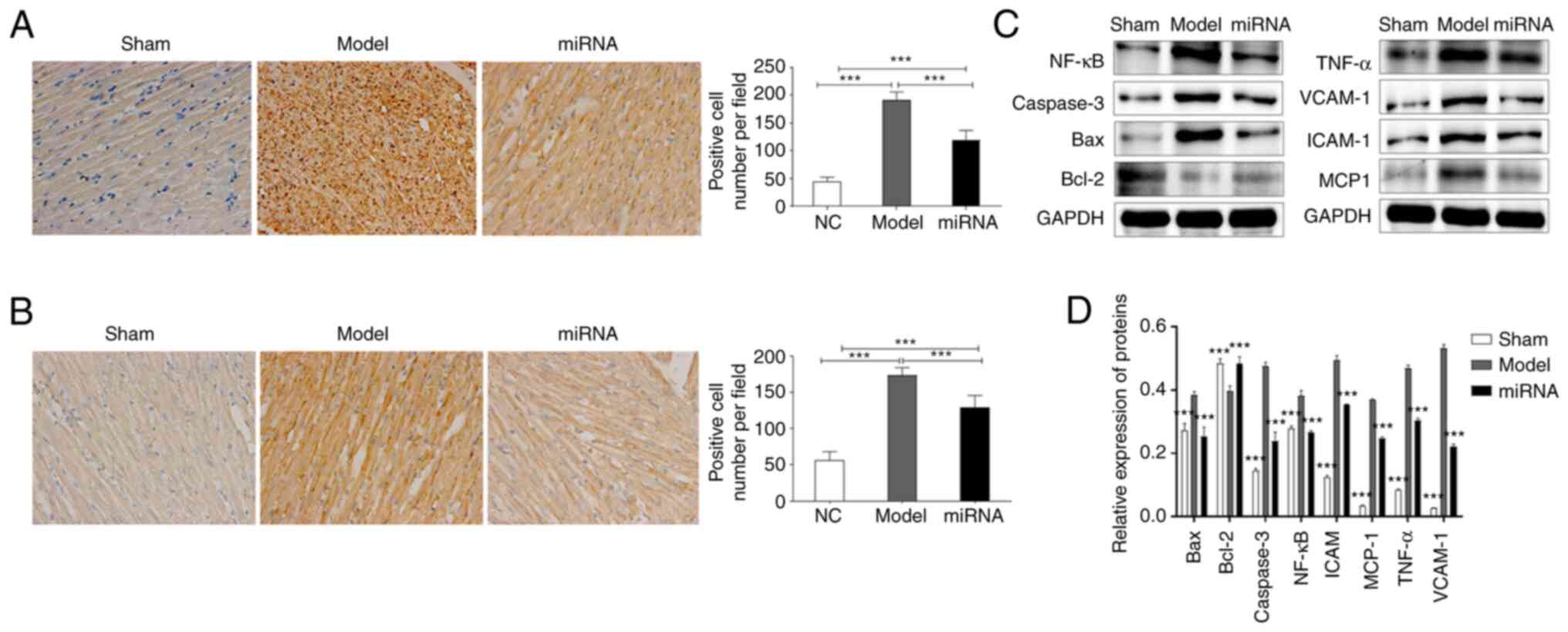
immunohistochemistry and western blotting. The staining showed that
myocardial cells in the model group were disordered and pink
protein mucous exudated, while myocardial lesions in the miRNA
group were significantly alleviated. Immunohistochemistry results
suggested that the proportion of NF-κB (Fig. 4A) and TNF-α (Fig. 4B) positive cells in the miRNA group
was significantly lower compared with that in the model group
(Fig. 4A and B). Subsequently, proteins were extracted
from rat cardiac tissue in each group. The results were consistent
with those of the in vitro studies, as the western blotting
results suggested that, compared with the sham group, the protein
expression levels of NF-κB, TNF-α, caspase-3, Bax, VCAM-1, ICAM-1
and MCP-1 were significantly upregulated in the model group
(Fig. 4C and D). By contrast, Bcl-2 expression was
downregulated in the model group compared with the sham group, but
upregulated in the miRNA group compared to the model group.
However, the miRNA group had significantly downregulated NF-κB,
TNF-α, caspase-3, Bax, VCAM-1, ICAM-1 and MCP-1 protein expression
levels compared with the model group (Fig. 4C and D).
Discussion
IRI is caused by decreased or blocked blood flow
after coronary artery reperfusion. IRI can lead to further damage
of ischemic tissues, cell death and even irreversible pathological
changes, such as organ dysfunction (16,17).
Moreover, IRI leads to a high mortality rate in patients with MI,
which is a serious clinical complication and affects the prognosis
of these patients. Oxidative stress, cardiomyocyte apoptosis and
cardiac dysfunction are all associated with IRI (18,19).
It has been previously reported that intervention in cardiomyocyte
apoptosis can restore IRI to a certain extent (19). The present research also suggested
that miR-24 can decrease cardiomyocyte apoptosis caused by IRI.
miRNAs are a type of non-coding RNA that regulate
post-transcriptional gene expression. In most multicellular
organisms, miRNAs perform a variety of functions, other than
protein translation, and are involved in physical processes and
disease development. Previous studies have revealed that miRNAs can
regulate inflammation, autophagy, apoptosis and oxidative stress,
and also serve an important role in the repair of myocardial damage
after IRI (20). For instance,
miR-703 protected against mouse cardiomyocyte injury by inhibiting
NOD-, LRR- and pyrin domain-containing protein 3/caspase-1 mediated
pyroptosis (21). It was also
reported that miR-346 reduced the infarct size and inhibited
cardiomyocyte apoptosis by targeting Bax after myocardial IRI in
rats (22). miR-760 protected
against myocardial IRI by inhibiting sodium hydrosulfide (23), while miR-141-3p interacts with
chromodomain-helicase-DNA-binding protein 8 and reduces the
expression of p21, which serves an important role in
hypoxia/reoxygenation-induced cardiomyocyte apoptosis (24). In the present study, miR-24
decreased the infarct size and cardiomyocyte apoptosis, as well as
improved myocardial function in rats following myocardial IRI.
NF-κB is an important inflammatory transcription
factor widely found in eukaryotic cells and is involved in the
transcriptional regulation of numerous apoptosis-related genes.
Previous studies have reported that NF-κB is essential in
cardiovascular diseases and is activated in the early pathogenesis
of ischemia (25). TNF-α, as an
inflammatory cytokine in the NF-κB pathway, is also involved in the
regulation of IRI (26). When
vascular endothelial cells undergo inflammatory activation, IL-8
and MCP-1 are induced to attract leukocytes, VCAM-1 and ICAM-1,
along with other cell adhesion molecules, in order to promote the
adhesion of inflammatory cells and lead to the occurrence of
vascular diseases, such as atherosclerosis (27). The ratio of Bax/Bcl-2 protein acts
as the molecular switch of apoptosis, and caspase-3 protein is the
executor of apoptosis. Consistently, the present study found that
the expression levels of NF-κB/TNF-α pathway-related proteins in
the miRNA group (NF-κB, caspase-3, Bax, Bcl-2, TNF-α, VCAM-1,
ICAM-1 and MCP-1) were significantly different from those in the
model group.
In conclusion, the present in vitro and in
vivo studies demonstrated that miR-24 improved myocardial
injury in rats by inhibiting the NF-κB/TNF-α pathway. However, due
to the intricate signaling pathways involved in myocardial IRI, the
role of miR-24 in additional signaling pathways associated with
myocardial IRI requires to be further elucidated.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Key Discipline Groups of
Shanghai Pudong New Area (grant no. PWZxq2017-01), the Medical and
Health Program of Pudong New Area Science and Technology
Development Fund (grant no. PKJ2017-Y38) and Shanghai Municipal
Health Commission of China (grant no. 202040159).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL conceived and designed the experiments. CL and MF
performed the experiments and wrote the manuscript. CL, MF, ZL and
WW analyzed the data. XL made substantial contributions to
proofreading the manuscript and gave final approval of the version
to be published. XL and CL confirmed the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments involved in this study were
approved by the Ethics Committee of Zhoupu Hospital affiliated to
Shanghai Medical University (2017-C-040-E01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xing J, Xie T, Tan W, Li R, Yu C and Han
X: microRNA-183 improve myocardial damager via NF-κB pathway: In
vitro and in vivo study. J Cell Biochem. 120:10145–10154.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kapur NK, Thayer KL and Zweck E:
Cardiogenic shock in the setting of acute myocardial infarction.
Methodist DeBakey Cardiovasc J. 16:16–21. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang J, Lu L, Chen S, Xie J, Lu S, Zhou Y
and Jiang H: PERK Overexpression-mediated Nrf2/HO-1 pathway
alleviates hypoxia/reoxygenation-induced injury in neonatal murine
cardiomyocytes via improving endoplasmic reticulum stress. Biomed
Res Int. 2020(6458060)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huang XW, Pan MD, Du PH and Wang LX:
Arginase-2 protects myocardial ischemia-reperfusion injury via
NF-κB/TNF-α pathway. Eur Rev Med Pharmacol Sci. 22:6529–6537.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Halushka PV, Goodwin AJ and Halushka MK:
Opportunities for microRNAs in the crowded field of cardiovascular
biomarkers. Annu Rev Pathol. 14:211–238. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang CS, Shao K, Liu CW, Li CJ and Yu BT:
Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte
apoptosis after acute myocardial infarction by upregulating
microRNA-24. Eur Rev Med Pharmacol Sci. 23:6691–6699.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang J, Zeng P, Yang J, Liu X, Ding J,
Wang H and Chen L: MicroRNA-24 regulates vascular remodeling via
inhibiting PDGF-BB pathway in diabetic rat model. Gene. 659:67–76.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen Z, Lu S, Xu M, Liu P, Ren R and Ma W:
Role of miR-24, furin, and transforming growth factor-β1 signal
pathway in fibrosis after cardiac infarction. Med Sci Monit.
23:65–70. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang J, Huang W, Xu R, Nie Y, Cao X, Meng
J, Xu X, Hu S and Zheng Z: MicroRNA-24 regulates cardiac fibrosis
after myocardial infarction. J Cell Mol Med. 16:2150–2160.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guo C, Deng Y, Liu J and Qian L:
Cardiomyocyte-specific role of miR-24 in promoting cell survival. J
Cell Mol Med. 19:103–112. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Meloni M, Marchetti M, Garner K,
Littlejohns B, Sala-Newby G, Xenophontos N, Floris I, Suleiman MS,
Madeddu P, Caporali A, et al: Local inhibition of microRNA-24
improves reparative angiogenesis and left ventricle remodeling and
function in mice with myocardial infarction. Mol Ther.
21:1390–1402. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Long L, Han X, Ma X, Li K, Liu L, Dong J,
Qin B, Zhang K, Yang K and Yan H: Protective effects of fisetin
against myocardial ischemia/reperfusion injury. Exp Ther Med.
19:3177–3188. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Saddala MS, Lennikov A, Mukwaya A, Yang Y,
Hill MA, Lagali N and Huang H: Discovery of novel L-type
voltage-gated calcium channel blockers and application for the
prevention of inflammation and angiogenesis. J Neuroinflammation.
17(132)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
D'Ignazio L, Shakir D, Batie M, Muller HA
and Rocha S: HIF-1β positively regulates NF-κB activity via direct
control of TRAF6. Int J Mol Sci. 21(3000)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sharma R, Kambhampati SP, Zhang Z, Sharma
A, Chen S, Duh EI, Kannan S, Tso MO and Kannan RM: Dendrimer
mediated targeted delivery of sinomenine for the treatment of acute
neuroinflammation in traumatic brain injury. J Control Release.
323:361–375. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Krzywonos-Zawadzka A, Franczak A, Sawicki
G and Bil-Lula I: Mixture of MMP-2, MLC, and NOS inhibitors affects
NO metabolism and protects heart from cardiac I/R injury. Cardiol
Res Pract. 2020(1561478)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Deng LC, Alinejad T, Bellusci S and Zhang
JS: Fibroblast growth factors in the management of acute kidney
injury following ischemia-reperfusion. Front Pharmacol.
11(426)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhao Z, Tang Z, Zhang W, Liu J, Li B and
Ding S: Inactivated pseudomonas aeruginosa protects against
myocardial ischemia reperfusion injury via Nrf2 and HO-1. Exp Ther
Med. 19:3362–3368. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lin F, Xu L, Huang M, Deng B, Zhang W,
Zeng Z and Yinzhi S: β-Sitosterol protects against myocardial
ischemia/reperfusion injury via targeting PPARγ/NF-κB signalling.
Evid Based Complement Alternat Med: Mar 28, 2020 (Epub ahead of
print). doi: 10.1155/2020/2679409.
|
|
20
|
Zhang ZH, Wang YR, Li F, Liu XL, Zhang H,
Zhu ZZ, Huang H and Xu XH: Circ-camk4 involved in cerebral
ischemia/reperfusion induced neuronal injury. Sci Rep.
10(7012)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wei X, Peng H, Deng M, Feng Z, Peng C and
Yang D: MiR-703 protects against hypoxia/reoxygenation-induced
cardiomyocyte injury via inhibiting the NLRP3/caspase-1-mediated
pyroptosis. J Bioenerg Biomembr. 52:155–164. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lv X, Lu P, Hu Y and Xu T: miR-346
inhibited apoptosis against myocardial ischemia-reperfusion injury
via targeting Bax in rats. Drug Des Devel Ther. 14:895–905.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ren L, Wang Q, Ma L and Wang D:
MicroRNA-760-mediated low expression of DUSP1 impedes the
protective effect of NaHS on myocardial ischemia-reperfusion
injury. Biochem Cell Biol. 98:378–385. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yao B, Wan X, Zheng X, Zhong T, Hu J, Zhou
Y, Qin A, Ma Y and Yin D: Critical roles of microRNA-141-3p and
CHD8 in hypoxia/reoxygenation-induced cardiomyocyte apoptosis. Cell
Biosci. 10(20)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li X, Yang J, Yang J, Dong W, Li S, Wu H
and Li L: RP105 protects against myocardial ischemia-reperfusion
injury via suppressing TLR4 signaling pathways in rat model. Exp
Mol Pathol. 100:281–286. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang S, Zhao Y, Song J, Wang R, Gao L,
Zhang L, Fang L, Lu Y and Du G: Total flavonoids from Anchusa
italica Retz. Improve cardiac function and attenuate cardiac
remodeling post myocardial infarction in mice. J Ethnopharmacol.
257(112887)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Iwashima T, Kudome Y, Kishimoto Y, Saita
E, Tanaka M, Taguchi C, Hirakawa S, Mitani N, Kondo K and Iida K:
Aronia berry extract inhibits TNF-α-induced vascular endothelial
inflammation through the regulation of STAT3. Food Nutr Res: Aug
16, 2019 (Epub ahead of print). doi: 10.29219/fnr.v63.3361.
|