Introduction
Excessive fat accumulation in obesity is a serious
aesthetic problem (1). The regional
distribution of local fat is a well-known risk factor of obesity
rather than body mass index (BMI) (2). Abdominal adiposity, with subcutaneous
or visceral fat deposition, is implicated in several medical
conditions such as metabolic syndrome, cardiovascular disease and
lower quality of life (3).
Aesthetic treatment for localized adiposity is performed mainly for
abdominal subcutaneous adipose tissue (4). Liposuction, suction-assisted
lipectomy, is the most commonly used technique of plastic surgery
procedures in North America (5).
Although fat tissue can be extracted by liposuction effectively,
complications may cause contour deformities, embolism and even
death (5). Injections of
phosphatidylcholine (PC) and sodium deoxycholate (DC) have been
widely used as a minimally invasive treatment for localized
adiposity (6). However, PC and DC
injection may have substantial side effects including fibrosis and
necrosis of the tissue (7).
Pharmacopuncture, a new acupuncture technique with the injection of
a herbs extract at the acupuncture point, could be a non-surgical
alternative in the treatment of localized adiposity (8).
LIPOSA, consisting of the tuber of Pinellia
ternata (Thunb.) Breitenb., the whole plant of Taraxacum
platycarpum Dahlst. and the root of Astragalus
membranaceus Bunge, is a newly developed formula of
pharmacopuncture treatment for localized adiposity. P.
ternata, T. platycarpum and A. membranaceus have
been used for treating metabolic disorders including obesity as
traditional Korean medicines (9-11).
In the theories of traditional Korean medicine, obesity can be
caused by ‘phlegm dampness’, ‘deficiency of Qi’ and ‘pathologic
dampness-heat’ (12). P.
ternata and T. platycarpum are known to be
effective herbs for dispelling ‘phlegm dampness’ and
‘dampness-heat’, respectively (12). A. membranaceus is one
of the most frequently prescribed ‘Qi tonifying’ herbs in
traditional Korean medicine (12).
P. ternata, T.
platycarpum and A. membranaceus can be used
for the treatment of obesity, however, the effects of
pharmacopuncture with these herbs on localized adiposity have not
been studied yet. In this study, we investigated the efficacy of
LIPOSA pharmacopuncture on localized adiposity by analyzing the fat
pad weight and histological changes of the fat tissues in obese
mice. To confirm the underlying mechanism of then LIPOSA
pharmacopuncture on the inhibition of local fat, lipolytic enzymes
including adipose triglyceride lipase (ATGL) and hormone-sensitive
lipase (HSL) and lipophagic molecules including LC3-Ⅱ,
autophagy-related gene (ATG) 5 and ATG7 were investigated in high
fat diet (HFD)-induced obese mice. Moreover, lipogenesis-related
factors such as acetyl-CoA carboxylase (ACC) and
phosphoenolpyruvate carboxykinase (PEPCK) and peroxisome
proliferator-activated receptors (PPAR)-γ as an adipogenetic
biomarker were evaluated in the inguinal fat tissues of the obese
mice.
Materials and methods
Preparation of LIPOSA
pharmacopuncture
LIPOSA pharmacopuncture compose A.
membranaceus, T. platycarpum and P. ternata.
A. membranaceus and T. platycarpum were extracted
with 20-folds of distilled water at 100˚C for 2 h by refluxing.
P. ternata was extracted with 15-folds of distilled water at
RT for 2 h. Each extract was filtered by 3 µm paper filers,
respectively, and then mixed. Mixed extracts were evaporated and
freeze-dried. The yields of mixed extracts were 8.65%. Dried
extracts were diluted in normal saline and compensated the pH range
from 6.8 to 7.2 by 1N NaOH solution.
Animal treatment
Male C57BL/6J mice (5 weeks old) were purchased from
Raonbio Inc. Mice were housed under temperature- and
humidity-controlled facility. After 1 week of housing, all mice
were fed high-fat diet (HFD) containing 60% fat for 8 weeks to
induce obesity. To monitor and compare any adverse effects of
LIPOSA pharmacopuncture with normal, 8 normal mice were fed
standard diet. Body weight was measured once a week until the end
of the animal experiments. Following 8 weeks of HFD, mice (n=8)
were divided into 3 groups in accordance with the weight of the
mice, which was LIPOSA 13.35, 26.7, 53.4 mg/ml. We conducted
previous experiments to determine the effective doses of LIPOSA.
Effective dose ranges of Pinellia ternate, Taraxacum
platycarpum and Astragalus membranaceus were 5-10, 2-4
and 15-20 mg/ml, respectively. Therefore, a tentative dose (26.7
mg/ml) with half dose (13.35 mg/ml) and double dose (53.4 mg/ml) of
LIPOSA were tested to establish the actual therapeutic dose of
LIPOSA in this study. Obese mice were used as self-control, vehicle
(normal saline) was injected in the right inguinal fat pad and
LIPOSA were injected in the left inguinal fat pad. The samples were
injected 100 µl each, 3 times a week for 2 weeks. Body weight and
food intake were monitored every week. No significance was observed
in body weight of LIPOSA-treated groups, suggesting that LIPOSA had
inhibitory effects against localized fat accumulation rather than
body weight reduction (Fig. S1).
During the treatment of LIPOSA, no significant differences of daily
food intake were shown in LIPOSA-treated mice (Table SI). In addition, there was no sign
of toxicity in all LIPOSA-treated mice. All animal procedures were
approved by Committee on Care and Use of Laboratory Animals of the
Kyung Hee University (KHUASP(SE)-18-070; Seoul, Korea).
Measurement of inguinal fat
weight
At the end of the 10 weeks, all animals under
anesthesia were scanned from total-body scanner (InAlyzer dual
X-ray absorptiometry; Medikors). Dual energy X-ray absorptiometry
(DXA) measures one time with low energy and one time with high
energy to separate the images into tissues in gram units by
separating them into fat and lean before analysis. Fat distribution
mice was visualized by body composition view by a mapping image
processed by a software in the device. Red, blue and white color
indicates the fat tissue, lean tissue and bone tissue,
respectively. After then, all mice were sacrificed under anesthesia
with 1% avertin (cat. no. T4,840-2; Sigma-Aldrich; Merck KGaA).
Blood samples were collected by orbital puncture. Mice are
euthanized by cervical dislocation. Inguinal fat pad was collected
from the thigh and weight was measured. The regions to determine
the weight of inguinal fat pad were from knee to tail based on line
of ventral spine. The inguinal fat pad weight by LIPOSA treatment
was calculated by relative intensity that the saline-treated fat
weight was converted to 1.
Histology
After 10 weeks of feeding experimental diets,
inguinal fat pad was collected from the C57BL/6 mice. Inguinal fat
tissues were fixed in 10% neutralized formalin. The dehydrated fat
tissue was then embedded in paraffin wax. Histological sections of
5 µm thickness were stained with hematoxylin and eosin (H&E).
Adipocyte size was evaluated in 6 mice from each group and 6 random
fields (magnification, x400) per mice. To measure cross-sectional
adipocyte area, micrographs were taken using a light microscope
(Nikon) and analyzed by using the ImageJ software.
Western blot analysis
Proteins were extracted by homogenizing inguinal fat
tissues in the tissue protein extraction reagent (T-PER) including
protease inhibitor cocktail. The homogenate was extracted in ice
for 2 h and centrifuged at 17,000 rpm for 30 min. Supernatant was
collected and quantified using Bradford assay. 20 µg of protein
samples were electrophoresed in 10% SDS-PAGE gels for 2 h at 100 V.
Proteins were transferred onto methanol-activated polyvinylidene
difluoride (PVDF) membrane using trans-blot turbo transfer system
(Bio-Rad Laboratories, Inc.). The membranes were blocked with 3%
BSA and washed with TBS-T buffer for 3 times. The membranes were
blotted with primary antibodies (1:1,000 dilution) for overnight at
4˚C and HRP conjugated secondary antibodies (1:3,000 dilution) were
incubated for 1 h at RT. The target protein bands were detected by
chemi-imaging system, Davinch-Chemi™.
Measurement of serum toxicity
Blood samples were separated by centrifuging the
blood at 17,000 rpm for 20 min to determine the serum toxicity by
enzyme-linked immunsorbent assay (ELISA). Serum biochemical
indicators including blood urea nitrogen (BUN), creatinine,
aspartate transaminase (AST) and alanine transaminase (ALT) were
analyzed by Mouse Blood Urea Nitrogen ELISA kit (cat. no.
MBS2611085; Mybiosource), Mouse Serum Creatinine ELISA kit (cat.
no. MBS3807501; Mybiosource). Mouse Aspartate Aminotransferase
ELISA kit (cat. no. MBS450720; Mybiosource) and Mouse Alanine
Aminotransferase ELISA kit (cat. no. MBS264717; Mybiosource),
respectively. Based on standard curve, serum BUN, creatinine, AST
and ALT was calculated.
Statistical analysis
Significance between vehicle and LIPOSA was
determined by paired Student's t-test. Serum BUN, creatinine, AST
and ALT measurements and body weight differences were analyzed
using one-way ANOVA and Tukey's multiple comparisons test. In all
analyses, P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of LIPOSA pharmacopuncture on
the inguinal fat weight in obese mice
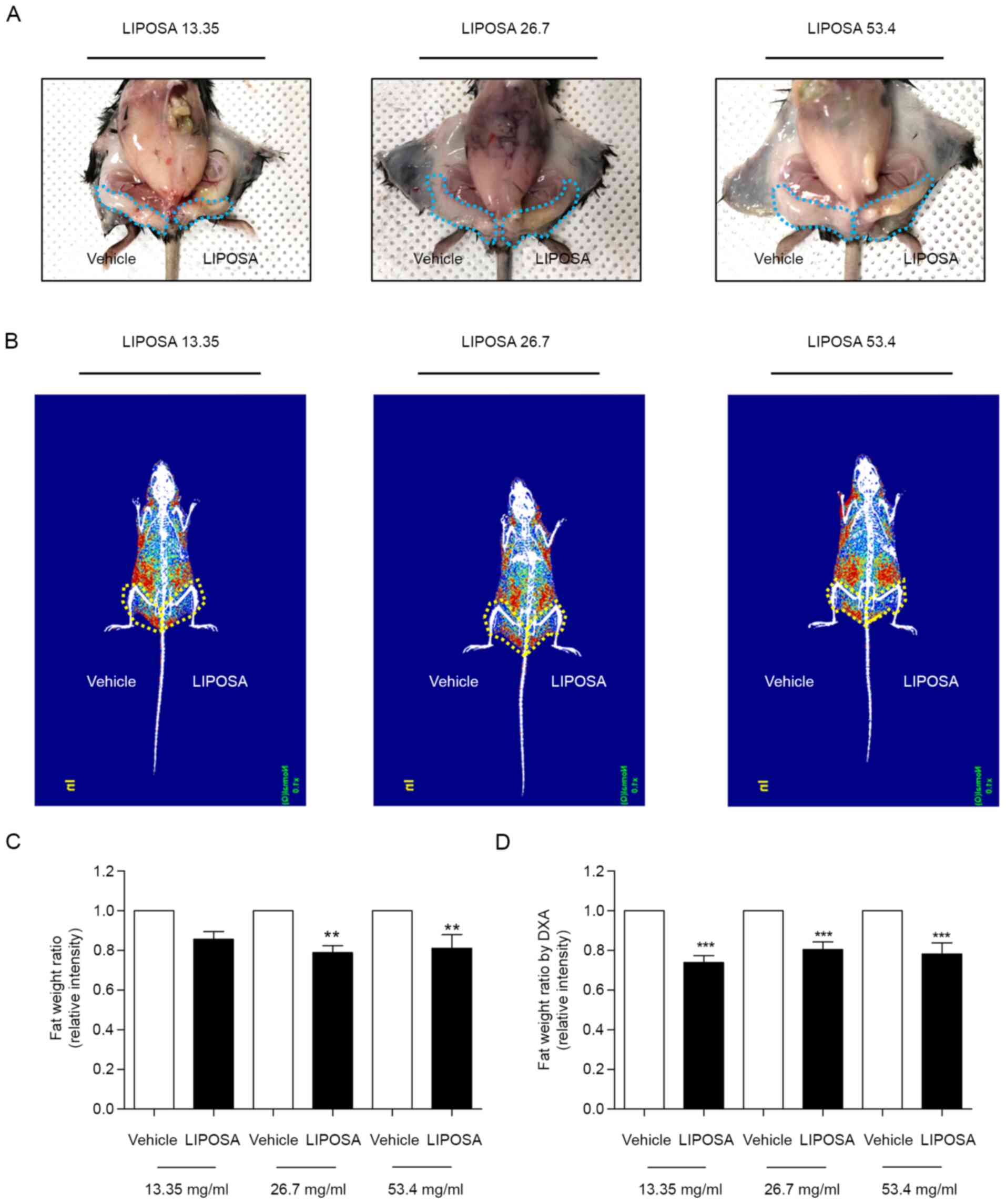
The left inguinal fat administered with LIPOSA was
significantly reduced compared to the vehicle side (Fig. 1A). Radiography of fat displayed by
red color showed that LIPOSA treatment remarkedly decreased the fat
deposition in inguinal fat pad (Fig.
1B). LIPOSA-treated groups exhibited decreases of inguinal fat
weight ratio. LIPOSA treatments with 13.35, 26.7 and 53.4 mg/ml
concentrations reduced the inguinal fat pad weight by 14.5, 21.2
and 19.0% in HFD-induced obese mice, respectively (Fig. 1C). Based on the DXA scan to measure
the exact weight of inguinal fat, the ratio of fat weight
administered with LIPOSA were significantly lower than that with
vehicle. Subcutaneous injection with 13.35, 26.7 and 53.4 mg/ml of
LIPOSA significantly attenuated the inguinal fat pads weight by
26.2, 19.6, and 21.8%, respectively (Fig. 1D).
Effects of LIPOSA pharmacopuncture on
histological changes of inguinal fat tissues in obese mice
As shown in H&E staining, fat diameter of in
inguinal fat pad was markedly reduced by LIPOSA treatment compared
to the vehicle side-fat pad (Fig.
2A). LIPOSA pharmacopuncture injection dose-dependently
decreased the inguinal fat adipocyte size about 48.5, 50.9 and
61.4% (Fig. 2B and C).
Effects of LIPOSA pharmacopuncture on
expressions of lipophagy-related factors in inguinal fat tissues in
obese mice
The protein levels of LC3-Ⅱ were significantly
increased by about 2.9-, 2.8- and 4.1-fold in the LIPOSA 13.35,
26.7 and 53.4 mg/ml-treated fat tissues compared to self-control,
right side. Compared with saline-injected right side, the
expressions of ATG5 were markedly upregulated to 3.1-, 3.0- and
4.6-folds in all three doses of LIPOSA group. Also, the ATG7
expressions were significantly increased by about 7.5-, 8.9- and
10.3-folds in the 13.35, 26.7 and 53.4 mg/ml of LIPOSA-treated fat
tissues (Fig. 3).
Effects of LIPOSA pharmacopuncture on
the expressions of lipolytic enzymes in inguinal fat tissues in
obese mice
The levels of phosphorylated ATGL were increased
about 1.5-, 1.8- and 1.5-folds in the inguinal fat tissues with
LIPOSA 13.35 mg/ml, LIPOSA 26.7 mg/ml and LIPOSA 53.4 mg/ml
compared to vehicle side-fat tissues. In similar, compared with
right side, the levels of phosphorylated HSL were elevated about
2.1-, 2.6- and 2.6-folds in the LIPOSA 13.35 mg/ml, LIPOSA 26.7
mg/ml and LIPOSA 53.4 mg/ml-treated inguinal fat tissues (Fig. 4).
Effects of LIPOSA pharmacopuncture on
the expressions of ACC, PEPCK and PPAR-γ in inguinal fat tissues in
obese mice
In Fig. 5, the
expression of phosphorylated ACC was significantly downregulated
69.5, 70.0 and 66.1%, respectively, in 13.35, 26.7 and 53.4 mg/ml
of LIPOSA-treated left inguinal fat pads. In addition, the levels
of PEPCK were remarkedly attenuated by 27.4, 21.3 and 54.8%,
respectively, in the LIPOSA 13.35, LIPOSA 26.7 and LIPOSA 53.4
groups. PPAR- γ expressions were lowly expressed by 38.7, 53.3 and
61.6% compared to right inguinal fat pads in dose-dependent
manner.
Effects of LIPOSA pharmacopuncture on
serum toxicity in obese mice
We evaluated levels of injury marker of liver and
kidney to assess potential toxic effect of LIPOSA (Table I). The levels of BUN were
25.80±2.17, 30.60±4.67 and 29.00±2.92 by LIPOSA injection.
Creatinine levels in serum were 0.27±0.05, 0.25±0.04 and 0.29±0.03
by injection of LIPOSA 13.35, 26.7 and 53.4 mg/ml, respectively.
The serum levels of AST were 187.50±40.83, 230.75±20.56,
173.25±44.30 and ALT were 33.00±7.66, 33.00±4.97, 29.00±5.23 in
each LIPOSA treated groups. There was no significant difference of
serum BUN, creatinine, AST and ALT levels in all three
LIPOSA-treated groups. We conducted the additional evaluation of
serum BUN, creatinine, AST (GOT) and ALT (GPT) in normal mice.
Also, the levels of blood urea nitrogen (BUN), creatinine, AST and
ALT were not changed by LIPOSA injection within normal range. We
suggested that no toxicities in the serum levels of BUN,
creatinine, AST and ALT by LIPOSA 13.35, 26.7 and 53.4 mg/ml
injection.
 | Table IChanges in concentrations of serum
BUN, creatinine, AST and ALT of mice after 2 weeks of LIPOSA
pharmacopuncture injection. |
Table I
Changes in concentrations of serum
BUN, creatinine, AST and ALT of mice after 2 weeks of LIPOSA
pharmacopuncture injection.
| Group | BUN | Creatinine | AST (GOT) | ALT (GPT) |
|---|
| Normal mice | 36.67±0.58 | 0.26±0.04 | 143±35.16 | 41.33±10.26 |
| 13.35 mg/ml
LIPOSA | 25.80±2.17 | 0.27±0.05 | 187.50±40.83 | 33.00±7.66 |
| 26.7 mg/ml
LIPOSA | 30.60±4.67 | 0.25±0.04 | 230.75±20.56 | 33.00±4.97 |
| 53.4 mg/ml
LIPOSA | 29.00±2.92 | 0.29±0.03 | 173.25±44.30 | 29.00±5.23 |
Discussion
Lipolytic stimulation of fat cells by lipolytic
injection or liposuction has been known to reduce localized body
fat, that is intended to slim down specific body parts (13). Adipose cells, known as adipocytes,
are the cells of adipose tissues that synthesize, store and release
fat into the blood (14). In the
condition of obesity by excessive fat intake, the size of
adipocytes is about 10 times bigger than the original size
(15). Enlargement of adipocytes in
specific regions forms ‘love handles’ known as fat deposits
(16). In this study, the potential
of LIPOSA pharmacopuncture as a localized lipolytic material was
investigated by comparing sample-injected left side and
saline-injected right side for self-control. Subcutaneous injection
with LIPOSA pharmacopuncture decreased the accumulation of inguinal
fat tissues. As shown in the x-ray images, the red-indicated fat
deposition was remarkedly reduced in the LIPOSA-treated site
compared to the saline-treated site. In addition, the diameter of
the adipocytes in the inguinal fat tissues was significantly
decreased by the LIPOSA injection at all concentrations. Those
results suggested that LIPOSA pharmacopuncture has a role as a
lipolytic material in localized fat depositions.
‘Lipolysis signaling’ is defined as the hydrolysis
of triacylglycerols stored in lipid droplets in the fat. There are
several potential mechanisms related to lipolytic stimulation,
which induces fat cell destruction (17). The neutral lipids in a lipid droplet
breakdown into free fatty acids by lysosomal degradation autophagy,
called lipophagy (18). The events
of the lipophagic process are coordinated by components of the
autophagic machinery, ATGs, with the generation of LC3-II. ATG7 has
been reported to regulate ATG5, leading to the conjugation of LC3
to a lipid. LC3-II activation in the lipid droplet forms the
autophagosomes following the degradation of lipid stores (19). LC3 is reported to regulate
ATGL-mediated lipid mobilization, although the contribution of
lipases and lipophagy to lipolytic process has not been clearly
known (20). Lipolysis by neutral
lipases such as ATGL and HSL releases fatty acids and glycerol on
triglycerides (21). The
triglycerides can be disrupted by lipolytic drugs without an issue
of body energy deposition (22). In
this study, subcutaneous injection with LIPOSA markedly increased
the expressions of lipophagic factors including ATG7, ATG5 and
LC3-II, and lipases including ATGL and HSL. Taken together, LIPOSA
pharmacopuncture regulated the lipolytic process, especially
lipophagy and lipase activation. Based on the results from the fat
weight and adipocyte diameter, we expected that LIPOSA
pharmacopuncture degraded fat cells containing triglycerides and
reduced the enlargement of lipid droplets through lipolysis and
lipophagy, which are therapeutic strategies to inhibit localized
body fat (Fig. 6).
Apart from lipolysis, molecules were investigated
that were involved in lipid accumulation. In addition to the
stimulation of lipolysis, strategies to regulate the lipid
metabolism by inhibiting the differentiation of adipocytes
(adipogenesis), release of glucose (gluconeogenesis) in adipose
tissue or synthesis of fatty acids (lipogenesis) can be targets for
obesity and obesity-related diseases (23). The phosphorylation of ACC, a
lipogenesis enzyme, has been known to induce the synthesis of fatty
acids (24). PPAR-γ is highly
expressed in white adipose tissues and associated with lipid
metabolism (25). Activation of
PPAR-γ mediates the differentiation of preadipocytes into mature
adipocytes (25). In terms of
PEPCK, it acts as a regulatory enzyme of gluconeogenesis in adipose
tissues (26). An increase in PEPCK
activity leads to adipocyte hypertrophy by free fatty acid
re-esterification, resulting in fat accumulation (26). LIPOSA pharmacopuncture was found to
decrease the expressions of ACC, PPAR-γ and PEPCK. Along with the
lipolytic effects, LIPOSA pharmacopuncture might inhibit fat
accumulation by regulating lipid metabolism.
Taken together, LIPOSA pharmacopunture breaks down
localized areas of fat by its lipolytic property. Triglyceride
accumulation in the inguinal fat pad was inhibited by LIPOSA
subcutaneous injection with its promotive effects on lipophagy and
lipase activation. In addition, lipid metabolism related to fat
accumulation including adipogenesis, gluconeogenesis and
lipogenesis was regulated by the LIPOSA treatment. LIPOSA
pharmacopuncture might reduce localized body fat as a lipolytic
injection.
Supplementary Material
Effects of LIPOSA on body weight. Body
weight flow was measured every week after treatments with LIPOSA.
Statistically significant difference was not observed between
normal mice and the LIPOSA-treated group after treatments with
LIPOSA. Quantitative data are shown as the mean ± standard error of
the mean.
Effects of LIPOSA pharmacopuncture on
food intake.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a National Research
Foundation of Korea Grant funded by the Korean Government (grant
no. NRF-2019R1I1A2A01063598).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL, MHK and WMY designed the study and drafted the
manuscript. SCJ, LYC and YKN performed the experiments and analyzed
the data. MHK and WMY assessed the authenticity of all the raw
data. YWM revised and edited the manuscript, supervised the project
and obtained the research grants for the current study. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the Committee
on Care and Use of Laboratory Animals of the Kyung Hee University
[approval no. KHUASP(SE)-18-070; Seoul, South Korea].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sarwer DB, Wadden TA and Foster GD:
Assessment of body image dissatisfaction in obese women:
Specificity, severity, and clinical significance. J Consult Clin
Psychol. 66:651–654. 1998.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Moya AP and Sharma D: A modified technique
combining vertical and high lateral incisions for abdominal-to-hip
contouring following massive weight loss in persistently obese
patients. J Plast Reconstr Aesthet Surg. 62:56–64. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Black DW, Shaw M, McCormick B and Allen J:
Pathological gambling: Relationship to obesity, self-reported
chronic medical conditions, poor lifestyle choices, and impaired
quality of life. Compr Psychiatry. 54:97–104. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Friedmann DP, Avram MM, Cohen SR, Duncan
DI, Goldman MP, Weiss ET and Young VL: An evaluation of the patient
population for aesthetic treatments targeting abdominal
subcutaneous adipose tissue. J Cosmet Dermatol. 13:119–124.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Berry MG and Davies D: Liposuction: A
review of principles and techniques. J Plast Reconstr Aesthet Surg.
64:985–992. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Reeds DN, Mohammed BS, Klein S, Boswell CB
and Young VL: Metabolic and structural effects of
phosphatidylcholine and deoxycholate injections on subcutaneous
fat: A randomized, controlled trial. Aesthet Surg J. 33:400–408.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Noh Y and Heo CY: The effect of
phosphatidylcholine and deoxycholate compound injections to the
localized adipose tissue: An experimental study with a murine
model. Arch Plast Surg. 39:452–456. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim SY, Shin IS and Park YJ: Effect of
acupuncture and intervention types on weight loss: A systematic
review and meta-analysis. Obes Rev. 19:1585–1596. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Huang YC, Tsay HJ, Lu MK, Lin CH, Yeh CW,
Liu HK and Shiao YJ: Astragalus membranaceus-polysaccharides
ameliorates obesity, hepatic steatosis, neuroinflammation and
cognition impairment without affecting amyloid deposition in
metabolically stressed APPswe/PS1dE9 mice. Int J Mol Sci.
18(2746)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim YJ, Shin YO, Ha YW, Lee S, Oh JK and
Kim YS: Anti-obesity effect of Pinellia ternata extract in Zucker
rats. Biol Pharm Bull. 29:1278–1281. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang HY and Lee SG: Effects of dandelion
(Teraxacum platycarpum) with various extracting method on
antioxidative capacity, lipid metabolism in diet-induced obese
rats. Korean J Orient Physiol Pathol. 25:48–54. 2011.(In
Korean).
|
|
12
|
Heo J: Dongeuibogam. 1613.
|
|
13
|
Pinto H: Local fat treatments:
Classification proposal. Adipocyte. 5:22–26. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bray GA, Fruhbeck G, Ryan DH and Wilding
JP: Management of obesity. Lancet. 387:1947–1956. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Engin A: Fat cell and fatty acid turnover
in obesity. Adv Exp Med Biol. 960:135–160. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Haczeyni F, Bell-Anderson KS and Farrell
GC: Causes and mechanisms of adipocyte enlargement and adipose
expansion. Obes Rev. 19:406–420. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee HJ, Lee MH, Lee SG, Yeo UC and Chang
SE: Evaluation of a novel device, high-intensity focused ultrasound
with a contact cooling for subcutaneous fat reduction. Lasers Surg
Med. 48:878–886. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cingolani F and Czaja MJ: Regulation and
functions of autophagic lipolysis. Trends Endocrinol Metab.
27:696–705. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Singh R and Cuervo AM: Lipophagy:
Connecting autophagy and lipid metabolism. Int J Cell Biol.
2012(282041)2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Martinez-Lopez N, Garcia-Macia M, Sahu S,
Athonvarangkul D, Liebling E, Merlo P, Cecconi F, Schwartz GJ and
Singh R: Autophagy in the CNS and periphery coordinate lipophagy
and lipolysis in the brown adipose tissue and liver. Cell Metab.
23:113–127. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kim JH, Kim OK, Yoon HG, Park J, You Y,
Kim K, Lee YH, Choi KC, Lee J and Jun W: Anti-obesity effect of
extract from fermented Curcuma longa L. through regulation of
adipogenesis and lipolysis pathway in high-fat diet-induced obese
rats. Food Nutr Res. 60(30428)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ryden M and Arner P: Subcutaneous
adipocyte lipolysis contributes to circulating lipid levels.
Arterioscler Thromb Vasc Biol. 37:1782–1787. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kojta I, Chacinska M and
Blachnio-Zabielska A: Obesity, bioactive lipids, and adipose tissue
inflammation in insulin resistance. Nutrients.
12(1305)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Janovska A, Hatzinikolas G, Staikopoulos
V, McInerney J, Mano M and Wittert GA: AMPK and ACC
phosphorylation: Effect of leptin, muscle fibre type and obesity.
Mol Cell Endocrinol. 284:1–10. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Marion-Letellier R, Savoye G and Ghosh S:
Fatty acids, eicosanoids and PPAR gamma. Eur J Pharmacol.
785:44–49. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Franckhauser S, Muñoz S, Pujol A, Casellas
A, Riu E, Otaegui P, Su B and Bosch F: Increased fatty acid
re-esterification by PEPCK overexpression in adipose tissue leads
to obesity without insulin resistance. Diabetes. 51:624–630.
2002.PubMed/NCBI View Article : Google Scholar
|