Introduction
Hepatic ischemia-reperfusion injury (HIRI) occurs in
hepatectomy, severe liver trauma surgery and liver transplantation
(1). It results in postoperative
liver dysfunction and failure (2).
Reactive oxygen species (ROS) are one of the key factors
influencing HIRI (3,4). ROS are continuously produced during
hepatic ischemia-reperfusion and excessive levels ROS damage
hepatocytes (5,6). In response to ROS damage, liver cells
undergo inflammation and apoptosis (7). The liver forms a complex oxidative
stress response system and upregulates expression levels of
protective genes, such as heme oxygenase-1 (HO-1), to decrease
injury to hepatocytes when exposed to ROS (8). Previous studies have shown that
pre-treatment drugs, such as sulforaphane and baicalein (BAI), can
also regulate the antioxidant pathway in the liver (9,10).
BAI is one of the most abundant flavonoids and the
primary active component of Scutellaria baicalensis
(11,12). Previous studies have confirmed that
BAI has anti-bacterial, ROS scavenging, anti-inflammatory and
anti-tumor effects (13-16).
Moreover, accumulated data have shown that BAI protects against
several types of liver disease, such as alcoholic liver disease
(17), non-alcoholic fatty liver
disease (18,19), chemical-induced liver fibrosis
(20) and immunological liver
injury (21). Although certain
studies by Liu et al (22,23)
addressed the beneficial effects of BAI on HIRI, no in vivo
study has determined the mechanism underlying the protective
effects of BAI on HIRI via the nuclear factor E2-related factor 2
(Nrf2)/antioxidant response element (ARE) pathway.
Nrf2 has been found to regulate antioxidant proteins
by interacting with ARE, which is a key endogenous antioxidant
stress pathway (9). There are
>200 coding endogenous protective genes regulated by the
Nrf2/ARE signaling pathway (1).
These protective genes include antioxidant and phase II
detoxification enzymes, such as catalase, HO-1, superoxide
dismutase (SOD), and NAD(P)H dehydrogenase quinone (NQO)-1 and -2
(24-26).
According to previous studies, the Nrf2/ARE pathway serves a key
role in protection against HIRI in mice (8,27,28).
However, whether the Nrf2/ARE pathway also contributes to the
protection provided by BAI against HIRI remains unclear. The
present study investigated the role of the Nrf2/ARE pathway in the
protection effect provided by BAI against HIRI and its underlying
mechanism.
Materials and methods
Animals
A total of 72 male C57BL/6 mice (age 6-8 weeks; body
weight, 19-21 g) were obtained from the Animal Center of Guangxi
Medical University (Nanning, China). Three mice were housed per
individual standard cages under controlled conditions (22±1˚C,
60±10% relative humidity and 12 h light/dark cycles), with free
access to water and food. BAI was obtained from Yuanye Bio (cat.
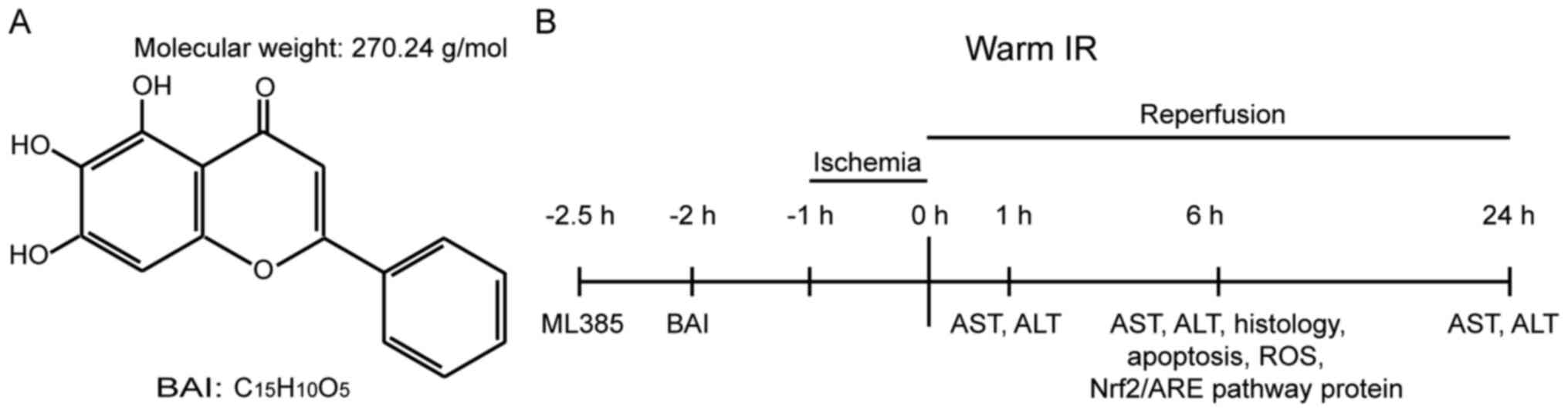
no. S25956), with a >98% purity (Fig. 1A). Nrf2 inhibitor ML385 (cat. no.
SML1833) was obtained from Sigma-Aldrich (Merck KGaA). Mice were
randomly assigned into different groups (n=6/group) as follows: i)
Sham, following intraperitoneal injection of DMSO, the abdominal
cavity was opened and closed without IR; ii) BAI (100 mg/kg),
following intraperitoneal injection of BAI (100 mg/kg; dissolved in
DMSO), the abdominal cavity was opened and closed without IR; iii)
IR, DMSO was injected intraperitoneally before IR; iv) BAI (10
mg/kg) + IR, BAI (10 mg/kg) was injected intraperitoneally before
IR; v) BAI (50 mg/kg) + IR, BAI (50 mg/kg) was injected
intraperitoneally before IR; vi) BAI (100 mg/kg) + IR, BAI (100
mg/kg) was injected intraperitoneally before IR; vii) ML385 (30
mg/kg) + IR, ML385 (30 mg/kg) was injected intraperitoneally before
IR; and viii) ML385 (30 mg/kg) + BAI (100 mg/kg) + IR, ML385 (30
mg/kg) and BAI (100 mg/kg) were injected intraperitoneally before
IR. The IR and BAI (100 mg/kg) + IR groups were further divided
into reperfusion subgroups, with 6 mice per subgroup, (1, 6 and 24
h). Mice were euthanized at 1, 6 and 24 h post-HIRI. All animals
were treated humanely and all animal experiments were approved by
the Animal Experimental Ethics Committee of the First Affiliated
Hospital of Guangxi Medical University (Nanning, China).
HIRI model
General anesthesia was induced in all mice by
continuous spontaneous inhalation of 2-5% volatile anesthetic
isoflurane via a mask. A mouse model of HIRI was established as
described by Tsung et al (29). The midline incision of the upper
abdomen was performed to separate and expose the hepatic portal.
The branches of the left and middle portal veins and hepatic
arteries were clipped using a non-invasive vascular clamp,
resulting in 70% warm ischemia. The color of the liver lobe in the
blocked area changed from bright red to pale white, indicating
successful induction of hepatic ischemia. After 60 min, the clamp
was released and blood flow was restored. In the Sham and BAI (100
mg/kg) groups, only the liver was exposed and blood flow was not
blocked. After 1, 6 and 24 h reperfusion, mice were anesthetized
via inhalation of 3% isoflurane for 3-5 min and 0.8-1 ml blood
samples from the inferior vena cava and liver tissue were
collected. Following blood collection, all mice were euthanized in
a closed chamber via inhalation of 5% volatile isoflurane for 10
min. Mouse death was confirmed by cessation of heartbeat and nerve
reflex. The procedure is shown in Fig.
1B.
Liver function assessment
Blood samples were stored at room temperature (RT)
for 1 h and then were centrifuged at 1,413 x g, 4˚C for 15 min.
Serum was collected and stored at -80˚C before testing. The levels
of alanine aminotransferase (ALT) (cat. no. C001-e; Changchun Huili
Biotech Co., Ltd.) and aspartate aminotransferase (AST) (cat. no.
C002-e, Changchun Huili Biotech Co., Ltd.) were determined using a
7180 Biochemical Analyzer (Hitachi, Ltd.).
Histopathological observation and
evaluation of liver injury
Ischemic liver lobes were fixed in 10% formalin at
RT for 24 h and embedded in paraffin. Then, 5 µm thick paraffin
slices were dewaxed with xylene twice (10 min each), fully hydrated
with ethanol twice (5 min each) and rinsed with tap water. Sections
were stained with hematoxylin solution (kit cat. no. C0105S;
Beyotime Institute of Biotechnology) at RT for 3-5 min, rinsed with
tap water and treated with 1% hematoxylin differentiation solution
at RT for 6-25 sec. Sections were treated with 0.6% ammonia water
solution for bluing at RT for 90 min, rinsed with tap water and
immersed in 85 and 95% ethanol at RT for 5 min each. Sections were
stained with 0.5% eosin dye (kit cat. no. C0105S; Beyotime
Institute of Biotechnology) at RT for 5 min. The sections were then
dehydrated and hyalinized in xylene before being sealed in neutral
gum. Liver damage was determined under a light microscope (Olympus
Corporation) (magnification, x400). The degree of liver injury was
evaluated by two independent pathologists blinded to the
experimental groups according to the classification standard
described by Suzuki et al (30). This Suzuki grading standard
consists of five grades (0-4) according to vacuolization of
hepatocyte cytoplasm, necrosis and tissue congestion.
TUNEL detection of apoptosis
TUNEL was used to detect hepatocyte apoptosis
(Colorimetric TUNEL Apoptosis Assay kit; cat. no. C1098; Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. Ischemic liver lobes were fixed in 10% formalin at RT for
24 h and embedded in paraffin. The 5 µm thick paraffin slices were
dewaxed with xylene twice for 10 min each, hydrated with ethanol
twice for 5 min each, followed by dehydration using 95, 90, 80 and
70% ethanol (5 min each). Proteinase K working solution was added
at 37˚C for 20 min. Sections were immersed in 3%
H2O2 at RT for 20 min, then washed three
times with PBS (pH 7.4) in a Rocker device. A total of 50 μl TUNEL
reaction mixture was added at 37˚C for 1 h.
Streptavidin-horseradish peroxidase and Tris-buffered saline
containing 0.1% (v/v) Tween-20 (TBST) were mixed at a ratio of
1:200, added to cover the tissue and incubated at 37˚C for 30 min.
The slides were placed in PBS (pH 7.4) and washed with shaking on a
decolorizing shaker three times (5 min each). Then,
diaminobenzidine coloring solution (0.2 ml) provided in the kit was
added to marked tissue at RT for 5 min. Sections were
counterstained with hematoxylin staining solution at RT for 1 min
and washed in water, dried and sealed with neutral resin (cat. no.
G8590; Beijing Solarbio Science & Technology Co., Ltd.).
TUNEL-positive hepatocytes (brown-yellow nucleus) were counted in
>3 high power histological fields under a light microscope
(Olympus Corporation; magnification, x400) and the percentage was
calculated.
Oxidative stress evaluation
Ischemic liver tissue was perfused with normal
saline three times at 4˚C for 3 min and ground into 10% homogenate.
The homogenate was centrifuged at 1,413 x g and 4˚C for 10 min. The
total protein concentration of the supernatant was determined via
BCA method. The content of SOD (cat. no. S0103; Beyotime Institute
of Biotechnology) and malondialdehyde (MDA; cat. no. S0131S;
Beyotime Institute of Biotechnology) in liver tissue homogenate
from each group was determined according to the manufacturer's
instructions. Frozen sections (10 µm) were prepared from fresh
liver tissue and the ROS content was determined by a fluorescent
dye dihydroethidium (DHE) (cat. no. D7008; Sigma-Aldrich; Merck
KGaA). Tissue at RT was marked liquid blocker pen. Spontaneous
fluorescence quenching reagent was added at RT for 5 min before
washing in running tap water for 10 min. ROS staining solution (DHE
10 µmol/l) was added to the tissue area at 37˚C for 30 min in the
dark. Sections were washed three times with PBS (pH 7.4) in a
Rocker device (5 min each), incubated with DAPI solution (cat. no.
G1012; Wuhan Servicebio Technology Co., Ltd.) at RT for 10 min in
the dark and washed three times with PBS (pH 7.4) in a Rocker
device (5 min each). Samples were mounted with anti-fade mounting
medium and images were captured using a fluorescence microscope
(magnification, x400). DAPI glows blue under UV at excitation
wavelength 330-380 nm and emission wavelength 420 nm; DHE itself
displays blue fluorescence (absorption/emission, 355/420 nm) in
cell cytoplasm while the oxidized form ethidium displays red
fluorescence (absorption/emission, 510/590 nm) upon DNA
intercalation. Nuclei appear blue by labeling with DAPI.
ROS-positive cells were labelled by fluorescein are red.
Western blotting
Total protein of HO-1 and NQO-1 from each group was
extracted using RIPA lysis buffer (cat. no. R0010; Beijing Solarbio
Science & Technology Co., Ltd.). The level of Nrf2
nucleoprotein was measured in nuclear lysate by a nuclear protein
extraction kit (cat. no. R0050; Beijing Solarbio Science &
Technology Co., Ltd.). The protein concentration was determined
using a BCA kit (cat. no. P0010S; Beyotime Institute of
Biotechnology). A total of 20 µg/lane protein samples were
separated using 10% SDS-PAGE and transferred to PVDF membranes
(cat. no. IPVH00010; Merck KGaA). Membranes were blocked with 5%
skimmed milk at RT for 1 h. The membranes were incubated with
primary antibodies at 4˚C overnight. The primary antibodies were as
follows: Anti-Nrf2 (1:1,000; cat. no. T55136; Abmart Pharmaceutical
Technology Co., Ltd.), anti-HO-1 (1:1,000; cat. no. PY5393; Abmart
Pharmaceutical Technology Co., Ltd.), anti-NQO-1 (1:1,000; cat. no.
T56710; Abmart Pharmaceutical Technology Co., Ltd.), anti-Histone
H3 (1:1,000; cat. no. T56587; Abmart Pharmaceutical Technology Co.,
Ltd.) and anti-GAPDH (1:20,000; cat. no. 10494-1-AP; ProteinTech
Group, Inc.). Membranes were then washed three times in
Tris-buffered saline containing 0.1% (v/v) Tween-20 (TBST) and
incubated with horseradish-conjugated goat-anti-rabbit secondary
antibody (1:10,000; cat. no. SA00001-2; ProteinTech Group, Inc.) at
RT for 1 h. Membranes were washed again. ECL reagent (cat. no.
P0018FM, Beyotime Institute of Biotechnology) was used to visualize
the bands. Bands were detected with a GeneSys System (Bio Rad
Laboratories, Inc.). Band intensities were measured using Image J
v1.8.0 software (National Institutes of Health).
Statistical analysis
SPSS 13.0 (SPSS, Inc.) and GraphPad Prism 5.0
software (GraphPad Software, Inc.) were used for statistical
analysis and presentation of the data. Shapiro-Wilk test confirmed
that data were normally distributed. The data are presented as mean
± SD of at least three replicates. One- or two-way ANOVA followed
post hoc Tukey's or Holm-Sidak test, respectively, were performed
for multiple group comparisons. Two-sided P<0.05 was considered
to indicate a statistically significant difference.
Results
BAI ameliorates liver injury induced
by IR
The activity of ALT and AST in serum and
histological examination were used to assess liver injury. Levels
of AST and ALT in the IR group were significantly increased,
indicating impaired liver function (Fig. 2A and B). Serum ALT and AST levels were
significantly decreased by pre-treatment with medium- and high-dose
BAI (50 and 100 mg/kg) before IR, which indicated that BAI exerted
a significant protective effect on liver function. Consistent with
the alterations in serum ALT and AST levels, compared with the Sham
and BAI (100 mg/kg) group, a larger necrotic area was observed in
the IR group by HE staining (Fig.
2C). Compared with the I/R group, pre-treatment with medium-
and high-dose BAI (50 and 100 mg/kg) before IR significantly
decreased liver necrosis. Pathological results were verified by
Suzuki score (Fig. 2D). Suzuki
scores indicated that the average injury level in the I/R group was
significantly higher than that in the Sham and BAI (100 mg/kg)
groups. However, compared with the I/R group, the BAI (50 mg/kg) +
IR and BAI (100 mg/kg) + IR groups showed significant alleviation
of average injury levels, while there was no significant difference
between injury levels in the I/R and BAI (10 mg/kg) + IR groups.
Following reperfusion (1, 6 and 24 h), pre-treatment with BAI
significantly decreased the levels of ALT and AST (Fig. 2E and F) compared with the corresponding IR
group. These results suggested that BAI protected the liver from
HIRI.
BAI decreases oxidative stress and
hepatic apoptosis following HIRI
The aforementioned results indicated that medium-
and high dose BAI (50 and 100 mg/kg) had protective effects on HIRI
and 100 mg/kg had a better protective effect. The levels of ALT and
AST peaked at 6 h after IR; therefore, this time point was selected
for subsequent experiments. To determine the protective effect of
100 mg/kg BAI, both blood and liver tissue samples from the Sham,
BAI (100 mg/kg), IR and BAI (100 mg/kg) + IR groups were used for
further study. To assess the effect of BAI on oxidative damage to
the liver, liver tissue was stained with DHE probe to detect liver
ROS content. Compared with the Sham and BAI (100 mg/kg) groups, IR
liver tissue samples showed notably higher expression levels of
intracellular ROS (Fig. 3A).
Pre-treatment with BAI suppressed ROS levels. Pre-treatment with
BAI (100 mg/kg) before IR significantly decreased liver cell
apoptosis compared with the IR group (Fig. 3B and C). Moreover, compared with the Sham and
BAI (100 mg/kg) groups, the MDA content in liver tissue of the IR
group was significantly increased; this was downregulated
significantly by BAI (100 mg/kg) pre-treatment (Fig. 3D). By contrast to MDA, the activity
of SOD in liver tissue was decreased significantly in the IR group
compared with the Sham group (Fig.
3E). Pre-treatment with BAI increased the activity of SOD in
liver tissue independently of IR.
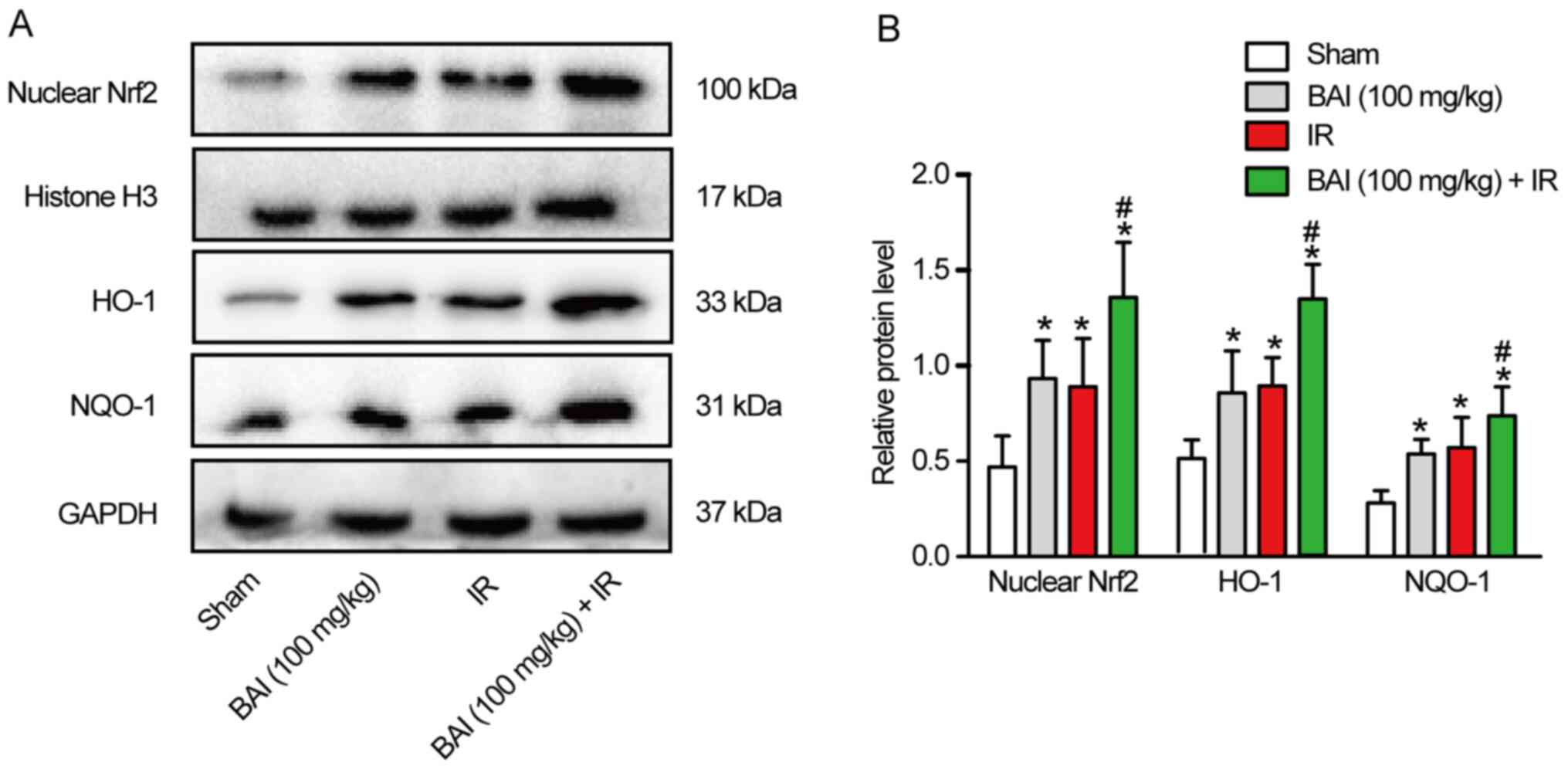
BAI upregulates Nrf2/ARE pathway
protein expression levels in liver
Western blotting was used to detect the expression
levels of Nrf2/ARE pathway-associated protein. Compared with the
Sham group, the expression levels of Nrf2 nucleoprotein and total
HO-1 and NQO-1 protein increased significantly in the IR and BAI
(100 mg/kg) groups (Fig. 4A and
B). Compared with the IR group,
the expression levels of Nrf2 nucleoprotein and total HO-1 and
NQO-1 protein in the BAI (100 mg/kg) + IR group were further
increased following pre-treatment with BAI. However, there was no
significant difference in the expression levels of Nrf2
nucleoprotein and total HO-1 and NQO-1 protein between the IR and
BAI (100 mg/kg) groups.
Nrf2/ARE pathway inhibitor reverses
the protective effect of BAI on HIRI
To confirm the role of the Nrf2/ARE pathway in
BAI-treated mice following HIRI, the Nrf2 inhibitor ML385 was used.
Compared with the IR group, the levels of Nrf2 nucleoprotein and
total HO-1 and NQO-1 protein in the ML385 + IR group decreased
significantly (Fig. 5A and
B). Moreover, compared with the
BAI 100 mg/kg+ IR group, the expression of Nrf2 nucleoprotein and
total HO-1 and NQO-1 protein in the ML385 + BAI (100 mg/kg) + IR
group was significantly decreased. ML385 also aggravated liver
injury (Fig. 5C, F and G),
hepatocyte apoptosis (Fig. 5F and
H) and oxidative stress injury
(Fig. 5D-F). These data suggested
that the Nrf2/ARE pathway served a significant role in the
protective effect of BAI against HIRI.
 | Figure 5Nrf2/ARE pathway inhibitor reverse
the protective effect of BAI on hepatic IR injury. Samples were
collected 6 h after liver perfusion in all groups. (A) Levels of
Nrf2 nucleoprotein, total HO-1, and NQO-1 protein were analyzed by
western blotting. GAPDH and histone H3 were used as the internal
controls. (B) Nrf2 nucleoprotein expression relative to histone H3
and HO-1 and NQO-1 protein expression relative to GAPDH. (C) Serum
ALT and AST levels. (D) MDA content and (E) SOD activity were
measured in liver tissue. (F) Pathological damage in the liver was
measured by HE and TUNEL staining and ROS activity was measured by
fluorescent-labeled DHE staining. Apoptotic cells are indicated by
red arrows. Scale bar=50 µm. (G) Suzuki score. (H) Percentage of
TUNEL-positive hepatocytes. *P<0.05 vs. IR;
#P<0.05 vs. IR + BAI 100 mg/kg. BAI, baicalein; IR,
ischemia/reperfusion; Nrf2, nuclear factor E2-related factor 2;
ARE, antioxidant response elements; HO01, heme oxygenase-1; NQO-1,
NAD(P)H dehydrogenase quinone-1; MDA, malonaldehyde; SOD,
superoxide dismutase; ALT, alanine aminotransferase; AST, aspartate
aminotransferase; HE, hematoxylin and eosin; ROS, reactive oxygen
species. |
Discussion
BAI is the most abundant flavonoid in S.
baicalensis and a key component of traditional Chinese medicine
(31). Previous studies have shown
that BAI attenuates HIRI via inhibition of the NF-κB pathway and
induction of autophagy (22,23).
Here, BAI pre-treatment significantly attenuated HIRI. BAI
significantly decreased the elevated levels of AST and ALT and
ameliorated the necrotic area of hepatocytes induced by HIRI.
Additionally, pre-treatment with BAI inhibited oxidative damage and
hepatocyte apoptosis. Pre-treatment with BAI promoted activation of
the Nrf2/ARE pathway and alleviated liver injury during HIR, while
Nrf2 inhibitor ML385 partially reversed the protective effect of
BAI on HIRI. The present data indicated that BAI served a
protective role in HIRI by regulating the Nrf2/ARE pathway to
alleviate oxidative damage.
Multiple factors are involved in the occurrence and
development of HIRI, such as oxygen free radical production, cell
apoptosis, inflammatory reaction and calcium overload (32,33).
Oxidative stress induced by large amounts of ROS during reperfusion
not only serves an important role in the early stage of hepatocyte
injury but also participates in the later stage of inflammation
(1). Excessive ROS beyond the
clearance capacity of the liver damage lipids and proteins in
cells, leading to cell death (34). The present results showed that the
levels of ALT and AST in serum were increased following HIRI.
Histological examination revealed that HIRI resulted in hepatocyte
apoptosis, congestion and necrosis. This damage may be due to the
formation of lipid peroxides by combination of excessive ROS and
lipids in the cell membrane, resulting in destruction of the
hepatocyte membrane (35,36). The end product of lipid peroxides
is MDA, which reflects the degree of cell peroxidation (37). SOD scavenges free radicals, which
decreases oxidative damage caused by IR (38). BAI exhibits a well-known
antioxidant capacity (39). Dong
et al (10) found that BAI
alleviates liver oxidative damage induced by high-level glucose.
Pre-treatment with BAI increased the activity of SOD and decreased
levels of ROS and MDA in liver tissue. Furthermore, pre-treatment
with BAI significantly decreased the elevated levels of AST and ALT
and ameliorated the necrotic area of hepatocytes induced by HIRI.
These results showed that the protective effect of BAI against HIRI
was mediated by alleviating oxidative damage to the liver.
The underlying mechanism of HIRI is complicated and
not fully understood. Our previous study showed that [D-Ala2,
D-Leu5]-Enkephalin significantly inhibits HIR-induced oxidative
stress by activation of the Nrf2/HO-1 pathway (27). Numerous endogenous antioxidant
protection genes, such as SOD (25) and phase II detoxification enzymes,
such as HO-1, are regulated by the Nrf2/ARE pathway (40). HO-1 and NQO-1 are two widely
studied phase II enzymes regulated by Nrf2 that serve an important
role in antioxidation (38,41).
Qin et al revealed that some hepatic metabolic enzyme genes
of the Nrf2/ARE pathway, such as NQO1 and HO-1, were activated in
BAI-treated hepatocytes (42). Shi
et al also demonstrated that pre-treatment with BAI
activates the Nrf2/ARE pathway to alleviate acetaminophen-induced
oxidative damage (43). Kim et
al (44) reported that
baicalin alleviates liver IR injury by upregulating HO-1
expression. At the same time, baicalin serves an anti-inflammatory
and antiapoptotic role by inhibiting activation of NF-κB and
caspases 3 and 8(44). Baicalin
and BAI are primary flavonoids extracted from the dry roots of
S. baicalensis (45). The
molecular formula of BAI is
C15H10O5, while that of baicalin
is C21H18O11. The two molecules
have similar pharmacological effects, such as anti-inflammatory,
antioxidant and antiapoptotic effects. Qin et al (31) showed that BAI positively regulates
the Nrf2/Kelch-like ECH-associated protein 1 (Keap1) pathway via
Keap1-independent and -dependent pathways. In accordance with
previous studies (9,27), HIR lead to an increase in
expression levels of Nrf2 nucleoprotein and total HO-1 and NQO-1
protein, which indicated that the antioxidant pathway was activated
when the liver was subjected to IR. Moreover, pre-treatment with
BAI further increased the expression levels of Nrf2 nucleoprotein
and downstream antioxidative enzymes, such as SOD, HO-1 and NQO-1.
These results indicated that pre-treatment with BAI promoted Nrf2
nuclear translocation and increased the antioxidant capacity of the
liver. The protective role of BAI in HIRI was disrupted by the Nrf2
inhibitor ML385, which supported the key role of Nrf2/ARE in
BAI-provided protection against HIRI.
Several S. baicalensis-derived mixtures or
pure compounds have been approved as clinical therapeutic drugs in
China; for example, BAI capsules are used to treat hepatitis
(46). Hepatitis patients often
develop liver cancer, and these patients with liver cancer and
hepatitis often need extensive hepatectomy (47). HIRI is a common pathological
process occurring in extensive hepatectomy (1). Therefore, it is worth studying
whether BAI can be used before surgery in patients with hepatitis
related liver cancer to decrease ischemia-reperfusion injury, as it
is not only able to decrease ischemia-reperfusion injury, but also
to decrease the adverse effect of hepatitis on the perioperative
period. Nevertheless, there are certain limitations to the present
study. The role of Keap1, an endogenous inhibitor of Nrf2, was not
investigated. The dose selection of BAI was based on previous
research (22). The maximum dose
of BAI was 100 mg/kg, which exhibited the most notable protective
effect. Whether a higher BAI dose has a better protective effect
requires further study. Based on the pharmacokinetics of BAI in
vivo, the time and frequency of preoperative BAI should also be
investigated in future.
The present findings showed that alleviation of
oxidative stress by regulating the Nrf2/ARE pathway contributed to
the protective effect of BAI against HIRI and BAI may be a
promising therapeutic drug for the management of HIRI.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 91949122 and 81771674), the 111
Project (grant no. D17011), the Guangxi Key Research and
Development Plan (grant no. 2018AD03001) and the Self-Funded
Scientific Research Project of Guangxi Zhuang Autonomous Region
Health Commission (grant no. Z20200575).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH, GY and YZh designed the study. YZh, ZT, YZe, SC
and GH performed the experiments. HH, YZe, JW, YZh, ZT and CQ
analyzed the data. YZh and ZT wrote the original draft of the
manuscript. SH and GY revised the manuscript. YZh and ZT confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal
Experimental Ethics Committee of the First Affiliated Hospital of
Guangxi Medical University (approval no. 2017-KY-81771674-002). All
the animals used were treated humanely.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhai Y, Petrowsky H, Hong JC, Busuttil RW
and Kupiec-Weglinski JW: Ischaemia-reperfusion injury in liver
transplantation - from bench to bedside. Nat Rev Gastroenterol
Hepatol. 10:79–89. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Busuttil R: Liver ischaemia and
reperfusion injury. Br J Surg. 94:787–788. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Casillas-Ramírez A, Mosbah IB, Ramalho F,
Roselló-Catafau J and Peralta C: Past and future approaches to
ischemia-reperfusion lesion associated with liver transplantation.
Life Sci. 79:1881–1894. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Elias-Miró M, Jiménez-Castro MB, Rodés J
and Peralta C: Current knowledge on oxidative stress in hepatic
ischemia/reperfusion. Free Radic Res. 47:555–568. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jaeschke H and Woolbright BL: Current
strategies to minimize hepatic ischemia-reperfusion injury by
targeting reactive oxygen species. Transplant Rev (Orlando).
26:103–114. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
van Golen RF, van Gulik TM and Heger M:
Mechanistic overview of reactive species-induced degradation of the
endothelial glycocalyx during hepatic ischemia/reperfusion injury.
Free Radic Biol Med. 52:1382–1402. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hoek JB and Pastorino JG: Ethanol,
oxidative stress, and cytokine-induced liver cell injury. Alcohol.
27:63–68. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yi Z, Deng M, Scott MJ, Fu G, Loughran PA,
Lei Z, Li S, Sun P, Yang C, Li W, et al: Immune-responsive gene
1/itaconate activates nuclear factor erythroid 2-related factor 2
in hepatocytes to protect against liver ischemia-reperfusion
injury. Hepatology. 72:1394–1411. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chi X, Zhang R, Shen N, Jin Y, Alina A,
Yang S and Lin S: Sulforaphane reduces apoptosis and oncosis along
with protecting liver injury-induced ischemic reperfusion by
activating the Nrf2/ARE pathway. Hepatol Int. 9:321–329.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dong Y, Xing Y, Sun J, Sun W, Xu Y and
Quan C: Baicalein Alleviates Liver Oxidative Stress and Apoptosis
Induced by High-Level Glucose through the Activation of the
PERK/Nrf2 Signaling Pathway. Molecules. 25(599)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liau P, Wu M and Lee C: Scutellaria
baicalensisInhibitory Effects of Root Extract on Linoleic Acid
Hydroperoxide-induced Lung Mitochondrial Lipid Peroxidation and
Antioxidant Activities. Molecules. 24(2143)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang YS, Cho JG, Hwang ES, Yang JE, Gao W,
Fang MZ, Zheng SD and Yi TH: Enhancement of Protective Effects of
Radix Scutellariae on UVB-induced Photo Damage in Human HaCaT
Keratinocytes. Appl Biochem Biotechnol. 184:1073–1093.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee W, Ku SK and Bae JS: Anti-inflammatory
effects of Baicalin, Baicalein, and Wogonin in vitro and in vivo.
Inflammation. 38:110–125. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lu Y, Joerger R and Wu C: Study of the
chemical composition and antimicrobial activities of ethanolic
extracts from roots of Scutellaria baicalensis Georgi. J
Agric Food Chem. 59:10934–10942. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lin H, Hao Y, Wan X, He J and Tong Y:
Baicalein inhibits cell development, metastasis and EMT and induces
apoptosis by regulating ERK signaling pathway in osteosarcoma. J
Recept Signal Transduct Res. 40:49–57. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
de Oliveira MR, Nabavi SF, Habtemariam S,
Erdogan Orhan I, Daglia M and Nabavi SM: The effects of baicalein
and baicalin on mitochondrial function and dynamics: A review.
Pharmacol Res. 100:296–308. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang H, Zhang Y, Bai R, Wang M and Du S:
Baicalin Attenuates Alcoholic Liver Injury through Modulation of
Hepatic Oxidative Stress, Inflammation and Sonic Hedgehog Pathway
in Rats. Cell Physiol Biochem. 39:1129–1140. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xi Y, Wu M, Li H, Dong S, Luo E, Gu M,
Shen X, Jiang Y, Liu Y and Liu H: Baicalin Attenuates High Fat
Diet-Induced Obesity and Liver Dysfunction: Dose-Response and
Potential Role of CaMKKβ/AMPK/ACC Pathway. Cell Physiol Biochem.
35:2349–2359. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xin HG, Zhang BB, Wu ZQ, Hang XF, Xu WS,
Ni W, Zhang RQ and Miao XH: Treatment with baicalein attenuates
methionine-choline deficient diet-induced non-alcoholic
steatohepatitis in rats. Eur J Pharmacol. 738:310–318.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sun H, Che QM, Zhao X and Pu XP:
Antifibrotic effects of chronic baicalein administration in a CCl4
liver fibrosis model in rats. Eur J Pharmacol. 631:53–60.
2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang Y, Shan L, Hua Y, Wang D, Zeng H,
Liu R, Zhang W and Hu Z: Baicalein selectively induces apoptosis in
activated lymphocytes and ameliorates concanavalin a-induced
hepatitis in mice. PLoS One. 8(e69592)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu A, Huang L, Fan H, Fang H, Yang Y, Liu
S, Hu J, Hu Q, Dirsch O and Dahmen U: Baicalein pretreatment
protects against liver ischemia/reperfusion injury via inhibition
of NF-κB pathway in mice. Int Immunopharmacol. 24:72–79.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu A, Huang L, Guo E, Li R, Yang J, Li A,
Yang Y, Liu S, Hu J, Jiang X, et al: Baicalein pretreatment reduces
liver ischemia/reperfusion injury via induction of autophagy in
rats. Sci Rep. 6(25042)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ruiz S, Pergola PE, Zager RA and Vaziri
ND: Targeting the transcription factor Nrf2 to ameliorate oxidative
stress and inflammation in chronic kidney disease. Kidney Int.
83:1029–1041. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wu P, Yan Y, Ma LL, Hou BY, He YY, Zhang
L, Niu ZR, Song JK, Pang XC, Yang XY, et al: Effects of the Nrf2
Protein Modulator Salvianolic Acid A Alone or Combined with
Metformin on Diabetes-associated Macrovascular and Renal Injury. J
Biol Chem. 291:22288–22301. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang H, Liu YY, Jiang Q, Li KR, Zhao YX,
Cao C and Yao J: Salvianolic acid A protects RPE cells against
oxidative stress through activation of Nrf2/HO-1 signaling. Free
Radic Biol Med. 69:219–228. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhou Y, Zhang J, Lei B, Liang W, Gong J,
Zhao C, Yu J, Li X, Tang B and Yuan S: DADLE improves hepatic
ischemia/reperfusion injury in mice via activation of the Nrf2/HO-1
pathway. Mol Med Rep. 16:6214–6221. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lu MC, Ji JA, Jiang ZY and You QD: The
Keap1-Nrf2-ARE Pathway As a Potential Preventive and Therapeutic
Target: An Update. Med Res Rev. 36:924–963. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tsung A, Sahai R, Tanaka H, Nakao A, Fink
MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, et al: The
nuclear factor HMGB1 mediates hepatic injury after murine liver
ischemia-reperfusion. J Exp Med. 201:1135–1143. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Suzuki S, Toledo-Pereyra LH, Rodriguez FJ
and Cejalvo D: Neutrophil infiltration as an important factor in
liver ischemia and reperfusion injury. Modulating effects of FK506
and cyclosporine. Transplantation. 55:1265–1272. 1993.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Qin S, Deng F, Wu W, Jiang L, Yamashiro T,
Yano S and Hou DX: Baicalein modulates Nrf2/Keap1 system in both
Keap1-dependent and Keap1-independent mechanisms. Arch Biochem
Biophys. 559:53–61. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang Q, Lai Y, Deng J, Wang M, Wang Z,
Wang M, Zhang Y, Yang X, Zhou X and Jiang H: Vagus Nerve
Stimulation Attenuates Hepatic Ischemia/Reperfusion Injury via the
Nrf2/HO-1 Pathway. Oxid Med Cell Longev.
2019(9549506)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhao Y, Cai H, Zhou P, Lin S, Pan Y and
Liang X: Protective effect of ulinastatin on hepatic ischemia
reperfusion injury through autophagy activation in Chang liver
cells. J Cell Biochem. 120:14960–14970. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Reyes-Gordillo K, Shah R and Muriel P:
Oxidative Stress and Inflammation in Hepatic Diseases: Current and
Future Therapy. Oxid Med Cell Longev. 2017(3140673)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Konishi T and Lentsch AB: Hepatic
Ischemia/Reperfusion: Mechanisms of Tissue Injury, Repair, and
Regeneration. Gene Expr. 17:277–287. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ibrahim SG, El-Emam SZ, Mohamed EA and Abd
Ellah MF: Dimethyl fumarate and curcumin attenuate hepatic
ischemia/reperfusion injury via Nrf2/HO-1 activation and
anti-inflammatory properties. Int Immunopharmacol.
80(106131)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Suji G and Sivakami S: Malondialdehyde, a
lipid-derived aldehyde alters the reactivity of Cys34 and the
esterase activity of serum albumin. Toxicol In Vitro. 22:618–624.
2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu D, Wang H, Zhang Y and Zhang Z:
Protective Effects of Chlorogenic Acid on Cerebral
Ischemia/Reperfusion Injury Rats by Regulating Oxidative
Stress-Related Nrf2 Pathway. Drug Des Devel Ther. 14:51–60.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gong WY, Zhao ZX, Liu BJ, Lu LW and Dong
JC: Exploring the chemopreventive properties and perspectives of
baicalin and its aglycone baicalein in solid tumors. Eur J Med
Chem. 126:844–852. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wu G, Zhu L, Yuan X, Chen H, Xiong R,
Zhang S, Cheng H, Shen Y, An H, Li T, et al: Britanin Ameliorates
Cerebral Ischemia-Reperfusion Injury by Inducing the Nrf2
Protective Pathway. Antioxid Redox Signal. 27:754–768.
2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Siegel D, Gustafson DL, Dehn DL, Han JY,
Boonchoong P, Berliner LJ and Ross D: NAD(P)H:quinone
oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol.
65:1238–1247. 2004.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Qin S, Chen J, Tanigawa S and Hou DX: Gene
expression profiling and pathway network analysis of hepatic
metabolic enzymes targeted by baicalein. J Ethnopharmacol.
140:131–140. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shi L, Hao Z, Zhang S, Wei M, Lu B, Wang Z
and Ji L: Baicalein and baicalin alleviate acetaminophen-induced
liver injury by activating Nrf2 antioxidative pathway: The
involvement of ERK1/2 and PKC. Biochem Pharmacol. 150:9–23.
2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kim SJ, Moon YJ and Lee SM: Protective
effects of baicalin against ischemia/reperfusion injury in rat
liver. J Nat Prod. 73:2003–2008. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liang W, Huang X and Chen W: The Effects
of Baicalin and Baicalein on Cerebral Ischemia: A Review. Aging
Dis. 8:850–867. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu H, Ye F, Sun Q, Liang H, Li C, Li S,
Lu R, Huang B, Tan W and Lai L: Scutellaria baicalensis
extract and baicalein inhibit replication of SARS-CoV-2 and its
3C-like protease in vitro. J Enzyme Inhib Med Chem. 36:497–503.
2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hwang S, Lee SG, Ahn CS, Kim KH, Moon DB,
Ha TY, Song GW, Jung DH, Park JI, Ryu JH, et al: Prevention of
hepatitis B recurrence after living donor liver transplantation:
Primary high-dose hepatitis B immunoglobulin monotherapy and rescue
antiviral therapy. Liver Transpl. 14:770–778. 2008.PubMed/NCBI View Article : Google Scholar
|