Introduction
Radioactive particle implantation is widely used to
treat various types of tumor, such as lung cancer, thoracic
esophageal squamous cell carcinoma and hepatocellular carcinoma
(1-3),
as the ultrastructure of tumor cells can be ruptured and
disintegrated by X-rays and γ-rays of radioactive particles within
their effective killing radius (4). Low-energy and -dose radioactive
125I seed implantation is a precise radiotherapy
technique used for the treatment of malignant tumors, such as
hepatocellular carcinoma, lung cancer, head and neck squamous cell
carcinoma and pancreatic cancer. These seeds can be implanted
within a close range of the tumor target area with the aid of
computer technology that is used to scan and locate the tumor
(5). The half-life of
125I is 59.6 days, which is beneficial for clinical
applications due to its relatively long half-life and shelf-life
(6). However, there is controversy
surrounding the quantitative evaluation of 125I seed
efficacy, especially in terms of particle activity and the time
within which the particle effect is greatest (7,8). To
the best of our knowledge, there are few quantitative evaluations
that have investigated the therapeutic effect of 125I in
precise clinical treatment.
Numerous studies examining the efficacy of
125I seed implantation have been conducted under
positron emission tomography (PET)/computed tomography (CT)
guidance (9-11);
PET/CT images accurately distinguish between surviving tumor cells
(characterized by high metabolism of fluorodeoxyglucose) and
necrotic areas without metabolism (12).
The standard uptake value (SUV), a mathematically
derived ratio of tissue radioactivity concentration and the
injected dose of radioactivity per kilogram of patient body weight,
is an important semi-quantitative parameter in PET/CT to measure
the response of cancer to treatment (13). The tumor inhibition rate can be
described as the change in tumor length change before and after
treatment (14). Additionally,
this pathological parameter can be evaluated using the ratio of
Bcl-2/Bax expression levels. Apoptosis and anti-apoptosis imbalance
are key processes in tumorigenesis; Bcl-2 serves a role in
inhibiting apoptosis and Bax antagonizes Bcl-2 (15,16).
The expression levels of Bcl-2 are positively correlated with the
degree of tumor malignancy. Therefore, overexpression of Bcl-2 is
an important marker for tumor cell development, such as oral and
maxillofacial squamous cell carcinoma and colorectal cancer
(17,18). The VX2 tumor cell line,
a squamous cell carcinoma derived from rabbit papilloma induced by
the ShPoe virus (19), is similar
to human tumors, such as lung cancer, in terms of biochemical,
biological and morphological characteristics; thus, it has been
widely used in clinical research, such as in imaging diagnostics
and interventional radiology (20,21).
In the present study, a paired VX2 tumor
model in a rabbit hind leg muscle was established. 125I
seeds with 0.4 mCi initial activity were implanted into the left
hind legs and seeds with 0.7 mCi initial activity were implanted
into the right hind legs. PET/CT scans were taken before
125I implantation and 72 h and 2- and 4-weeks
post-implantation. Changes in tumor length, SUV and the ratio of
Bcl-2 to Bax were evaluated. The effectiveness of 125I
seeds with different levels of activity were compared and the
therapeutic effects were observed. The present study aimed to
provide a reference for optimal particle activity and quantitative
evaluation of the therapeutic effects of specific, individualized,
clinical treatment using the radioactive particle
125I.
Materials and methods
Animal model
New Zealand White rabbits (n=30; age, 3-4 months;
weight, 2-3 kg; 15 male and 15 female rabbits) were allowed free
access to food and water and housed individually under conditions
of 18-26˚C, 30-70% relative humidity and a 12/12 h dark/light
cycle. All procedures were provided by the Animal Experimental
Center of Shanxi Cancer Hospital (Taiyuan, China). The
VX2 tumor block was provided by BeNa Culture Collection;
Beijing Beina Chunglian Institute of Biotechnology. The health
states of rabbits were recorded every 2 days following
implantation. No adverse effects were observed in the animals. The
VX2 solid tumor tissues were cut into ~2 mm3
tumor blocks, resuspended in physiological saline and injected into
the bilateral thigh muscles of white rabbits. After 2 weeks, solid
nodules were found at the inoculation site (~20 mm in size),
indicating successful preparation of the rabbit tumor model.
Rabbits were anesthetized with 3% (40 mg/kg) sodium pentobarbital
for 20 min. Rabbits were euthanized by anesthesia followed by
pentobarbital overdose. The experimental process was approved by
the Medical Ethics Committee of the Jincheng Anthracitic Coal
Mining Group General Hospital (Jincheng, China).
18F-fluorodeoxyglucose
(FDG) PET/CT imaging
PET/CT (Biography mCTs; Siemens AG) is a 52-ring,
large aperture scanner. PET was performed using a three-dimensional
(3D) collection mode with a layer thickness of 3.75 mm, a matrix of
128x128 and a collection rate of 3 min/bed. The CT acquisition
conditions were as follows: Voltage, 120 kV; current, 200 mA;
spiral time, 0.8 seconds/circles; bed speed, 22.5 mm/sec and
matrix, 512x512. Image merging and management was performed using
an Xeleris workstation (version 4.1; GE Healthcare).
18F-FDG with a radiochemical purity >95% was provided
by the PET/CT office at the Center of Jincheng Anthracitic Coal
Mining Group General Hospital. VX2 tumor model rabbits
were fasted for 4-6 h and subsequently anesthetized with 3% (40
mg/kg) sodium pentobarbital before imaging. 18F-FDG at
0.5 mCi/Kg was injected into the ear vein of the rabbits; PET/CT
scanning was performed after 60 min. The tumor position was
determined by PET/CT imaging and 125I seeds (0.4 mCi or
0.7 mCi) were implanted at the tumor sites. PET/CT scans were
performed again at 72 h and 2 and 4 weeks to obtain the tumor
index, which included SUV and tumor length.
Particle implantation
The rabbits were implanted with 0.4 mCi
125I seeds into the left hind leg, while 0.7 mCi seeds
were implanted into the right hind leg. The implanting doses were
calculated using the Radiation Therapy Planning System (cat. no.
KL-SIRPS-3D-800; Beijing ASTRO Technology Development Co.,
Ltd.).
Tumor inhibition rate assessment
The standard uptake values were measured using the
Ellipsoid Isocontour 3D measurement method featured in the PET/CT
software (PETViewer 2.0; Informer Technologies, Inc.), and the
tumor lengths were measured on PET images. SUV=2.5 in the target
area of the PET/CT image was set as the background deduction
parameter to outline the region of interest (ROI). The ROI peak (1
cm3) was measured. SUV variation was calculated as
follows: ΔSUV=(SUVinitial
-SUVfinal)/SUVinitial x100%. Tumor inhibition
rate was calculated as follows: (Tumor
lengthinitial-tumor lengthfinal)/tumor
lengthinitial x100%.
Pathological observation
Each rabbit was sacrificed by pentobarbital overdose
(injection of 100 mg/kg sodium pentobarbital) and two tumors in
total, one from the left and one from the right leg, were
harvested. The tumor tissue samples were selected from 5-10 mm
around the 125I seed region and obtained by tumor
puncture. The removed tissue samples were fixed overnight in 10%
neutral buffered formalin, embedded in paraffin, cut into
5-µm-thick sections, and placed on Superfrost Plus slides (Thermo
Fisher Scientific, Inc.). Sections were subjected to hematoxylin
staining for 5 min and eosin staining for 2 min at 20˚C. For
immunohistochemistry, deparaffinized and rehydrated slides using
xylene and ethanol gradient, respectively, were subjected to
antigen retrieval via autoclaving in a 10 mM citric acid buffer (pH
6.0). Upon cooling to room temperature for 30 min slides were
blocked with 0.3% H2O2 for 20 min, washed in
PBS, and then blocked with 1% BSA in PBS at 25˚C. Slides were
incubated with diluted primary antibodies Bax (1:200; cat. no.
EL600961-100; EterLife) and Bcl-2 (1:150; cat. no. EL600995-100;
EterLife) overnight at 4˚C, and were subsequently incubated with
diluted HRP-conjugated secondary goat anti-rabbit IgG antibody for
2 h at 37˚C (1:500; cat. no. EL990003; EterLife). The expression
levels of apoptosis-associated proteins Bcl-2 and Bax were detected
based on the histology slide. The positive expression of Bcl-2 and
Bax was identified as the precipitation of brown-yellow particles
by IHC staining in the cytoplasm. A total of six high-power fields
(magnification, x400) were randomly selected from each section and
200 cells were manually counted using a cell counter in each field
to evaluate the percentage of tumor-positive cells. The expression
intensities of Bcl-2 and Bax were measured according to the
tumor-positive cells in the field of view and the ratio of Bcl-2
and Bax (Bcl-2/Bax) was evaluated.
Statistical analysis
The statistical analysis was conducted using SPSS
13.0 software (SPSS, Inc.). Data are presented as the mean ± SD of
three experimental repeats. Unpaired student's t-test was performed
to compare tumor indexes, including tumor length, SUV and Bcl-2 and
Bax expression in the left and right legs, before 125I
implantation. Spearman's correlation coefficients were calculated
to evaluate the association between the average tumor length,
Bcl-2, Bax and SUV. P<0.05 was considered to indicate a
statistically significant difference. Following 125I
implantation, the association between ΔSUV, tumor inhibition rate
and Bcl-2/Bax in the 0.4 and 0.7 mCi 125I groups was
analyzed by IHC staining.
Results
Pathological observation
Following 125I seed implantation, tumor
cell proliferation, according to growth speed records (time taken
by the cells to cover the entire culture dish, examined via
semi-quantitative analysis; data not shown), decreased, necrotic
areas appeared, peripheral fibrous connective tissue was destroyed
and tumor angiogenesis decreased. PET/CT scans (Fig. 1A) showed that the metabolic rate
and volume of tumor tissue decreased over time. Following
125I seed implantation, liquefactive necrotic areas
gradually appeared in the center of the tumor tissue (showing no
metabolic signals) and the necrotic area was notably large
(Fig. 1B and C).
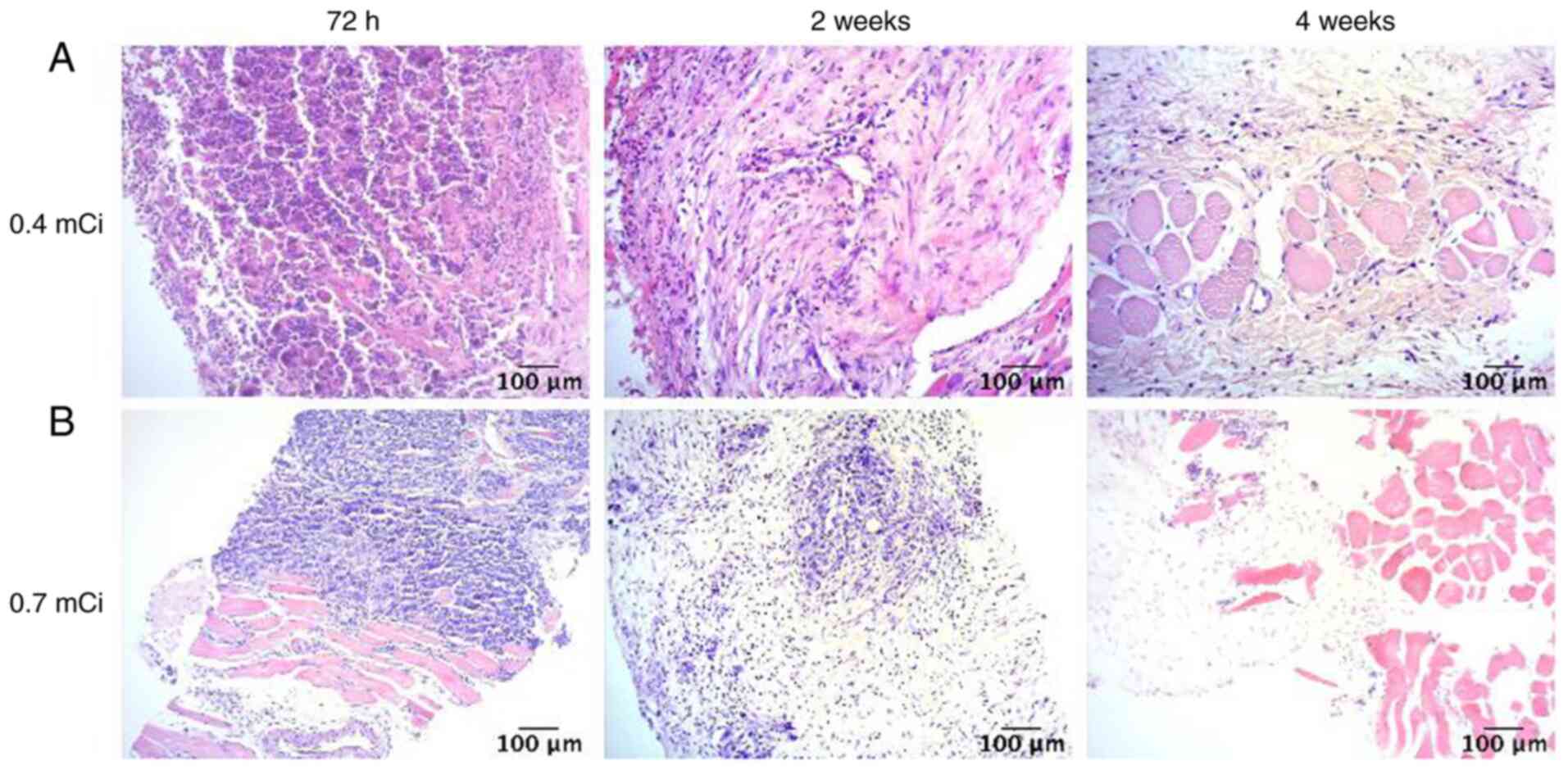
The results of HE staining (Fig. 2) showed that, following
125I seed implantation, the tumor tissue demonstrated
notable liquefaction of the necrotic areas. Furthermore, the number
of tumor cells notably decreased and the surrounding fibrous
connective tissue was extensively destroyed. Compared with the 0.4
mCi group, the proliferation signal of tumor cells in the 0.7 mCi
group decreased more notably, which indicated that there were fewer
tumor cells following implantation of 0.7 mCi 125I.
Tumor index results
Bcl-2 and Bax expression levels were observed via
immunohistochemistry (Fig. 3). The
expression intensity of Bcl-2 decreased over time, while the
expression intensity of Bax increased, which indicated that
apoptosis increased during treatment.
Fig. 4 shows the
statistical tumor index results, including the mean ± SD tumor
length, SUV and Bcl-2 and Bax intensity before and after
125I implantation. Before treatment, t-test showed no
significant difference in the distribution of the tumor length and
SUV in the left and right legs. Following treatment, the mean tumor
length and SUV in both groups decreased. At 2 and 4 weeks, SUV was
significantly decreased. For both groups, a significant decrease in
the average SUV was observed at 2 weeks after treatment, while it
was more pronounced in the 0.7 mCi group (Fig. 4B). Following implantation, Bcl-2
expression decreased and Bax expression increased (Fig. 4C and D). In addition, the decrease of Bcl-2 in
the 0.7 mCi group was larger than that in the 0.4 mCi group. Bcl-2
expression continuously decreased over 4 weeks. By contrast, the
increase of Bax in the 0.7 mCi group was higher than that of the
0.4 mCi group. These quantitative data demonstrated that the
treatment was effective and that 0.7 mCi 125I seeds were
more effective as a tumor therapy compared with 0.4 mCi seeds.
Fig. 5 presents the
association between variations in tumor indexes, including ΔSUV,
tumor inhibition rate and Bcl-2/Bax at different time points
following 125I implantation. At 72 h, ΔSUV, tumor
inhibition rate and Bcl-2/Bax data were relatively dispersed. The
data became concentrated as the time elapsed from 2 to 4 weeks.
These results indicated that the effect of each implantation varied
greatly at the beginning but after 4 weeks, all 30 rabbits
demonstrated similar treatment effects. Meanwhile, in the 0.7 mCi
group, each tumor index indicator showed a better distribution
(smaller intragroup differences) in data analysis, which also
indicated that 0.7 mCi 125I seeds held greater
therapeutic potential in terms of treatment effect.
Discussion
Since the 1960s, research on permanent intertissue
implantation of radioactive particles for the treatment of
malignant tumors has demonstrated notable advancements (22,23).
However, there is currently no uniform dose standard for
125I seeds, which is the most widely used therapeutic
radioactive particle for permanent intertissue implantation in
clinical practice, particularly for solid tumor therapy. Its
half-life is 59.49 days and it decays by electron capture to an
excited state of 125Te. This state is not the metastable
125mTe, but rather a lower energy state that decays
immediately by gamma decay with a maximum energy of 35 keV. The
state of low energy makes it possible for tumor treatment for its
appropriate effects, such as fewer side effects and more treatment
effects (22,23). To determine the optimal standard
dose of 125I seeds for solid tumor therapy and provide a
theoretical basis for the optimal clinical application of
radioactive particles such as 125I seeds, the present
study used New Zealand White rabbits to construct a VX2
solid tumor xenograft model in the hind leg. Theoretically,
125I seeds do not affect the SUV of PET/CT, because they
have distinct mechanisms. PET provides detailed information about
the function and metabolism of the tumor lesion. PET/CT
image-guided surgery utilizes 18F-FDG to monitor the
biochemical activity of the tumor (24,25).
125I seeds at 0.4 mCi were implanted into the left hind
leg and 125I seeds at 0.7 mCi into the right hind leg of
each rabbit. Changes in tumor length, SUV and the ratio of
Bcl-2/Bax were compared before and after 125I seed
implantation by PET/CT scans to assess the therapeutic effect of
125I seeds on solid tumors. Using comparative analysis,
it was shown that, compared with 125I seeds at 0.4 mCi
activities, treating solid tumors with 125I seeds with
0.7 mCi activity exhibited a more obvious therapeutic effect, as
demonstrated by decreased tumor length, SUV and Bcl-2 expression
and increased Bax following treatment. 125I seeds at 0.7
mCi activity resulted in a more consistent treatment effect, which
indicated that 125I seeds at 0.7 mCi may be more
beneficial in clinical applications.
There is controversy surrounding the effect of
125I seed implantation at different activity levels and
durations for solid tumor treatment, for which there is no unified
standard for comparative analysis. To the best of our knowledge,
there are few literature reports on the safety of 125I
seeds at different activity levels in the treatment of solid
tumors. Therefore, upper and lower prescription doses have not been
established for solid tumor treatment using radioactive
125I seeds in terms of interstitial implantation.
Several studies have reported that different doses
of 125I seeds implanted into tissue have different
effects on tumor eradication (26-28).
In the present study, 125I seeds with 0.7 mCi activity
caused tumors to shrink in a short period of time, while
125I seeds with 0.4 mCi activity did not completely
inhibit tumor growth.
With respect to the comprehensive and periodic
treatment of solid tumors, the selection of 125I seeds
with 0.7 mCi activity is more consistent with results previously
reported by Wang et al (29), who found that low-activity
particles had a lower dose inhibition rate for tumor tissues
compared with high-activity particles, and that the radiation
released by the particles decayed rapidly with increasing distance.
Thus, low-activity radioactive particles do not completely inhibit
the proliferation of tumor tissue, unless very high doses are
selected in clinical practice. However, increasing the dosage of
low-activity particles in the treatment of solid tumors results in
greater potential for tumor radiotherapy side effects, such as the
damage of normal tissues (30).
Pan et al found that 32P-CP-PLLA particles
significantly inhibit the glucose metabolism of VX2
tumors, thereby promoting tumor cell apoptosis. They also confirmed
the association between tumor SUV metabolism and radioactive
particle dose (31). The present
results showed that most tumor cells were necrotic after using
125I seeds to treat VX2 tumors; there was no
metabolic signal in the treatment area and the number of active
tumor cells decreased. Following treatment of VX2 solid
tumor xenografts with 125I seeds for 72 h, PET/CT (which
can detect morphological changes of VX2 solid tumors)
was used to evaluate the therapeutic effect of 125I
seeds on solid tumors. Through comparative analysis of changes in
tumor length, SUV and the expression of molecules associated with
tumor metabolism, such as Bcl-2 and Bax, the present study
demonstrated that the therapeutic effect of 0.7 mCi 125I
seeds was better than that of 0.4 mCi 125I seeds.
Through immunohistochemical staining and
pathological observation, the present study showed that
125I radioactive seeds participate in the apoptosis of
tumor cells by regulating expression of tumor metabolism-associated
molecules, such as Bcl-2 and Bax, to inhibit solid tumor growth.
The ratio of Bcl-2/Bax expression was notably downregulated
following treatment. More importantly, there was a correlation
between decreased Bcl-2/Bax expression content ratio and activity
of radioactive 125I seeds, indicating that, compared
with 0.4 mCi 125I seeds, 0.7 mCi 125I seeds
exhibited a better therapeutic effect on solid tumors. The present
study observed the short-term therapeutic effects of 0.4 mCi and
0.7 mCi 125I radioactive seeds VX2 solid
tumors. Additionally, there were no adverse effects in either
treatment group. This is consistent with studies that 0.7 mCi seed
activity is commonly used and dosage as high as 0.8-2.5 mCi is safe
(32-37).
The present study had certain limitations. First,
this was a paired comparison and further studies should compare
differences between groups using more samples. Experiments should
be performed using the left leg as a control with 0 mCi seeds and
implanting the right leg with 0.7 or 0.4 mCi 125I seeds
to demonstrate the therapeutic effect of each activity level. The
4-week therapeutic effects of 125I seeds on solid tumors
are still unknown. In addition, the small number of experimental
groups, as well as the small sample size, may lead to biased
conclusions. In future, the 4-week therapeutic effects of
125I seeds of different activity levels in the treatment
process of solid tumors should be studied. Future investigations
should increase the number of experimental groups and sample size
to generate high-quality research data.
In conclusion, low-activity 125I
implantation was effective for VX2 tumor treatment and
0.7 mCi 125I seeds may be more suitable in the clinic
than 0.4 mCi seeds.
Acknowledgements
Not applicable.
Funding
The present study was supported by Scientific Research Projects
of Shanxi Health Commission (grant no. 2018139).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY, ZW, JW, FW, QF and RZ participated in study
conception and design, performed the experiments and
analyzed/interpreted data. ZW, JW and RZ confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by Jincheng
Anthracitic Coal Mining Group General Hospital (approval no.
2018139; Jincheng, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheng J, Ma S, Yang G, Wang L and Hou W:
The mechanism of computed tomography-guided 125I particle in
treating lung cancer. Med Sci Monit. 23:292–299. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lin L, Wang J, Jiang Y, Meng N, Tian S,
Yang R, Ran W and Liu C: Interstitial 125I seed implantation for
cervical lymph node recurrence after multimodal treatment of
thoracic esophageal squamous cell carcinoma. Technol Cancer Res
Treat. 14:201–207. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gaopeng L, Shengli D, Ye LU, Miaoyun L,
Jian G and Qibin T: The cooperative effect of p53 and Rb in local
nanotherapy in a rabbit VX2 model of hepatocellular carcinoma. Int
J Nanomedicine. 8:3757–3768. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Leth T, Von Oettingen G, Lassen-Ramshad
YA, Lukacova S and HøYer M: Survival and prognostic factors in
patients treated with stereotactic radiotherapy for brain
metastases. Acta Oncologica. 54:107–114. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Quintás-Cardama A, Daver N, Kim H, Dinardo
C, Jabbour E, Kadia T, Borthakur G, Pierce S, Shan J,
Cardenas-Turanzas M, et al: A prognostic model of therapy-related
myelodysplastic syndrome for predicting survival and transformation
to acute myeloid leukemia. Clin Lymphoma Myeloma Leukemia.
14:401–410. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Třeška V, Duras P, Mírka H, Skalický T and
Sutnar A: [Chemoembolization with Drug Eluting Beads (TACE DEB) in
patients with primary unresectable hepatocellular carcinoma (HCC)].
Rozhledy v chirurgii: měsíník eskoslovenské chirurgické spolenosti.
93:63–69. 2014.PubMed/NCBI
|
|
7
|
Hutchings M: FDG-PET response-adapted
therapy: Is 18F-fluorodeoxyglucose positron emission tomography a
safe predictor for a change of therapy? Hematol Oncol Clin North
Am. 28:87–103. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lim AJ, Brandon AH, Fiedler J, Brickman
AL, Boyer CI, Raub WA Jr and Soloway MS: Quality of life: Radical
prostatectomy versus radiation therapy for prostate cancer. J Urol.
154:1420–1425. 1995.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gao F, Li C, Gu Y, Huang J and Wu P:
CT-guided 125I brachytherapy for mediastinal metastatic lymph nodes
recurrence from esophageal carcinoma: Effectiveness and safety in
16 patients. Eur J Radiol. 82:e70–e75. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nakamura R, Kikuchi K, Tanji S, Yabuuchi
T, Uwano I, Yamaguchi S, Ariga H and Fujioka T: Narrow safety range
of intraoperative rectal irradiation exposure volume for avoiding
bleeding after seed implant brachytherapy. Radiat Oncol.
7(15)2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang F, Wu K, Gao F, Zhang W, Shi F and
Li C: Refractory nasopharyngeal carcinoma: Positron emission
tomography combined with computed tomography-guided 125I seed
implantation therapy after repeated traditional radiochemotherapy.
Otolaryngol Head Neck Surg. 149:417–423. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li C, Yao L, Gong J, Pang H, Shan Q and
Wang Z, Lu J and Wang Z: Efficacy of gefitinib combined with
125I radioactive particles in the treatment of
transplanted lung cancer tumors in nude mice. Cardiovasc Intervent
Radiol. 43:1364–1370. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Toba H, Sakiyama S, Otsuka H, Kawakami Y,
Takizawa H, Kenzaki K, Kondo K and Tangoku A:
18F-fluorodeoxyglucose positron emission tomography/computed
tomography is useful in postoperative follow-up of asymptomatic
non-small-cell lung cancer patients. Interact Cardiovasc Thorac
Surg. 15:859–864. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zou JF: Effect of 125I radioactive
particle implantation combined with TACE treatment on serum markers
apoptotic molecules in tumor tissue of patients with primary
hepatocellular carcinoma. J Hainan Medical University. 22:101–104.
2016.
|
|
15
|
Lu KV, Chang JP, Parachoniak CA, Pandika
MM, Aghi MK, Meyronet D, Isachenko N, Fouse SD, Phillips JJ,
Cheresh DA, et al: VEGF inhibits tumor cell invasion and
mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell.
22:21–35. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aggarwal S, Devaraja K, Sharma SC and Das
SN: Expression of vascular endothelial growth factor (VEGF) in
patients with oral squamous cell carcinoma and its clinical
significance. Clinica Chimica Acta. 436:35–40. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li M, Wang Z, Xing Y, Yu J, Tian L, Zhang
D and Xin Z: A multicenter study on expressions of vascular
endothelial growth factor, matrix metallopeptidase-9 and tissue
inhibitor of metalloproteinase-2 in oral and maxillofacial squamous
cell carcinoma. Iran Red Crescent Med J. 16(e13185)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Diaz-Rubio E, Gomez-Espana A, Massuti B,
Sastre J, Abad A, Valladares M, Rivera F, Safont MJ, Martínez de
Prado P, Gallén M, et al: First-line XELOX plus bevacizumab
followed by XELOX plus bevacizumab or single-agent bevacizumab as
maintenance therapy in patients with metastatic colorectal cancer:
The phase III MACRO TTD study. Oncologist. 17:15–25.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Verma A, Um SW, Koh WJ, Suh GY, Chung MP,
Kwon OJ and Kim H: Long-term tolerance of airway silicone stent in
patients with post-tuberculosis tracheobronchial stenosis. ASAIO J.
58:530–534. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dedic-Hagan J, Teh AY, Liang E, Collett N
and Woo HH: Migration of a strand of four seeds in low-dose-rate
brachytherapy. BMJ Case Rep: May 30, 2014 (Epub ahead of print).
doi: 10.1136/bcr-2014-204515.
|
|
21
|
Shin DY, Han SW, Oh DY, Im SA, Kim TY and
Bang YJ: Prognostic implication of (18)F FDG-PET in patients with
extrahepatic metastatic hepatocellular carcinoma undergoing
systemic treatment, a retrospective cohort study. Cancer Chemother
Pharmacol. 68:165–175. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Salem R, Gordon AC, Mouli S, Hickey R,
Kallini J, Gabr A, Mulcahy MF, Baker T, Abecassis M, Miller FH, et
al: Y90 radioembolization significantly prolongs time to
progression compared with chemoembolization in patients with
hepatocellular carcinoma. Gastroenterology. 151:1155–1163.e2.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Assi R, Kantarjian H, Ravandi F and Daver
N: Immune therapies in acute myeloid leukemia: A focus on
monoclonal antibodies and immune checkpoint inhibitors. Curr Opin
Hematol. 25:136–145. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Caresia Aroztegui AP, Garcia Vicente AM,
Alvarez Ruiz S, Delgado Bolton RC, Orcajo Rincon J, Garcia Garzon
JR, de Arcocha Torres M and Garcia-Velloso MJ: Oncology Task Force
of the Spanish Society of Nuclear Medicine and Molecular Imaging:
18F-FDG PET/CT in breast cancer: Evidence-based recommendations in
initial staging. Tumour Biol: Oct 12, 2017 (Epub ahead of print).
doi: 10.1177/1010428317728285.
|
|
25
|
Dietlein M, Klussmann JP and Drzezga A:
18F-FDG PET/CT in head and neck tumors. Nuklearmedizin. 59:8–11.
2020.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
26
|
Wang H, Peng R, Li X, Wang Y, Jiang Y, Ji
Z, Guo F, Tian S, Sun H, Fan J and Wang J: The dosimetry evaluation
of 3D printing non-coplanar template-assisted CT-guided 125I seed
stereotactic ablation brachytherapy for pelvic recurrent rectal
cancer after external beam radiotherapy. J Radiat Res. 62:473–482.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Qu A, Jiang P, Wei S, Jiang Y, Ji Z, Sun
H, Li W, Shao Y, Fan J and Wang J: Accuracy and dosimetric
parameters comparison of 3D-printed non-coplanar template-assisted
computed tomography-guided iodine-125 seed ablative brachytherapy
in pelvic lateral recurrence of gynecological carcinomas. J Contemp
Brachytherapy. 13:39–45. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li J, Wang J, Meng N, Qu A, Yuan H, Liu C,
Ran W and Jiang Y: Image-guided percutaneous (125)I seed
implantation as a salvage treatment for recurrent soft tissue
sarcomas after surgery and radiotherapy. Cancer Biother Radiopharm.
26:113–120. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang W, Qin H, Zhu X, et al: The study of
different activity ~(125)I seeds implantation to therapy rabbit
liver VX2 tumor. Journal of Anhui Medical University. 50:778–781.
2015.(In Chinese).
|
|
30
|
Bradley JD, Paulus R, Komaki R, Masters G,
Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A,
et al: Standard-dose versus high-dose conformal radiotherapy with
concurrent and consolidation carboplatin plus paclitaxel with and
without cetuximab for patients with stage IIIA and IIIB
non-small-cell lung cancer (RTOG 0617): A randomized, two-by-two
factorial phase 3 study. Lancet Oncol. 16:187–199. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pan D, Yang M, Xu Y, Wang L, Liu L and
Huang P: Experimental study of CT guided ³²P-CP-PLLA microparticle
implantation in the treatment of rabbit VX2 lung tumor. Zhongguo
Fei Ai Za Zhi. 14:1–6. 2011.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
32
|
He X, Liu M, Zhang M, Sequeiros RB, Xu Y,
Wang L, Liu C, Wang Q, Zhang K and Li C: A novel three-dimensional
template combined with MR-guided (125)I brachytherapy for recurrent
glioblastoma. Radiat Oncol. 15(146)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li CC, Chi JL, Ma Y, Li JH, Xia CQ, Li L,
Chen Z and Chen XL: Interventional therapy for human breast cancer
in nude mice with 131I gelatin microspheres (¹³¹I-GMSs) following
intratumoral injection. Radiat Oncol. 9(144)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao GS, Liu S, Yang L, Li C, Wang RY,
Zhou J and Zhang YW: Evaluation of radioactive (125)I seed
implantation for the treatment of refractory malignant tumours
based on a CT-guided 3D template-assisted technique: Efficacy and
safety. BMC Cancer. 20(718)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yao LH, Su L, Liu L, Sun HT and Wang JJ:
Stenting of the portal vein combined with different numbers of
Iodine-125 seed strands: Dosimetric analyses. Chin Med J (Engl).
130:2183–2189. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Huo X, Huo B, Wang H, Wang L, Cao Q, Zheng
G, Wang J, Chai S, Zhang Z, Yang K, et al: Implantation of computed
tomography-guided Iodine-125 seeds in combination with chemotherapy
for the treatment of stage III non-small cell lung cancer. J
Contemp Brachytherapy. 9:527–534. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yang Z, Zhang Y, Xu D, Maccauro G, Rossi
B, Jiang H, Wang J, Sun H, Xu L, Chen Y and Liu X: Percutaneous
vertebroplasty combined with interstitial implantation of 125I
seeds in banna mini-pigs. World J Surg Oncol. 11(46)2013.PubMed/NCBI View Article : Google Scholar
|