Introduction
Traumatic brain injury (TBI) has been recognized as
a serious public health issue and a key contributor to disability
and death, with a huge economic burden worldwide (1,2).
High incidence (1/1,000 in China between 1983 and 1985) of TBI is
predominantly reported in low- and middle-income countries as well
as developing countries, including China and Iran (1-3).
The prevalence of TBI has witnessed a rapidly growing trend due to
the considerable increase in road accidents, such as motor vehicle
collisions (2). Although an
increasing number of randomized controlled trials have included
intracranial pressure monitoring, therapeutic hypothermia, surgical
methods and drug administration in recent years, long-term outcomes
have not substantially improved, especially after drug intervention
(2-8).
Therefore, it is important to further clarify the
physiopathological processes of TBI and identify novel efficient
pharmacological targets for TBI treatment. It is widely
acknowledged that the pathophysiology of TBI encompasses several
types of pathological and physiological changes, mainly involving
primary brain injury and secondary brain injury, which leads to
neuronal death, neurological deficits and mortality after TBI
(9). Primary brain injury, which
is a direct physical injury to the brain tissue, is difficult to
prevent and cannot be usually reversed, and leads to brain tissue
disorganization, intracerebral hemorrhage and blood-brain barrier
(BBB) damage (1,10,11).
Secondary brain injury includes calcium overload, oxidative stress,
neuroinflammation, autophagy, lipid peroxidation, apoptosis and
necroptosis, and can be reversed (10,11).
Necroptosis has recently been identified as a
pathway of modulated necrosis and a mechanism of
caspase-independent programmed cell death, requiring the proteins
mixed lineage kinase domain-like (MLKL) and receptor-interacting
protein kinase-3 (RIP3), and is triggered by death receptors
(12). Accumulating evidence
indicates that necroptosis performs an important function in
central nervous system disorders, such as TBI (13-15),
intracerebral hemorrhage (16,17),
ischemic stroke (18), Alzheimer's
disease, Parkinson's disease and amyotrophic lateral sclerosis
(19). The activation of a TNF
ligand family member, such as RIP1 and MLKL, is the most upstream
signaling activity necessary for necroptosis induction (20,21).
The activation of RIP1 contributes to necroptosis via inducting
RIP1-RIP3-MLKL complex (22).
Necroptosis is common in early brain injury (EBI) and may be a
mechanism of TBI.
In previous years, it has become widely acknowledged
that hydrogen-rich saline or hydrogen gas can protect the human
body from numerous diseases, such as neurodegenerative disorders,
spontaneous subarachnoid hemorrhage, stroke and
ischemia-reperfusion damage, by modulating neuronal apoptosis,
inflammatory response and oxidative stress (23-26).
A previous study has indicated that hydrogen may selectively
minimize peroxynitrites and hydroxyl radicals, and subsequently
have a crucial function exhibiting cytoprotective,
anti-inflammatory, anti-apoptotic and antioxidant properties
(27). However, the
neuroprotective benefits of hydrogen-rich saline treatment on TBI
are controversial. Heme oxygenase-1 (HO-1) is an essential element
of the cellular defense system that is activated by
oxidant-stimulated damage and acts against it (28,29).
In the central nervous system (CNS), HO-1 exerts anti-necroptotic,
anti-neuroinflammatory and neuroprotective functions (27,30).
In our previous study, HO-1 was demonstrated to regulate neuronal
death in acute CNS disease (31).
Therefore, treatments that target HO-1 may be potentially effective
at preventing necroptosis, oxidative stress and inflammation
following TBI. Nonetheless, the specific mechanisms regarding the
potential neuroprotective benefits of hydrogen-rich saline
treatment are unknown. The present study examined whether
neuroprotective benefits of hydrogen-rich saline treatment in a
mouse model of TBI occur via effects on neuroinflammation and
necroptosis, and whether neuroprotection is dependent on the
reactive oxygen species (ROS)/HO-1 pathway.
Materials and methods
Animals
All the animal experiments conducted in the present
study were completed in compliance with guidelines for the
appropriate handling of laboratory animals formulated by the
National Institutes of Health (32). Approval of the experiments was
provided by the Ethics Committee of Wuxi Clinical College of Anhui
Medical University (Wuxi, China). A total of 36 healthy adult male
C57BL/6J mice (age, 8-10 weeks; Anhui Medical University, Hefei,
China) weighing between 22-25 g were used when conducting all the
experiments for the current study. The mice were maintained in
animal care facilities (temperature, 25±2˚C; humidity, 55±5%) with
a 12/12 h dark/light cycle and an unrestricted supply of water and
food.
Animal TBI model
The Feeney weight-drop model of focal injury was
strictly followed when developing the TBI model (33,34).
Briefly, anesthetization of the mice was performed using 1% sodium
pentobarbital (40 mg/kg) injected intraperitoneally. The mice were
subsequently placed in a brain stereotaxic apparatus. While being
operated, a heating pad was utilized to ensure that the rectal
temperature remained at 37±0.5˚C. The coordinates utilized when
making a burr hole in the left hemisphere were set as follows: 0.2
mm posterior, 1 mm lateral and 2.2 mm ventral of bregma's
horizontal plane. To visualize the dura mater, the bone fap was
removed. The dura was subsequently placed under weight-drop
equipment that included an impact sensor pedal; (Anhui Zhenghua
Biological Instrument Equipment Co., Ltd.). Metal with a tip
diameter of 3-mm and weight of 240 g was dropped onto the dura
mater from a distance of 1 cm above the dura via a catheter. The
scalp was subsequently closed and the mice were removed from the
stereotaxic apparatus. Subsequently, a medical bone wax was
utilized to cover the hole. The animals in the sham cohort received
comparable surgical treatments but without the weight-drop
procedure. At 72 h following TBI, the mice were sacrificed with 100
mg/kg sodium pentobarbital via an intraperitoneal injection. Brain
tissue samples were collected after the mice were sacrificed. Fresh
specimens (cerebral cortex) and serum were stored in liquid
nitrogen (-196˚C) or hippocampus was stored in 4% formalin-fixed
(4˚C) for ≥48 h.
Drug preparation and
administration
After the TBI model was established successfully,
the mice received intraperitoneal injections every day for 72 h
that contained either plain saline (control) or hydrogen-rich (5
ml/kg; experimental). Hydrogen-rich saline was prepared in
accordance with the procedure described in previous studies
(35,36). Briefly, purified hydrogen was
dissolved in normal saline for 2 h at an elevated pressure of 0.4
MPa. The physiological concentration was maintained at 1.73 ml
hydrogen per 100 ml saline (average, >6 mmol/l). Hydrogen-rich
saline was stored at 4˚C in an aluminum bag without dead volume
under atmospheric pressure. Every week, fresh hydrogen-rich saline
was synthesized to guarantee a consistent concentration. The
content of hydrogen in saline was evaluated and detected by gas
chromatography as reported in a previous study (37).
Neurobehavioral assessment
By employing a previously published neurological
grading system (38,39), the degree of brain injury was
assessed 72 h following TBI via the determination of neurological
function. The scoring system consisted of reflex, sensory, balance
and motor tests. The neurological scores ranged between 0 and 18
and were calculated by summing the individual scores (Table SI). A behavioral assessment was
conducted on all mice in each cohort, with a higher score
indicating impaired neurological function.
Brain water-content measurement
As reported previously, the degree of brain edema
was examined by quantifying the brain water content utilizing the
standard wet-dry technique (39-41).
A total of 72 h following TBI the mice were euthanized, and their
whole brain was extracted and divided into the contralateral and
ipsilateral cortex, contralateral and ipsilateral basal ganglia and
cerebellum (wet weight). The dry weight of each cohort's brain
specimen was then determined after dehydrating each part at 105˚C
for 24 h. The proportion of brain water content was equal to (wet
weight-dry weight)/wet weight x100%.
Cytokine measurements
ELISA was utilized to measure cerebral cortex NF-κΒ
(cat. no. ab176663), cerebral cortex TNF-α (cat. no. ab208348),
cerebral cortex IL-6 (cat. no. ab222503) and cerebral cortex IL-1β
(cat. no. ab197742; all from Abcam). The cerebral cortex was
extracted from mice brain. The procedure was conducted in
accordance with the manufacturer's protocols.
Analysis of ROS
The brain tissue intracellular ROS synthesis was
evaluated utilizing the non-fluorescent diacetylated
2',7'-dichlorofluorescein diacetate (DCFH-DA) probe (Sigma-Aldrich;
Merck KGaA) that becomes highly fluorescent upon oxidation and was
used according to the guidelines provided by the manufacturer.
Fresh cerebral cortex was used to form a single cell suspension.
The cells were harvested at a concentration of 2x106
cells/ml, then DCFH-DA (10 µM final concentration) was added and
the mixture was incubated at 37˚C for 15 min. The DCFH results were
examined via flow cytometry (CytoFLEX; Beckman Coulter, Inc.).
CytExpert (version 2.4; Beckman Coulter, Inc.) was used to analyze
the data.
Analysis of MDA, superoxide dismutase
(SOD) and glutathione (GSH)
The malondialdehyde (MDA) Assay Kit
(excitation/emission, 532/553 nm; cat. no. ab118970; Abcam) was
utilized to detect serum MDA levels in compliance with the
instructions provided by the manufacturer. The serum was extracted
from mice venous blood. The SOD Assay Kit (cat. no. A001-3-2;
Nanjing Jiancheng Bioengineering Institute) and GSH (42) Assay Kit (cat. no. A005-1-2; Nanjing
Jiancheng Bioengineering Institute) were utilized to detect serum
SOD and serum GSH levels in compliance with the manufacturer's
instructions (43,44).
TUNEL staining
To evaluate neuronal death in the brain cortex,
TUNEL staining was utilized. The procedure was performed with a
TUNEL staining kit (cat. no. 11684817910; Roche Diagnostics GmbH)
according to the protocols provided by the manufacturer. In each 4%
formalin-fixed (4˚C; duration, ≥48 h) for specimen,
paraffin-embedded sections (10-µm) were cut from formalin-fixed
tissue. Subsequently, 50 µl TUNEL reaction mixture was added. The
negative control used did not include the TUNEL reaction mixture.
Subsequently, the slides were subjected to incubation in a
humidified dark chamber at a temperature of 37˚C for 60 min. Then,
a primary antibody against the neuronal marker NeuN (1:200; rabbit
polyclonal; cat. no. ab128886; Abcam) diluted in PBS was added,
followed by incubation overnight at 4˚C. Subsequently, incubated
with FITC goat anti-mouse IgG secondary antibodies (1:100; cat. no.
BA1101; Boster Biological Technology Co., Ltd.) at temperature of
37˚C for 1.5 h. Next, the slides were incubated at ambient
temperature for 5 min in the dark with DAPI for staining of the
nuclei, followed by imaging using a fluorescence microscope
(magnification, x200). The validation of the cell count was
performed using four high-power fields randomly selected, and the
data obtained from each field were averaged.
Western blot analysis
Western blot analysis was conducted as indicated
previously (40). Cerebral cortex
specimens were collected and extracted. Cerebral cortex samples
were collected, homogenized and total protein was extracted using
RIPA buffer (CoWin Biosciences). The protein concentrations were
quantified utilizing a BCA Protein Assay kit (Beyotime Institute of
Biotechnology) and proteins (40 µg/lane) were separated using 10%
SDS-PAGE. Then, the proteins were transferred onto Immobilon
nitrocellulose membranes. Blocking of the membranes was performed
with 5% non-fat milk for 1 h at room temperature. The membranes
were then subjected to incubation using the following primary
antibodies overnight at 4˚C: Anti-β-actin (1:1,000; rabbit
polyclonal; cat. no. ab8227; Abcam), anti-RIP1 (1:1,000; rabbit
polyclonal; cat. no. ab106393; Abcam), anti-nuclear factor
erythroid 2-related factor 2 (Nrf2; 1:1,000; rabbit polyclonal;
cat. no. ab31163; Abcam), anti-HO-1 (1:1,000; rabbit polyclonal;
cat. no. ab13243; Abcam) and anti-RIP3 (1:1,000; rabbit polyclonal;
cat. no. ab62344; Abcam). After washing the membranes using 0.5%
TBS-Tween-20 three times, the membranes were incubated with
HRP-conjugated goat anti-rabbit IgG secondary antibodies (1:2,000;
cat. no. 7074s; Cell Signaling Technology, Inc.) at room
temperature for 1.5 h. The signals were developed using an enhanced
chemiluminescence reagent (MilliporeSigma) according to the
manufacturer's instructions. A Bio-Rad imaging system (Bio-Rad
Laboratories, Inc.) was utilized to identify protein bands that
were then measured using ImageJ software (version 1.52; National
Institutes of Health).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
TRIzol® reagent (Thermo Fisher
Scientific, Inc.) was utilized to isolate total RNA from the
cerebral cortex of brain specimens as per the guidelines provided
by the manufacturer, which was then quantified utilizing a
NanoDrop™ 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). The RevertAid First Strand cDNA Synthesis Kit
(cat. no. K1622; Thermo Fisher Scientific Inc.) was subsequently
utilized to perform reverse transcription of RNA to cDNA according
to the manufacturer's protocol. Each specimen's mRNA levels were
quantified via qPCR utilizing SYBR Green Master Mix (Toyobo Life
Science). The expression levels of all genes were normalized to
that of β-actin. The following qPCR thermocycling criteria were
used: 45˚C (2 min) and 95˚C (10 min), followed by 40 cycles of
denaturation at 95˚C (15 sec), annealing at 60˚C (1 min) and
extension at 72˚C (1 min). The analysis of all specimens was
performed in triplicate. The primers used are listed as follows:
Nrf2 forward, 5'-CAGTGCTCCTATGCGTGAA-3' and reverse,
5'-GCGGCTTGAATGTTTGTCT-3'; HO-1 forward,
5'-TGACAGAAGAGGCTAAGACCG-3' and reverse,
5'-AGTGAGGACCCACTGGAGGA-3'; and GAPDH forward,
5'-ATGGGTGTGAACCACGAGA-3' and reverse, 5'-CAGGGATGATGTTCTGGGCA-3'
(45).
Statistical analysis
All experiments were performed with at least three
experimental repeats, and the data are presented as the mean ± SEM.
Neurological scores are presented as the median and interquartile
range. Statistical analyses were implemented using GraphPad Prism 6
(GraphPad Software, Inc.) and SPSS 14.0 (SPSS, Inc.). Differences
between multiple groups were analyzed using one-way ANOVA followed
by Tukey's post hoc test. Neurological scores were analyzed using a
non-parametric test (Kruskal-Wallis followed by Dunn's post hoc
test). P<0.05 was considered to indicate a statistically
significant difference.
Results
Hydrogen-rich saline alleviates
neurological deficits and brain edema after TBI
Modified neurological severity scores were employed
to assess neurological impairments to understand the
neuroprotection of hydrogen-rich saline following TBI. To assess
brain damage, the wet-dry technique was employed to measure brain
water content 72 h after TBI. The findings illustrated that TBI was
associated with a considerable increase in the brain water content,
which was alleviated after hydrogen-rich saline treatment (Fig. 1B). Comparable findings in
neurological scores indicated that the scores were considerably
higher in animals suffering from hydrogen-rich saline treatment
compared with the TBI group, and that hydrogen-rich saline
treatment substantially improved the neurological function
(Fig. 1A).
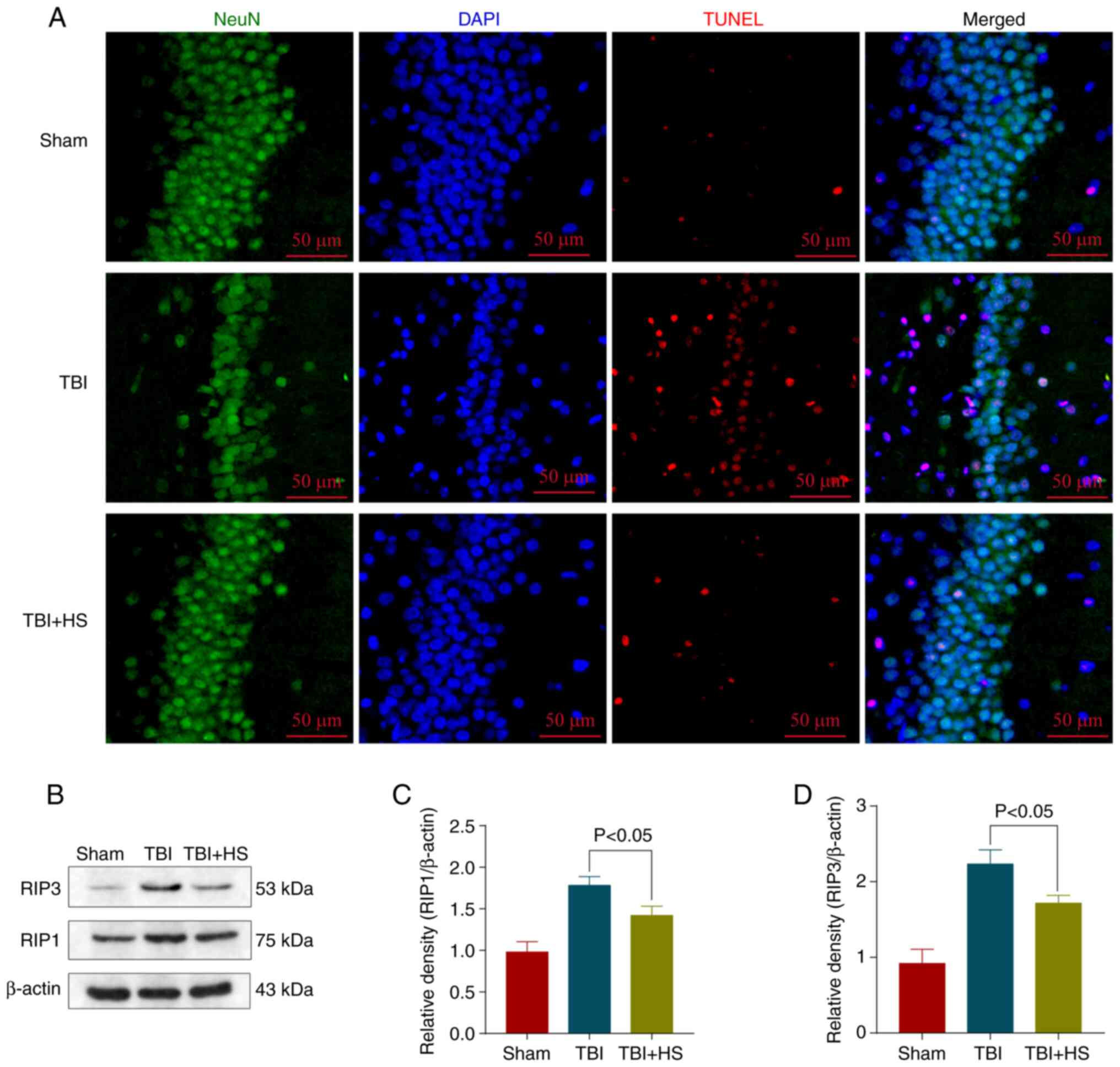
Hydrogen-rich saline alleviates
neuronal necroptosis after TBI
Neuronal necroptosis is the main factor resulting in
EBI after TBI (46). Therefore, a
TUNEL assay was conducted to examine the degree of cell death in
TBI mice treated or non-treated with hydrogen-rich saline 72 h
following model establishment. Neuronal death in the hippocampus
decreased upon hydrogen-rich saline treatment compared with the TBI
group (Fig. 2A). The expression
levels of necroptosis-related proteins were additionally detected
via western blotting (Fig. 2B).
The results of western blotting also demonstrated that
hydrogen-rich saline significantly reduced the expression levels of
the necroptosis-related proteins RIP1 and RIP3 compared with the
TBI group (Fig. 2C and D). These findings indicated that
hydrogen-rich saline exhibited neuroprotective benefits after
TBI.
Hydrogen-rich saline alleviates
neuroinflammation after TBI
Previous studies have demonstrated that
neuroinflammation exhibits a vital function in EBI after TBI and
enhanced neuroinflammation can aggravate EBI (10,47-49).
Activation of the inflammatory process can induce the release of
inflammatory cytokines, which include NF-κB, TNF-α IL-6 and IL-1β
(39,50). Therefore, ELISA was employed to
examine the hippocampal levels of NF-κB, TNF-α IL-6 and IL-1β. The
findings revealed that the inflammatory cytokines were increased
substantially after TBI, while they were significantly decreased
after hydrogen-rich saline treatment (Fig. 3A-D). Thus, these results suggested
that hydrogen-rich saline exhibited a potent anti-inflammatory
activity against TBI-induced neuroinflammation.
Hydrogen-rich saline inhibits
TBI-induced oxidative stress and decreases ROS levels
To clarify whether oxidative stress performs a
crucial function in TBI and whether hydrogen-rich saline can
regulate oxidative stress, the oxidative stress-related biomarker
levels of GSH, MDA and SOD were detected in each cohort. The
findings illustrated that MDA increased following TBI, but
considerably decreased following hydrogen-rich saline treatment
(Fig. 4A). Additionally, SOD and
GSH decreased after TBI but increased considerably following
hydrogen-rich saline treatment (Fig.
4B and C). ROS are considered
to be a biomarker of oxidative stress activation initiating
programmed cell and neuronal death (31,51).
ROS levels were detected using the DCF-DA probe. The results
demonstrated that ROS levels were increased after TBI in the
hippocampus, while decreased after hydrogen-rich saline
administration (Figs. 4D and
S1).
Hydrogen-rich saline regulates
necroptosis via the ROS/HO-1 signaling pathway after TBI
It was explored whether necroptosis inhibition
occurred through the ROS/HO-1 signaling pathway after hydrogen-rich
saline treatment. The mRNA expression levels of HO-1 and Nrf2 were
detected via RT-qPCR. The findings illustrated that the expression
levels of HO-1 and Nrf2 were considerably reduced in the TBI
cohort, and were elevated following hydrogen-rich saline
administration (Fig. 5A and
B). The protein expression levels
of HO-1 and Nrf2 were also detected via western blotting (Fig. 5C). Quantification of the protein
levels of HO-1 and Nrf2 in the brain cortex relatively to the
β-actin loading control revealed that hydrogen-rich saline
increased HO-1 and Nrf2 expression after TBI in mice (Fig. 5D and E). Thus, the present findings
collectively demonstrated that hydrogen-rich saline may inhibit
TBI-induced necroptosis by regulating the ROS/HO-1 signaling
pathway.
Discussion
The present study examined the therapeutic value of
hydrogen-rich saline for ameliorating EBI in a TBI mouse model. The
findings indicated that hydrogen-rich saline was a neuroprotective
agent that can attenuate EBI following TBI. The results
demonstrated that hydrogen-rich saline could achieve the following:
i) Ameliorate neurological dysfunction following TBI; ii) relieve
brain damage in a mouse TBI model; iii) relieve neuroinflammation
after TBI and decrease brain inflammatory damage; and iv) prevent
necroptosis after TBI and alleviate neuronal death. Furthermore,
the anti-neuroinflammatory and anti-necroptotic roles of
hydrogen-rich saline may be associated with the ROS/HO-1
pathway.
Hydrogen-rich saline or hydrogen gas can easily
penetrate the BBB via gaseous diffusion, which is acknowledged to
achieve protective effects in several CNS disorders, such as TBI,
neurodegenerative diseases, intracranial hemorrhage and ischemic
stroke (23-26).
Hydrogen gas or hydrogen-rich saline serves an important
antioxidant role with high tissue transferability, and it has been
demonstrated that H2 is safe for patients and animals
(37). The anti-oxidative stress
and anti-inflammatory effects of hydrogen-rich saline or hydrogen
gas are mediated by selective suppression of toxic ROS, which
include peroxynitrite and hydroxyl radical (25). Liu et al (52) also reported that H2 can
markedly improve cognitive dysfunction and survival rates, decrease
inflammatory response and oxidative stress, and increase
antioxidant enzyme activities in the serum and hippocampus in a
mouse model of sepsis. In the intracerebral hemorrhage model, it
has also been demonstrated that hydrogen performs a neuroprotective
function against EBI following ICH, alleviating brain edema and
neurologic deficits by regulating oxidative stress,
neuroinflammation and apoptosis (53). In the hypoxic-ischemic brain injury
neonatal rat model, H2 inhalation could alleviate brain
damage and improve early neurological outcomes through
anti-inflammatory, anti-apoptotic and antioxidant responses via the
MAPK/HO-1/peroxisome proliferator-activated receptor γ coactivator
1a pathway (54). In the TBI
model, molecular hydrogen water could also reverse the controlled
cortical impact-induced brain edema through preserved or increased
ATP levels (55). Tian et
al (56) demonstrated that
hydrogen-rich water could alleviate inflammation and decrease brain
damage, exhibiting a neuroprotective function against TBI. Yuan
et al (57) also discovered
that hydrogen-rich water can inhibit oxidative stress and activate
the Nrf2 pathway, thereby improving TBI-induced brain damage. Both
aforementioned studies explored the neuroprotective effect of
hydrogen-rich water associated with inflammation and oxidative
stress without investigating further molecular mechanisms. However,
the current study also revealed that hydrogen-rich saline
considerably enhanced neurological function, alleviated brain
edema, increased neuronal survival and downregulated the protein
expression of RIP3 and RIP1, as well as the cytokines NF-κB, TNF-α,
IL-1β and IL-6, following TBI.
Necroptosis is a cell death type of modulated
necrosis, requiring the proteins MLKL and RIP3, and is stimulated
by death receptors (12).
Increasing evidence suggests that necroptosis performs an
instrumental function in CNS diseases, such as TBI (13-15).
Our previous studies have also demonstrated that necroptosis has an
integral function in the pathophysiology of neuronal death
following in vitro traumatic neuronal injury, and that the
potential regulatory mechanisms may be associated with the
Akt/GSK-3β and the metabotropic glutamate receptor 1 signaling
pathways (13,14). Therefore, necroptosis is a vital
mechanism in TBI, and necroptosis inhibition via drugs may decrease
neuronal death. Jia et al (58) reported that hydrogen can decrease
the expression levels of necroptosis-related proteins in the
hippocampus, including MLKL, phosphorylated MLKL and RIP3, thereby
partly preventing neuronal and astrocyte necroptosis in the
lithium-pilocarpine model of status epilepticus. Dong et al
(59) also reported that high
concentrations of hydrogen inhalation can alleviate skin
ischemia/reperfusion injury by regulating necroptosis and the
RIP-MLKL-serine/threonine-protein phosphatase PGAM5/dynamin-related
protein 1 necrotic pathway. In the present study, the results
confirmed that the mRNA and protein expression levels of RIP3 and
RIP1 were elevated 72 h after TBI, while hydrogen-rich saline
treatment could reduce the RIP3 and RIP1 expression levels.
The molecular mechanism of necroptosis and
neuroinflammation is complex, and the specific mechanisms of the
neuroprotective benefits of hydrogen-rich saline treatment are yet
to be elucidated. Wang et al (60) reported that rehmapicrogenin can
improve adriamycin-induced nephropathy in vitro and in
vivo by reducing ROS accumulation, thereby regulating the
expression levels of Nrf2. Nrf2 is an important transcriptional
regulation factor that can regulate the expression of >250 genes
and is characterized by its binding site ‘antioxidant response
element’; the majority of regulated genes by Nrf2 are involved in
oxidative stress and cell apoptosis, necroptosis, autophagy and
ferroptosis (31). Yu et al
(61) discovered that the
inhalation of 2% molecular hydrogen gas may enhance the survival
rates, reduce the lung edema and the lung injury score, and
ameliorate the injuries induced by inflammation and oxidative
stress in the septic mouse model, while Nrf2 knockout could reverse
or weaken the protection of H2 gas on lung damage, which
was also dependent on HO-1 and high mobility group protein B1.
Additionally, Chen et al (62) demonstrated that H2
decreased endothelial inflammation and injury and increased the
expression and activity of HO-1 in vivo and in vitro.
Nrf2 knockout or HO-1 inhibition reversed the protection of
H2, indicating that this was dependent on the activity
of the Nrf2/HO-1 signaling pathway. However, the exact mechanism
requires further elucidation.
In summary, the present findings demonstrated that
necroptosis, which is mediated by the RIP1 and RIP3 proteins, is a
key cellular regulatory mechanism that may lead to EBI following
TBI. The present study, for the first time to the best of our
knowledge, revealed that hydrogen-rich saline induced modulation of
necroptosis and neuroinflammation via the ROS/HO-1 pathway, and
also provided a novel insight into evaluating the biological
impacts as well as the mechanisms that underly neuroprotection and
inhibition of inflammation and necroptosis by hydrogen-rich
saline.
Supplementary Material
Reactive oxygen species levels as
detected via flow cytometry. TBI, traumatic brain injury; HS,
hydrogen-rich saline.
Neurological behavior scores.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH wrote the manuscript. YH, XF, YW, LS and JC
performed the experiments and prepared the figures. YH and JC
confirm the authenticity of all the raw data. YH and JC designed
the study and revised the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Research
Ethics Committee of Wuxi Clinical College of Anhui Medical
University (approval no. YXLL-2020-012; Wuxi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang JY, Gao GY, Feng JF, Mao Q, Chen LG,
Yang XF, Liu JF, Wang YH, Qiu BH and Huang XJ: Traumatic brain
injury in China. Lancet Neurol. 18:286–295. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen J, Li M, Chen L, Chen W, Zhang C,
Feng Y, Wang Y and Chen Q: The effect of controlled decompression
for severe traumatic brain injury: A randomized, controlled trial.
Front Neurol. 11(107)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen JH, Li PP, Yang LK, Chen L, Zhu J, Hu
X and Wang YH: Value of ventricular intracranial pressure
monitoring for traumatic bifrontal contusions. World Neurosurg.
113:e690–e701. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nichol A, French C, Little L, Haddad S,
Presneill J, Arabi Y, Bailey M, Cooper DJ, Duranteau J, Huet O, et
al: Erythropoietin in traumatic brain injury (EPO-TBI): A
double-blind randomised controlled trial. Lancet. 386:2499–2506.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hutchinson PJ, Kolias AG, Timofeev IS,
Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A,
Eynon CA, et al: Trial of decompressive craniectomy for traumatic
intracranial hypertension. N Engl J Med. 375:1119–1130.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cooper DJ, Nichol AD, Bailey M, Bernard S,
Cameron PA, Pili-Floury S, Forbes A, Gantner D, Higgins AM, Huet O,
et al: Effect of early sustained prophylactic hypothermia on
neurologic outcomes among patients with severe traumatic brain
injury: The POLAR randomized clinical trial. JAMA. 320:2211–2220.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wright DW, Yeatts SD, Silbergleit R,
Palesch YY, Hertzberg VS, Frankel M, Goldstein FC, Caveney AF,
Howlett-Smith H, Bengelink EM, et al: Very early administration of
progesterone for acute traumatic brain injury. N Engl J Med.
371:2457–2466. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Robertson CS, Hannay HJ, Yamal JM,
Gopinath S, Goodman JC and Tilley BC: Epo Severe TBI Trial
Investigators. Baldwin A, Rivera Lara L, Saucedo-Crespo H, et al:
Effect of erythropoietin and transfusion threshold on neurological
recovery after traumatic brain injury: A randomized clinical trial.
JAMA. 312:36–47. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang Y, Wang L, Hu T, Wang F, Han Z, Yin
Z, Ge X, Xie K and Lei P: Hydrogen improves cell viability partly
through inhibition of autophagy and activation of PI3K/Akt/GSK3β
signal pathway in a microvascular endothelial cell model of
traumatic brain injury. Neurol Res. 42:487–496. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li H, Lu C, Yao W, Xu L, Zhou J and Zheng
B: Dexmedetomidine inhibits inflammatory response and autophagy
through the circLrp1b/miR-27a-3p/Dram2 pathway in a rat model of
traumatic brain injury. Aging (Albany NY). 12:21687–21705.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang Y, Zhao M, Shang L, Zhang Y, Huang C,
He Z, Luo M, Wu B, Song P, Wang M and Duan F: Homer1a protects
against neuronal injury via PI3K/AKT/mTOR signaling pathway. Int J
Neurosci. 130:621–630. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vandenabeele P, Galluzzi L, Vanden Berghe
T and Kroemer G: Molecular mechanisms of necroptosis: An ordered
cellular explosion. Nat Rev Mol Cell Biol. 11:700–714.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Chen T, Yang LK, Zhu J, Hang CH and Wang
YH: The AMPAR antagonist perampanel regulates neuronal necroptosis
via Akt/GSK3β signaling after acute traumatic injury in cortical
neurons. CNS Neurol Disord Drug Targets. 20:266–272.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen T, Zhu J, Wang YH and Hang CH: Arc
silence aggravates traumatic neuronal injury via mGluR1-mediated ER
stress and necroptosis. Cell Death Dis. 11(4)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bao Z, Fan L, Zhao L, Xu X, Liu Y, Chao H,
Liu N, You Y, Liu Y, Wang X and Ji J: Silencing of A20 aggravates
neuronal death and inflammation after traumatic brain injury: A
potential trigger of necroptosis. Front Mol Neurosci.
12(222)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Laird MD, Wakade C, Alleyne CH Jr and
Dhandapani KM: Hemin-induced necroptosis involves glutathione
depletion in mouse astrocytes. Free Radic Biol Med. 45:1103–1114.
2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shen H, Liu C, Zhang D, Yao X, Zhang K, Li
H and Chen G: Role for RIP1 in mediating necroptosis in
experimental intracerebral hemorrhage model both in vivo and in
vitro. Cell Death Dis. 8(e2641)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang Y, Li M, Li X, Zhang H, Wang L, Wu
X, Zhang H and Luo Y: Catalytically inactive RIP1 and RIP3
deficiency protect against acute ischemic stroke by inhibiting
necroptosis and neuroinflammation. Cell Death Dis.
11(565)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yuan J, Amin P and Ofengeim D: Necroptosis
and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev
Neurosci. 20:19–33. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu C, Chen Y, Cui W, Cao Y, Zhao L, Wang
H, Liu X, Fan S, Huang K, Tong A and Zhou L: Inhibition of neuronal
necroptosis mediated by RIP1/RIP3/MLKL provides neuroprotective
effects on kaolin-induced hydrocephalus in mice. Cell Prolif.
54(e13108)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu Y, Zheng Z, Cao X, Yang Q, Norton V,
Adini A, Maiti AK, Adini I and Wu H: RIP1/RIP3/MLKL mediates
myocardial function through necroptosis in experimental autoimmune
myocarditis. Front Cardiovasc Med. 8(696362)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Linkermann A and Green DR: Necroptosis. N
Engl J Med. 370:455–465. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zou R, Wang MH, Chen Y, Fan X, Yang B, Du
J, Wang XB, Liu KX and Zhou J: Hydrogen-rich saline attenuates
acute lung injury induced by limb ischemia/reperfusion via
down-regulating chemerin and NLRP3 in rats. Shock. 52:134–141.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ning K, Liu WW, Huang JL, Lu HT and Sun
XJ: Effects of hydrogen on polarization of macrophages and
microglia in a stroke model. Med Gas Res. 8:154–159.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kumagai K, Toyooka T, Takeuchi S, Otani N,
Wada K, Tomiyama A and Mori K: Hydrogen gas inhalation improves
delayed brain injury by alleviating early brain injury after
experimental subarachnoid hemorrhage. Sci Rep.
10(12319)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ohno K and Ito M, Ichihara M and Ito M:
Molecular hydrogen as an emerging therapeutic medical gas for
neurodegenerative and other diseases. Oxid Med Cell Longev.
2012(353152)2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Takeuchi S, Mori K, Arimoto H, Fujii K,
Nagatani K, Tomura S, Otani N, Osada H and Wada K: Effects of
intravenous infusion of hydrogen-rich fluid combined with
intra-cisternal infusion of magnesium sulfate in severe aneurysmal
subarachnoid hemorrhage: Study protocol for a randomized controlled
trial. BMC Neurol. 14(176)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Schallner N, Pandit R, LeBlanc R III,
Thomas AJ, Ogilvy CS, Zuckerbraun BS, Gallo D, Otterbein LE and
Hanafy KA: Microglia regulate blood clearance in subarachnoid
hemorrhage by heme oxygenase-1. J Clin Invest. 125:2609–2625.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Kaiser S, Frase S, Selzner L, Lieberum JL,
Wollborn J, Niesen WD, Foit NA, Heiland DH and Schallner N:
Neuroprotection after hemorrhagic stroke depends on cerebral heme
oxygenase-1. Antioxidants (Basel). 8(496)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Afonso MB, Rodrigues PM, Simão AL,
Ofengeim D, Carvalho T, Amaral JD, Gaspar MM, Cortez-Pinto H,
Castro RE, Yuan J and Rodrigues CM: Activation of necroptosis in
human and experimental cholestasis. Cell Death Dis.
7(e2390)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen J, Wang Y, Wu J, Yang J, Li M and
Chen Q: The potential value of targeting ferroptosis in early brain
injury after acute CNS disease. Front Mol Neurosci.
13(110)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
National Research Council (US). Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: The National Academies Collection: Reports funded by
National Institutes of Health. In: Guide for the Care and Use of
Laboratory Animals. 8th edition. National Academies Press,
Washington, DC, 2011.
|
|
33
|
Flierl MA, Stahel PF, Beauchamp KM, Morgan
SJ, Smith WR and Shohami E: Mouse closed head injury model induced
by a weight-drop device. Nat Protoc. 4:1328–1337. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tian J, Yang L, Wang P, Yang L and Fan Z:
Exogenous CGRP regulates apoptosis and autophagy to alleviate
traumatic brain injury through Akt/mTOR signalling pathway.
Neurochem Res. 45:2926–2938. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhuang Z, Zhou ML, You WC, Zhu L, Ma CY,
Sun XJ and Shi JX: Hydrogen-rich saline alleviates early brain
injury via reducing oxidative stress and brain edema following
experimental subarachnoid hemorrhage in rabbits. BMC Neurosci.
13(47)2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Feng Y, Wang R, Xu J, Sun J, Xu T, Gu Q
and Wu X: Hydrogen-rich saline prevents early neurovascular
dysfunction resulting from inhibition of oxidative stress in
STZ-diabetic rats. Curr Eye Res. 38:396–404. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
38
|
Tang C, Shan Y, Hu Y, Fang Z, Tong Y, Chen
M, Wei X, Fu X and Xu X: FGF2 attenuates neural cell death via
suppressing autophagy after rat mild traumatic brain injury. Stem
Cells Int. 2017(2923182)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen J, Zhang C, Yan T, Yang L, Wang Y,
Shi Z, Li M and Chen Q: Atorvastatin ameliorates early brain injury
after subarachnoid hemorrhage via inhibition of pyroptosis and
neuroinflammation. J Cell Physiol. 236:6920–6931. 2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen JH, Wu T, Xia WY, Shi ZH, Zhang CL,
Chen L, Chen QX and Wang YH: An early neuroprotective effect of
atorvastatin against subarachnoid hemorrhage. Neural Regen Res.
15:1947–1954. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen J, Xuan Y, Chen Y, Wu T, Chen L, Guan
H, Yang S, He J, Shi D and Wang Y: Netrin-1 alleviates subarachnoid
haemorrhage-induced brain injury via the PPARγ/NF-KB signalling
pathway. J Cell Mol Med. 23:2256–2262. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hollingshead JR and Phillips RK:
Haemorrhoids: Modern diagnosis and treatment. Postgrad Med J.
92:4–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Das S, Chattopadhyay D, Chatterjee SK,
Mondal SA, Majumdar SS, Mukhopadhyay S, Saha N, Velayutham R,
Bhattacharya S and Mukherjee S: Increase in PPARγ inhibitory
phosphorylation by Fetuin-A through the activation of Ras-MEK-ERK
pathway causes insulin resistance. Biochim Biophys Acta Mol Basis
Dis. 1867(166050)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li Y, Liu Y, Wu P, Tian Y, Liu B, Wang J,
Bihl J and Shi H: Inhibition of ferroptosis alleviates early brain
injury after subarachnoid hemorrhage in vitro and in vivo via
reduction of lipid peroxidation. Cell Mol Neurobiol. 41:263–278.
2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wehn AC, Khalin I, Duering M, Hellal F,
Culmsee C, Vandenabeele P, Plesnila N and Terpolilli NA: RIPK1 or
RIPK3 deletion prevents progressive neuronal cell death and
improves memory function after traumatic brain injury. Acta
Neuropathol Commun. 9(138)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Huang GR and Hao FG: Dexmedetomidine
inhibits inflammation to alleviate early neuronal injury via
TLR4/NF-κB pathway in rats with traumatic brain injury. Crit Rev
Eukaryot Gene Expr. 31:41–47. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Li F, Wang X, Zhang Z, Zhang X and Gao P:
Dexmedetomidine attenuates neuroinflammatory-induced apoptosis
after traumatic brain injury via Nrf2 signaling pathway. Ann Clin
Transl Neurol. 6:1825–1835. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yang T, Feng X, Zhao Y, Zhang H, Cui H,
Wei M, Yang H and Fan H: Dexmedetomidine enhances autophagy via
α2-AR/AMPK/mTOR pathway to inhibit the activation of NLRP3
inflammasome and subsequently alleviates lipopolysaccharide-induced
acute kidney injury. Front Pharmacol. 11(790)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Fei W, Jiao W, Feng X, Chen X and Wang Y:
Intermittent hypoxia mimicking obstructive sleep apnea aggravates
early brain injury following ICH via neuroinflammation and
apoptosis. Mol Med Rep. 24(824)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Feng X, Ma W, Zhu J, Jiao W and Wang Y:
Dexmedetomidine alleviates early brain injury following traumatic
brain injury by inhibiting autophagy and neuroinflammation through
the ROS/Nrf2 signaling pathway. Mol Med Rep. 24(661)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Liu L, Xie K, Chen H, Dong X, Li Y and Yu
Y, Wang G and Yu Y: Inhalation of hydrogen gas attenuates brain
injury in mice with cecal ligation and puncture via inhibiting
neuroinflammation, oxidative stress and neuronal apoptosis. Brain
Res. 1589:78–92. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Choi KS, Kim HJ, Do SH, Hwang SJ and Yi
HJ: Neuroprotective effects of hydrogen inhalation in an
experimental rat intracerebral hemorrhage model. Brain Res Bull.
142:122–128. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang P, Zhao M, Chen Z, Wu G, Fujino M,
Zhang C, Zhou W, Zhao M, Hirano SI, Li XK and Zhao L: Hydrogen gas
attenuates hypoxic-ischemic brain injury via regulation of the
MAPK/HO-1/PGC-1a pathway in neonatal rats. Oxid Med Cell Longev.
2020(6978784)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Dohi K, Kraemer BC, Erickson MA, McMillan
PJ, Kovac A, Flachbartova Z, Hansen KM, Shah GN, Sheibani N,
Salameh T and Banks WA: Molecular hydrogen in drinking water
protects against neurodegenerative changes induced by traumatic
brain injury. PLoS One. 9(e108034)2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tian R, Hou Z, Hao S, Wu W, Mao X, Tao X,
Lu T and Liu B: Hydrogen-rich water attenuates brain damage and
inflammation after traumatic brain injury in rats. Brain Res.
1637:1–13. 2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yuan J, Wang D, Liu Y, Chen X, Zhang H,
Shen F, Liu X and Fu J: Hydrogen-rich water attenuates oxidative
stress in rats with traumatic brain injury via Nrf2 pathway. J Surg
Res. 228:238–246. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Jia R, Jia N, Yang F, Liu Z, Li R, Jiang
Y, Zhao J, Wang L, Zhang S, Zhang Z, et al: Hydrogen alleviates
necroptosis and cognitive deficits in lithium-pilocarpine model of
status epilepticus. Cell Mol Neurobiol. 39:857–869. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Dong XH, Liu H, Zhang MZ, Zhao PX, Liu S,
Hao Y and Wang YB: Postconditioning with inhaled hydrogen
attenuates skin ischemia/reperfusion injury through the
RIP-MLKL-PGAM5/Drp1 necrotic pathway. Am J Transl Res. 11:499–508.
2019.PubMed/NCBI
|
|
60
|
Wang M, Ke Y, Li Y, Shan Z, Mi W, Cao Y,
Feng W and Zheng X: The nephroprotective effects and mechanisms of
rehmapicrogenin include ROS inhibition via an oestrogen-like
pathway both in vivo and in vitro. Biomed Pharmacother.
138(111305)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Yu Y, Yang Y, Yang M, Wang C, Xie K and Yu
Y: Hydrogen gas reduces HMGB1 release in lung tissues of septic
mice in an Nrf2/HO-1-dependent pathway. Int Immunopharmacol.
69:11–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Chen H, Xie K, Han H, Li Y, Liu L, Yang T
and Yu Y: Molecular hydrogen protects mice against polymicrobial
sepsis by ameliorating endothelial dysfunction via an Nrf2/HO-1
signaling pathway. Int Immunopharmacol. 28:643–654. 2015.PubMed/NCBI View Article : Google Scholar
|