Introduction
The evolution of bones enhances an animal's ability
to escape hazards and bone stem cells continuously produce bone
cells to maintain bone homeostasis (1). The survival and aging of skeletal stem
cells are regulated by numerous physiological factors (2,3),
including secreted class 3 semaphorin (2), parathyroid hormone (4), hypoxia-inducible factor 1α (5) and non-coding RNA (6,7). The
formation and maintenance of osteoblasts is associated with
glutamine metabolism and pathological processes (1,8), such
as the development of leukemia (9)
and hematologic malignancy (10).
In clinical treatment, external stressors (including hypoxia and
radiation) damage cells and trigger an immune response (11). Non-coding RNA mediates cellular
immune responses against harmful stimuli (12,13)
and in bone marrow mesenchymal stem cells (BMSCs) (14).
Recently, research on the treatment of blood
diseases at the molecular level has received widespread attention
(15-17).
BMSCs are a type of multipotent stem cell that participate in the
formation of the bone marrow (BM) hematopoietic microenvironment
(18), which is more tolerant to
radiation than hematopoietic stem cells (19). BMSCs are important structural and
functional components of hematopoietic recovery and reconstruction,
following radiation injury (19).
Prior to organ transplants, patients are subjected to total body
irradiation (TBI) (20). Previous
studies have demonstrated that patients who receive TBI to destroy
infected tissues and cells prior to BM transplantation suffer
damage to healthy BM cells, such as BM stromal cells (21-23).
Furthermore, previous studies have reported that BMSC
transplantations stimulate hematopoiesis, accelerate lymphocyte
recovery and promote tissue repair (24,25).
However, in the early stage of transplantation, BMSCs may undergo a
variety of adverse reactions, including oxidative stress, hypoxia
and inflammatory reactions (26).
Irradiation has been reported to promote homing of BMSCs following
BMSC transplantation (27,28). Moreover, irradiation promotes
transplanted BMSCs to be implanted and induce the formation of new
bone (29). Therefore, the relevant
molecular mechanisms underlying irradiation-induced injury of BMSCs
require investigation to discover novel strategies for irradiation
injury.
Circular RNA (circRNA) is widely expressed in
mammalian cells and is a type of non-coding endogenous RNA molecule
that regulates gene expression (30). Numerous studies have demonstrated
that circRNA may serve important roles in the progression of
various diseases, including neurological disorders, diabetes and
types of cancer (31-33),
indicating that circRNAs may be an important biomarker for
predicting disease progression and prognosis. A previous study has
reported that the expression profiles of circRNAs in mouse BMSCs
were significantly altered following irradiation (34). Additionally, the expression levels
of circRNA-011235 and circRNA-016901 were significantly increased
in mouse BMSCs following irradiation (34). However, whether circRNA-016901
influences irradiation-induced injury of BMSCs and associated
molecular mechanisms remains unclear and requires further
exploration.
MicroRNAs (miRNAs or miRs) are a class of non-coding
small RNA molecules that are involved in various important
physiological and pathological processes (35), such as asthma (36), brain aging (37) and prostate cancer (38). MiRNAs act mainly by complementarily
pairing with the 3'-untranslated region (UTR) region of target gene
mRNA (39) to degrade or inhibit
its expression (40). Furthermore,
miRNAs serve important regulatory functions for growth and
development, and the occurrence and development of various
diseases, which included ischemic heart disease and chronic
lymphocytic leukemia (41). A
previous study has demonstrated that miR-1249-5p expression is
significantly increased in chronic intermittent hypoxia-treated
mouse aortic endothelial cells and is closely associated with
apoptosis and autophagy (42). As
previously reported, miR-1249 inhibits cell proliferation,
invasion, migration and epithelial-mesenchymal transition processes
in colorectal cancer (43).
Furthermore, microarray analysis revealed that miR-1249-5p was
upregulated in types of cancer, including cardiac myxoma and colon
cancer, indicating miR-1249-5p may serve a tumor-promoting function
(44,45). miR-1249-5p has been demonstrated to
be activated in hepatocellular carcinoma and involved in the
regulation of apoptosis (46).
Increasing evidence has revealed that circRNAs bind to miRNAs to
regulate miRNA function (47).
Bioinformatics analysis demonstrated that circ-016901 contained a
binding site for miR-1249-5p. However, the role of circ-016901 and
miR-1249-5p in regulating irradiation-induced injury of BMSCs
remains unclear.
Homeodomain-interacting protein kinase (HIPK) is a
serine/threonine protein kinase located in the nucleus and the HIPK
subfamily includes HIPK1, HIPK2 and HIPK3(48). As a transcription factor, HIPK2
regulates cell differentiation, proliferation, angiogenesis and
apoptosis and is associated with tumor development and progression
(49-51).
A recent study has reported that HIPK2 is a tumor suppressor gene
that affects the biological characteristics of various tumors,
including esophageal squamous cell carcinoma (52). Additionally, HIPK2 is a key
regulator of renal fibrosis and is a serum marker (53). A previous study has demonstrated
that HIPK2 activates p53 function under UV irradiation, thereby
promoting apoptosis (54).
Furthermore, HIPK2 has been predicted to be one of the candidate
target genes for miR-1249-5p through TargetScan bioinformatics
analysis. However, the function of the miR-1249-5p/HIPK2 axis in
irradiation-induced injury of BMSCs and the potential molecular
mechanisms remains to be elucidated.
The present study aimed to investigate the
expression and potential role of circ-016901, miR-1249-5p and HIPK2
on autophagy and apoptosis in irradiation-induced injury of BMSCs,
which may provide novel clinical targets to protect BMSCs from
irradiation injury.
Materials and methods
BMSC isolation, culturing and
identification
All animal procedures and euthanasia were reviewed
by local government authorities in accordance with the local Animal
Care Committee of Third XiangYa Hospital of Central South
University (Hunan, China) in line with the Helsinki
Declaration.
Adult male BALB/c mice (age, 8-10 weeks; weight,
18-25 g) were purchased from the laboratory animal center of the
Third Xiangya Hospital of Central South University, Hunan, China.
Mice were housed at 22±2˚C at 40-60% humidity with 12-h light/dark
cycles. Mice had free access to food and water. A total of 50%
volume/min CO2 flow rate was used for euthanasia
(55). When the volume of
CO2 reached at 70%, mice would lose consciousness
(recumbency without muscle tone) within 30 sec, thus mice were
euthanized using CO2 combined with cervical dislocation.
This method avoided the effect of abnormal hypoxia on the
experimental results. As all exogenous factors were identical when
mice were sacrificed, the anomalous effects of cell autophagy and
apoptosis caused by hypoxia on BMSCs can be excluded.
Tibias and femurs were surgically excised from mice
and immersed in 75% ethanol solution at 25˚C for a few seconds to
disinfect the medullary cavity proximal to the end of the tibias
and femurs which were touched by the scissors. Tibias and femurs
were washed in α modification minimum essential medium (α-MEM)
(cat. no. 36450; Stemcell Technologies, Inc.) supplemented with 10%
FBS (cat. no. F2442; Sigma-Aldrich; Merck KGaA) and 5 U/ml heparin
(cat. no. BP2524100; Thermo Fisher Scientific, Inc.) and bone
marrow was collected.
Cells were washed twice with heparin-free α-MEM
medium and seeded into petri dishes (seeding density,
2x106 cells/ml) after 24 h of bone marrow collection.
Cells were washed three times with PBS three days later to remove
unattached cells and cultured in DMEM medium (cat. no. 11965092;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS. Cells
were cultured in an incubator at 37˚C containing 5% CO2
for 24 h. At 80% confluence, BMSCs were digested with 0.25% trypsin
(cat. no. MFCD00130286; Sigma-Aldrich; Merch KGaA) at 37˚C for 2
min, adjusted to a density to 1x106 cells/ml, washed
twice with PBS and centrifuged at 300 x g for 5 min at 4˚C.
FITC-labeled cluster of differentiation (CD) 34 (cat. no. 553733;
BD Biosciences), CD45 (cat. no. 553080; BD Biosciences) and stem
cell antigen 1 (Sca-1) (cat. no. ab25031; Abcam) mixed with CD90
(cat. no. 1740-02; SouthernBiotech) were added to the cell
suspension and incubated for 15-25 min at 20-25˚C in the dark.
Following this, BMSCs were identified using flow cytometry (ZE5
Cell Analyzer; cat. no. 12004279; BioRad Laboratories, Inc.), data
were analyzed using FlowJo software (version 10.0; FlowJo LLC).
Following identification via flow cytometry,
positive cell ratios of CD34, CD45, Sca-1 and CD90 were 0.75, 0.96,
99.42 and 98.01%, respectively, indicating that the majority of
cells were CD34(-), CD45(-), Sca-1(+) and CD90(+). The
identification and characteristics of BMSCs were based on the
immunophenotype of mouse BMSCs (56). Isolated and identified cells were
used for subsequent studies.
Irradiation treatment
Single cell suspensions of BMSCs (seeding density,
2x106 cells/ml) were plated into a 96-well culture dish
until cells reached 80% confluence. Cells were divided into 4
groups and irradiated with 0 (control group), 2, 4 or 6 Gy for
various time points of 6, 12 and 24 h under room temperature
conditions (25˚C) in the X-ray radiation field of a linear
accelerator (2008 Synergy s/n 151765; Elekta) at a dose rate of 0.4
Gy/min with a source target distance of 100 cm.
Transfection
Cells in the logarithmic growth phase were
transfected (seeding density, 1.5x105 cells/ml).
Circ-016901 small interfering (si)RNA, miR-1249-5p mimics, HIPK2
siRNA or corresponding controls, scrambled siRNA (NC) or control
mimics (all GenePharma, Inc.) were transfected into BMSCs using a
Lipofectamine® 2000 Transfection Reagent kit (cat. no.
11668030; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The sequences and mass of all miRNA/siRNA
were listed in Table I. The
transfection was performed at 37˚C for 48 h. At 24 h
post-transfection, the transfection efficiency of siRNA and
overexpression was detected using reverse
transcription-quantitative PCR (RT-qPCR).
 | Table ISequences and mass of all miRNA/siRNA
in transfection. |
Table I
Sequences and mass of all miRNA/siRNA
in transfection.
| Name | Sequence
(5'→3') | Mass (µg) |
|---|
| circ-016901
siRNA |
GGGCTGGTTCTTCTTCACATA | 3.33 |
| Scrambled siRNA
negatice control |
GTGCTTATCAGCCGGTTTCTA | 3.33 |
| miR-1249-5p
mimics |
UCCUCCCUCCCCUACCCGGUUCAAG | 4 |
| miRNA control
mimics |
UUUGUACUACACAAAAGUACUG | 4 |
| HIPK2 siRNA |
GATCACTCCACCACGTAGACT | 3.33 |
Dual-luciferase reporter assay
circ-016901 and HIPK2 were determined to contain
binding sites for miR-1249-5p using the Starbase (version 2.0;
http://starbase.sysu.edu.cn/index.php), TargetScan
(release 7.2; http://www.targetscan.org/vert_72/) and IntaRNA
(version 2.0; http://rna.informatik.uni-freiburg.de/IntaRNA/Input.jsp)
databases. Subsequently, the miR-1249-5p mimics and control mimics
were subcloned into the Renilla luciferase reporter vector
psiCHECK-2 (Promega Corporation) according to the manufacturer's
instructions. Circ-016901 and HIPK2 wild-type (WT) and mutant (MUT)
Dual-luciferase reporter vectors were constructed and
co-transfected with miR-1249-5p mimic or NC control using
Lipofectamine® 2000 (cat. no. 11668030; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Following incubation for 48 h at 37˚C in a 5% CO2
incubator, cells were harvested and detected for luciferase
activity using Dual-Luciferase® Reporter 1000 Assay
system (Promega Corporation) and presented as the ratio of firefly
to Renilla luciferase activity.
Cell Counting Kit (CCK)-8 assay
The cells treated with varying doses and times of
irradiation were seeded at into 96-well plates (seeding density,
5x103 cells/well) and cultured in an incubator at 37˚C
containing 5% CO2. Cell proliferation was measured at 0,
24, 48 and 72 h following seeding using a CCK-8 Assay kit (cat. no.
ab228554; Abcam) according to the manufacturer’s protocol. Briefly,
10 µl of CCK-8 solution was added to each well. Following
incubation for 1 h at 37˚C in a 5% CO2 incubator, the
absorbance (optical density, OD) of each well was measured using a
microplate reader (BMG Labtech, GmbH) at a wavelength of 450 nm and
was used to calculate relative cell numbers over the ratio of
absorbance from ≥3 scopes.
Flow cytometry assay
Cells in each treatment group were supplemented with
serum-free DMEM medium to induce apoptosis. Briefly, cells were
digested with 0.25% trypsin at 37˚C for 2 min. to prepare a single
cell suspension and washed twice with ice cold PBS. Cells were
incubated with Annexin V-FITC (cat. no. ab14085; Abcam) for 10 min
at room temperature (25˚C). Following this, the probe solution, 50
µg/ml propidium iodide (PI; cat. no. ab14083; Abcam) was added
directly to the cell suspension. Cells were incubated for 30 min at
room temperature in the dark prior to flow cytometry analysis.
Subsequently, the cells were washed with fresh serum-free DMEM. The
fluorescent intensity was detected via flow cytometry (ZE5 Cell
Analyzer; cat. no. 12004279; Bio-Rad Laboratories, Inc.) and
apoptosis rate was measured a by Annexin V-PI Apoptosis Detection
kit (Abcam), according to the manufacturer's protocol. Data were
analyzed using FlowJo software (version 10.0; FlowJo LLC).
Caspase-3/7 activity assay
Following transfection, 100 µl of cell suspension
was added to 96-well plates (seeding density, 1x106
cells/ml). Caspase-Glo reagent (cat. no. G8200; Promega
Corporation) was added to each well, according to the 1:1
principle. Cells were gently centrifuged at 80-120 x g at 4˚C for
30 sec and incubated for 2 h at room temperature. Incubated sample
plates were then placed in a Veritas™ Microplate Luminometer
(Promega Corporation) to detect the fluorescence value of each
sample for data analysis.
RNA isolation and RT-qPCR
Total RNA was extracted from cells using
TRIzol® reagent (cat. no. 15596026; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
First-strand cDNA was synthesized via reverse transcription from
total RNA using the High-Capacity cDNA Reverse Transcription kit
(cat. no. 4368814; Thermo Fisher Scientific, Inc.), quality was
determined using OD260/OD280 by NanoDrop ND-1000 instrument
(Agilent Technologies, Inc.) and digested with Rnase R (cat. no.
19101; Qiagen China Co., Ltd.) to remove linear RNAs. qPCR was
performed using SYBR-Green PCR Master Mix (cat. no. 4367660; Thermo
Fisher Scientific, Inc.) on a ViiA 7 Real-time PCR system (cat. no.
4453545; Thermo Fisher Scientific, Inc.). Experiments were
performed in triplicate. The thermocycling conditions used for qPCR
were as follows: 50˚C UNG activation step for 2 min; denaturation
for 30 sec at 95˚C; followed by 40 cycles of denaturation for 5 sec
at 95˚C and annealing for 30 sec at 60˚C. GAPDH mRNA was used to
normalize the expression levels of target genes. U6 was used for
normalization of miR-1249-5p expression level. Relative mRNA
expression levels were calculated using the 2-ΔΔCq
method (57). Primer sequences used
in the present study were as follows: circ-016901 forward,
5'-ACAGCGCTAC ACTTGTTCCGA-3' and reverse, 5'-GACGATGCTA TCCAG
GAGAGGT-3'; miR-1249-5p forward, 5'-GAGGAGGG AGGGGATG-3' and
reverse, 5'-TCCAGTTTTTTTTT TTTTTTGAACTTG-3'; HIPK2 forward,
5'-CTTCAGGAG CCATCGCCTAC-3' and reverse, 5'-CTGTTG TGCGGG
AAGGTGTA-3'; light chain 3 (LC3) forward, 5'-ATGCCGTC CGAGAAG-3'
and reverse, 5'-TTACACAG CCATTGCTG-3'; Beclin-1 forward,
5'-CGGAATTCTATGGAAGGGTCTAA GACGTCC-3' and reverse,
5'-CGGGATCCTCATTTGTT ATAAAATTGTGAGGACA-3'; U6 forward, 5'-CTCGCTTC
GGCAGCACA-3' and reverse, 5'-AACGCTTCACGAAT TTGCGT-3'; and GADPH
forward, 5'-CACTGAGCAAG AGAGGCCCTAT-3' and reverse,
5'-GCAGCGAACTTTAT TGATGGTATT-3'.
Western blotting
Each group of cells was washed with PBS, centrifuged
at 300 x g at 4˚C for 5 min and the supernatant was discarded.
Cells were lysed on ice for 30 min using RIPA protein lysate (cat.
no. ab7937; Abcam) and centrifuged at 11,500 x g for 20 min at 4˚C.
Protease inhibitors (cat. no. 635673; Takara Bio, Inc.) were added
to cell lysates. Cells were then placed on ice for 30 min until
complete dissolution and the supernatant was transferred to a new
EP tube. Total protein concentration was determined using a Pierce™
BCA Protein Assay kit (cat. no. 23227; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Proteins were
separated via 12% SDS-PAGE (15 µg protein/gel lane) and transferred
to PVDF membranes (EMD Millipore). Membranes were blocked with 5%
skim milk for 1 h at 25˚C. Following this, primary antibodies were
added and cells were incubated overnight at 4˚C. After washing with
TBST, horseradish peroxidase-conjugated rabbit anti-mouse
immunoglobulin G secondary antibodies (1:5,000; cat. no. 61-6520;
Thermo Fisher Scientific, Inc.) was added and incubated for 1 h at
room temperature. Proteins bands were detected using ECL™ Western
Blotting Detection reagents (cat. no. RPN2209; Sigma-Aldrich; Merck
KGaA). Gray values of each protein bands were analyzed using ImageJ
software (version 1.46; National Institute of Health).
Additionally, the gray values obtained from each group were
compared with the gray values of the corresponding β-actin band to
analyze protein expression levels. The ratio of LC3-II/LC3-I were
calculated based on gray values of LC3-II and LC3-I. Primary
antibodies used were as follows: LC3 (1:1,000; cat. no. M152-3;
Medical & Biological Laboratories Co., Ltd.), Beclin-1
(1:1,000; cat. no. NB500-249; Novus Biologicals, Inc.), HIPK2
(1:1,000; cat.no. 5091; Cell Signaling Technology, Inc.) and
β-actin (1:5,000; cat. no. 3700S; New England BioLabs, Inc.).
Ethical statement
All animal procedures were in accordance with the
guidelines approved by the Institutional Animal Care and Use
Committee of Third XiangYa Hospital of Central South University,
Hunan, China (approval no. 2019-S534).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 19.0; IBM Corp.) and data are presented as mean ±
standard deviation. Differences between groups were analyzed using
one-way or two-way ANOVA followed by Bonferroni's post hoc test,
and unpaired Student's t-tests. All experiments were performed and
analyzed in triplicate. P<0.05 was considered to indicated a
statistically significant difference.
Results
Expression levels of circ-016901,
miR-1249-5p and HIPK2 are associated with irradiation in BMSCs
To investigate the effect of radiation on BMSCs, the
effects of different doses (2, 4 and 6 GY) of radiation on the
proliferation of BMSCs was examined using CCK-8 assays. Compared
with the control group, irradiation treatment significantly
decreased the cell proliferation of BMSCs in a dose-dependent
manner (Fig. 1A; P<0.01). The
mRNA and protein expression levels of autophagy-related genes
(including Beclin-1 and LC3) were detected at various irradiation
doses via RT-qPCR and western blotting, respectively. The results
indicated that the mRNA and protein expression levels of Beclin-1
were significantly decreased in irradiation-treated BMSCs in a
dose-dependent manner compared with the control group (Fig. 1B and C; P<0.01). Additionally, although the
mRNA expression of LC3 was significantly decreased, there was no
significant difference in the ratio of LC3-II/LC3-I compared with
the control group. Furthermore, irradiation treatment significantly
upregulated circ-016901 and HIPK2 expression, and downregulated
miR-1249-5p expression in a time- and dose-dependent manner in
BMSCs compared with the control group (Fig. 1D-F; P<0.01). Therefore, the 6 GY
dose and 24 h time point was used for all subsequent studies.
Furthermore, the results indicated that irradiation treatment
significantly increased caspase-3/7 activity (Fig. 1G) and increased apoptosis in BMSCs
and (Fig. 1H; P<0.01). The
current results indicated that circ-016901, miR-1249-5p and HIPK2
had potential regulatory effects in irradiation-induced injury of
BMSCs.
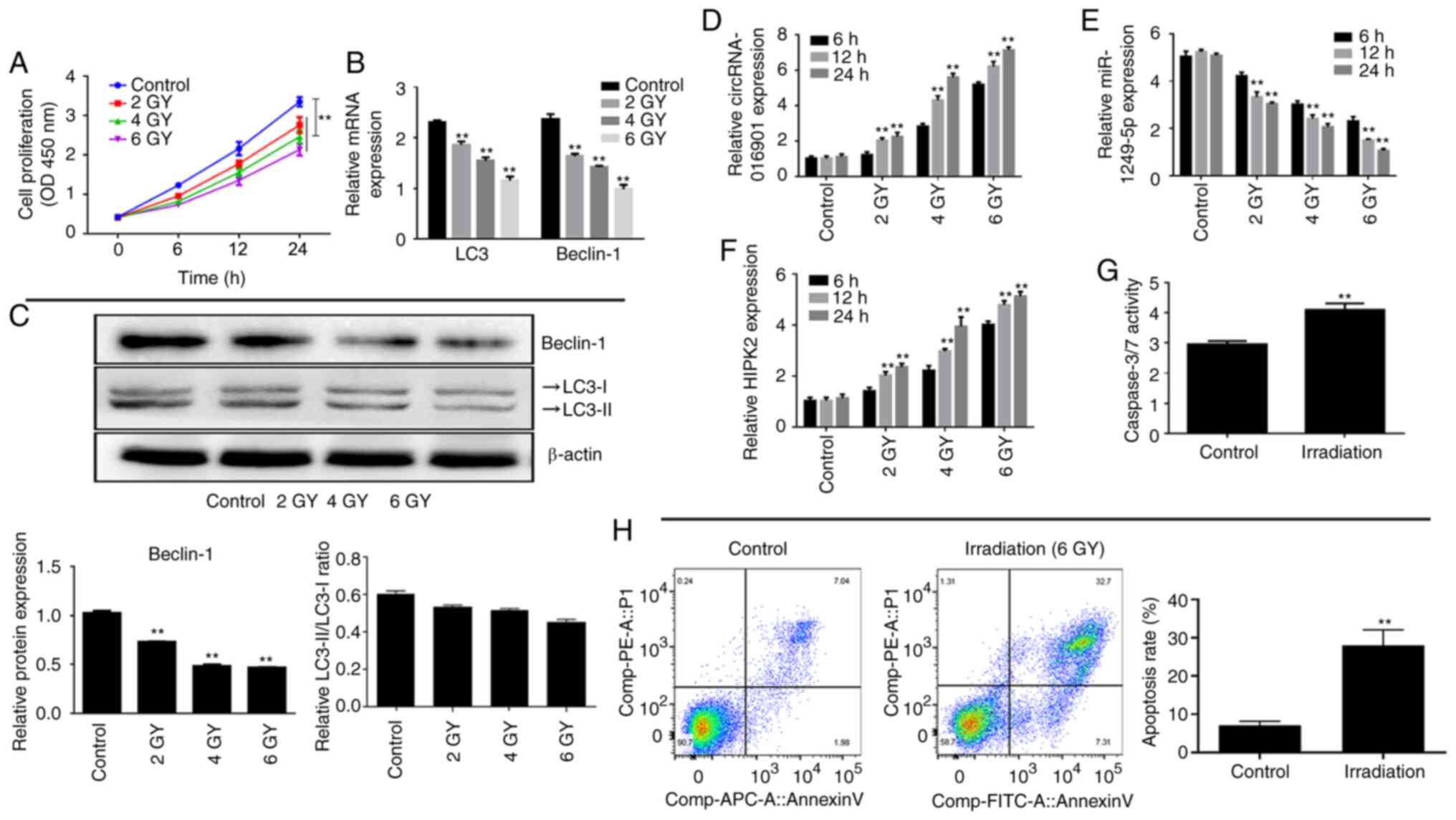 | Figure 1Expression levels of circ-016901,
miR-1249-5p and HIPK2 are associated with irradiation in BMSCs. (A)
Effect of various doses of irradiation on the proliferation of
BMSCs was measured by using Cell Counting Kit-8 assays. (B) mRNA
and (C) protein expression levels of Beclin-1 and LC3-II/I in BMSCs
treated with various doses of irradiation were detected using
RT-qPCR and western blotting, respectively. Expression levels of
(D) circ-016901, (E) miR-1249-5p and (F) HIPK2 in BMSCs treated
with various doses of irradiation were detected using RT-qPCR.
Apoptosis rates of BMSCs treated with irradiation was detected
using (G) caspase-3/7 and (H) flow cytometry. Data are presented as
mean ± standard deviation. Experiments were performed in
triplicate. **P<0.01 vs. the control group. circ,
circular RNA; miR, microRNA; HIPK2, homeodomain-interacting protein
kinase; BMSC, bone marrow mesenchymal stem cells; LC3, light chain
3; RT-qPCR, reverse transcription-quantitative PCR; OD, optical
density. |
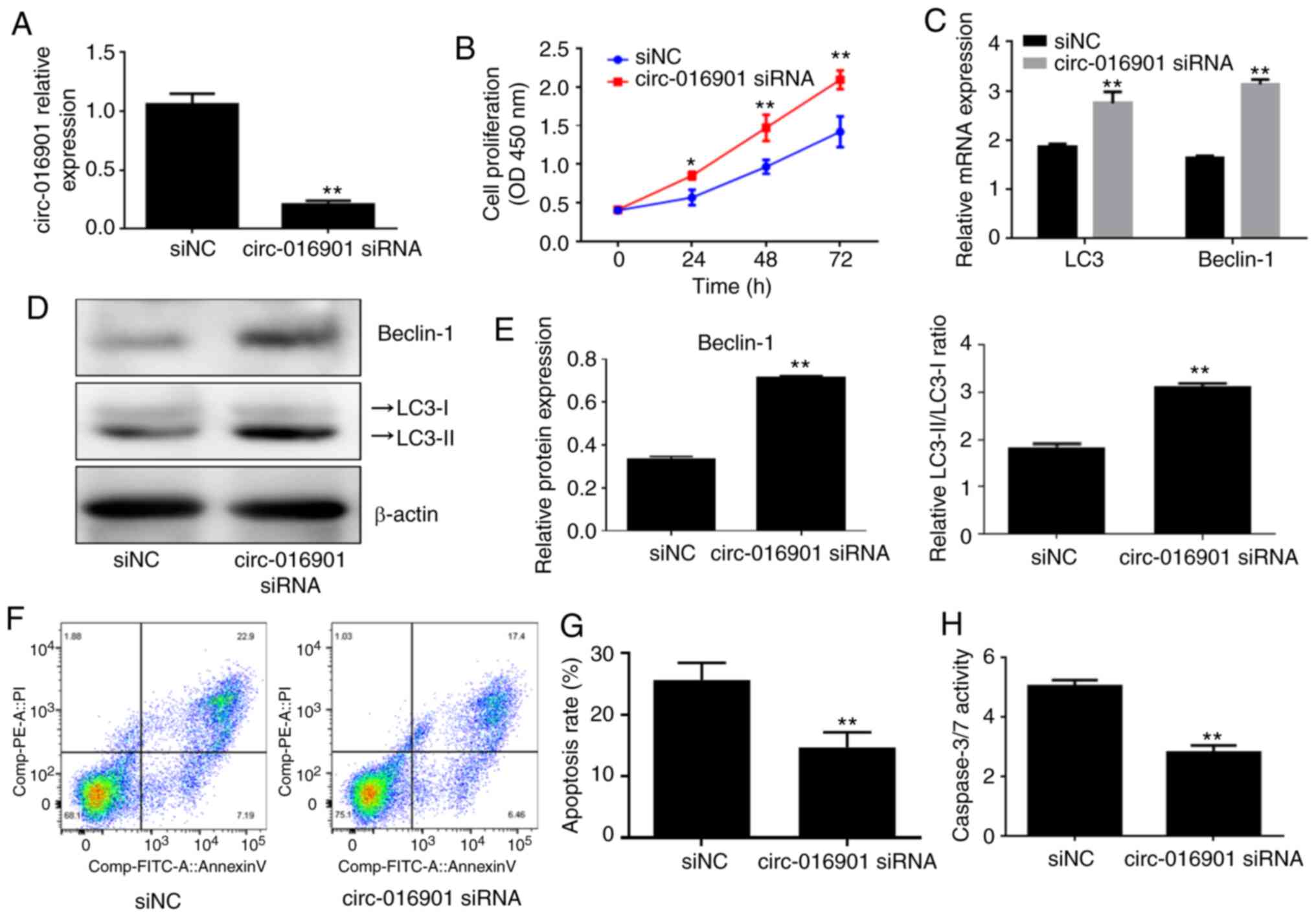
Silencing of circ-016901 attenuates
cell injury induced by irradiation in BMSCs
BMSCs were transfected with circ-016901 siRNA or
siNC prior to treatment with 6 GY irradiation for 24 h.
Transfection efficiency was verified by using RT-qPCR and the
results demonstrated that circ-016901 siRNA significantly
downregulated circ-016901 expression compared with the siNC group
(Fig. 2A; P<0.01). Furthermore,
CCK-8 assays revealed that circ-016901 silencing significantly
increased the proliferation of BMSCs treated with irradiation
compared with the siNC group (Fig.
2B; P<0.05. Circ-016901 siRNA significantly upregulated the
mRNA and protein expression levels of Beclin-1 and LC3 II/I in
irradiation-treated BMSCs compared with the siNC group (Fig. 2C-E; P<0.01). Additionally,
circ-016901 silencing significantly inhibited the apoptosis of
irradiation-treated BMSCs compared with the siNC group (Fig. 2F and G; P<0.01). Similar results were
obtained from the measurement of caspase-3/7 activity (Fig. 2H; P<0.01). These results
demonstrated that circ-016901 siRNA promoted cell proliferation and
autophagy, and inhibited apoptosis of irradiation-treated BMSCs,
indicating that circ-016901 silencing attenuated the cell injury
induced by irradiation of BMSCs.
circ-016901 regulates the
miR-1249-5p/HIPK2 axis in irradiation-treated BMSCs
Bioinformatics analysis using the online Targetscan
and Starbase databases revealed that miR-1249-5p had a binding site
for circ-016901 (Fig. 3A and
C) and the 3'-UTR of HIPK2 had a
binding site for miR-1249-5p (Fig.
3B). To investigate whether circ-016901 regulated the
miR-1249-5p/HIPK2 axis in irradiation-treated BMSCs, the expression
levels of miR-1249-5p and HIPK2 in BMSCs transfected with
circ-016901 siRNA were detected via RT-qPCR. The results
demonstrated that circ-016901 silencing significantly upregulated
the expression of miR-12249-5p (Fig.
3D; P<0.01) and downregulated HIPK2 expression (Fig. 3E; P<0.01) compared with the siNC
group. Additionally, western blotting analysis indicated that
circ-016901 silencing decreased HIPK2 protein expression compared
with the siNC group (Fig. 3F and
G; P<0.01). Furthermore, the
direct target association between miR-1249-5p, circ-016901 and
HIPK2 was verified using luciferase reporter assays. The
miR-1249-5p overexpression significantly decreased the relative
luciferase activity of WT-circ-016901 (Fig. 3H) and WT-HIPK2 (Fig. 3I) compared with the control mimic
group. However, there were no significant differences between
MUT-circ-016901 and MUT-HIPK2 and control mimics following
miR-1249-5p overexpression. These results indicated that
circ-016901 regulates the expression of miR-1249-5p, and that
miR-1249-5p directly modulates the expression of HIPK2 via binding
the HIPK2 3'-UTR. These results suggested that the
circ-016901/miR-1249-5p/HIPK2 axis may represent a regulatory
signaling pathway involved in the irradiation-induced injury of
BMSCs.
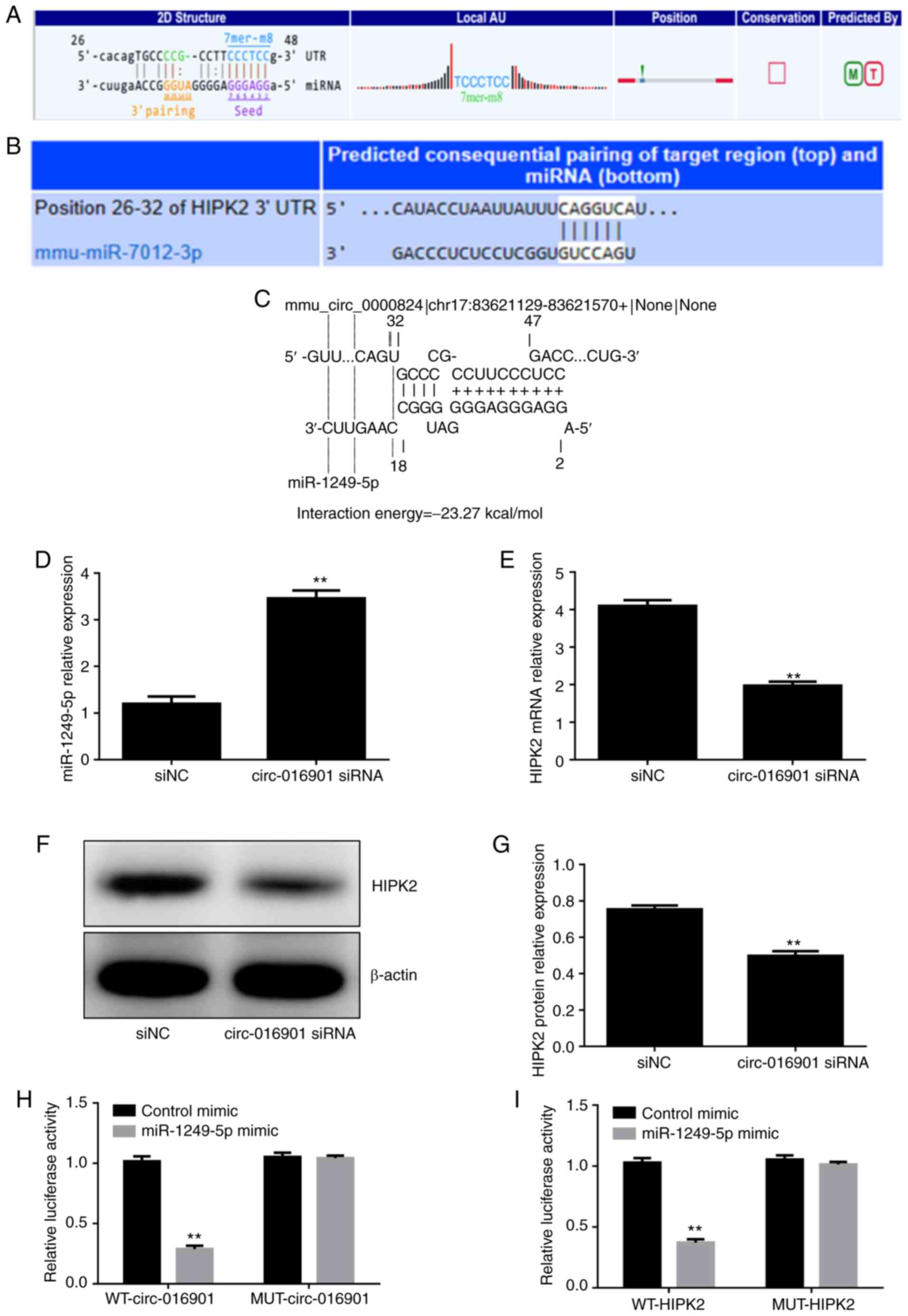 | Figure 3Circ-016901 regulates the
miR-1249-5p/HIPK2 axis in irradiation-treated BMSCs. (A) Starbase
predicted miR-1249-5p as a target for circ-016901. (B) TargetScan
predicted HIPK2 as a target for miR-1249-5p. (C) IntaRNA predicted
miR-1249-5p as a target for circ-016901. (D) Circ-016901 silencing
upregulated miR-1249-5p expression. Circ-016901 silencing
downregulated the (E) mRNA and (F and G) protein expression levels
of HIPK2. (H) Luciferase reporter assays indicated that there was
an interaction between circ-016901 and miR-1249-5p. (I) Luciferase
reporter assays demonstrated that there was an interaction between
HIPK2 and miR-1249-5p. Data are presented as mean ± standard
deviation. Experiments were performed in triplicate.
**P<0.01 vs. the siNC or control mimic groups. Circ,
circular; miR, microRNA; HIPK2, HIPK2, homeodomain-interacting
protein kinase; BMSCs, bone marrow mesenchymal stem cells; si,
small interfering; NC, negative control; UTR, untranslated region;
WT, wild-type; MUT, mutant. |
HIPK2 silencing mimics the effects of
miR-1249-5p in irradiation-treated BMSCs
To explore the possible regulatory role of
miR-1249-5p and HIPK2 in the circ-016901/miR-1249-5p/HIPK2 axis in
irradiation-treated BMSCs, miR-1249-5p mimics, HIPK2 siRNA and
corresponding controls (control mimic and siNC, respectively) were
transfected into BMSCs. miR-1249-5p mimics significantly
downregulated HIPK2 expression at the mRNA (Fig. 4A) and protein levels (Fig. 4B) compared with the control mimic
group (P<0.01). Furthermore, RT-qPCR was used to verify the
transfection efficiency of miR-1249-5p overexpression and HIPK2
silencing. The results demonstrated that miR-1249-5p overexpression
significantly increased miR-1249-5p expression (Fig. 4C; P<0.01), while HIPK2 silencing
decreased the expression of HIPK2 (Fig.
4D; P<0.01). Additionally, western blotting indicated that
HIPK2 silencing decreased HIPK2 protein expression compared with
the siNC group (Fig. 4E;
P<0.01). CCK-8 assays were used to determine the effect of
miR-1249-5p and HIPK2 on the proliferation of irradiation-treated
BMSCs and the results revealed that miR-1249-5p overexpression and
HIPK2 silencing significantly promoted the proliferation of
irradiation-treated BMSCs compared with their respective controls
(Fig. 4F; P<0.01). Furthermore,
miR-1249-5p mimics and HIPK2 siRNA efficiently inhibited the
apoptosis of irradiation-treated BMSCs compared with the control
mimic and siNC groups, respectively (Fig. 4G and H; P<0.01). RT-qPCR and western blotting
results indicated that miR-1249-5p overexpression and HIPK2
silencing significantly increased the mRNA and protein expression
levels of LC3 II/I and Beclin-1 compared with the control mimic and
siNC groups (Fig. 4I and J, respectively; P<0.05), indicating
that miR-1249-5p and HIPK2 promoted autophagy of
irradiation-treated BMSCs. These results demonstrated that the
miR-1249-5p/HIPK2 axis attenuated irradiation-induced injury of
BMSCs by promoting proliferation and autophagy, and inhibiting
apoptosis.
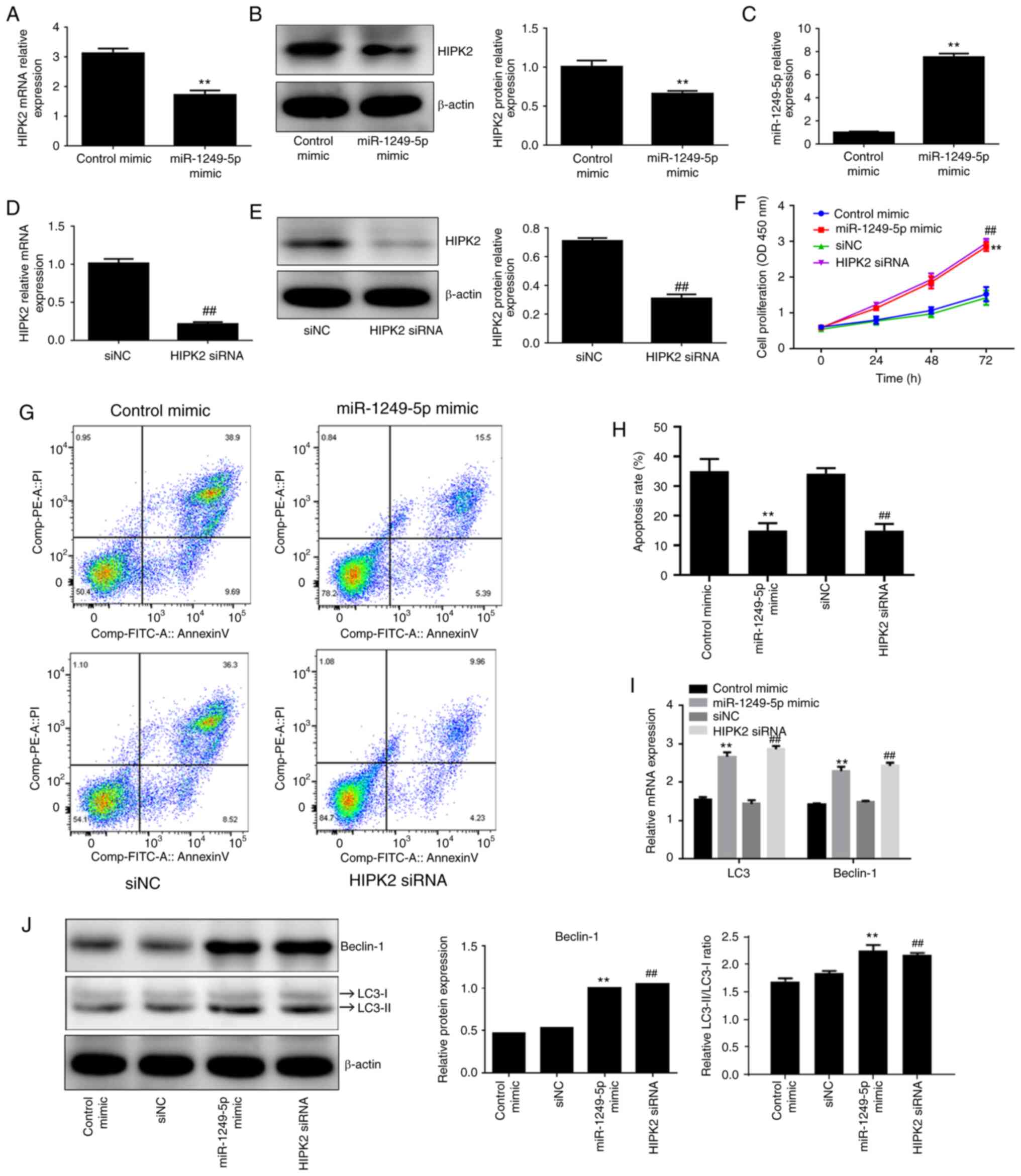 | Figure 4HIPK2 silencing mimics the effects of
miR-1249-5p in irradiation-treated BMSCs. Effect of miR-1249-5p
overexpression on the (A) mRNA and (B) protein expression levels of
HIPK2 was detected using RT-qPCR and western blotting,
respectively. (C) Transfection efficiency of miR-1249-5p
overexpression was detected using RT-qPCR. Transfection efficiency
of HIPK2 silencing on (D) mRNA and (E) protein expression levels
was detected using RT-qPCR and western blotting. (F) Effect of
miR-1249-5p overexpression and HIPK2 silencing on the proliferation
of irradiation-treated BMSCs was measured using Cell Counting Kit-8
assays. (G and H) Apoptosis rates of irradiation-induced BMSCs
following transfection with circ-016901 siRNA was detected using
flow cytometry. Effect of miR-1249-5p overexpression and HIPK2
silencing on the (I) mRNA and (J) protein expression levels of
Beclin-1 and LC3-II/I in irradiation-treated BMSCs were detected
using RT-qPCR and western blotting, respectively. Data are
presented as mean ± standard deviation. Experiments were performed
in triplicate. **P<0.01 vs. the siNC,
##P<0.01 vs. the control mimic groups. HIPK2,
homeodomain-interacting protein kinase; miR, microRNA; BMSCs, bone
marrow mesenchymal stem cells; RT-qPCR, reverse
transcription-quantitative PCR; circ, circular; si, small
interfering; LC3, light chain 3; NC, negative control; OD, optical
density. |
Discussion
In the clinical treatment of hematological
malignancies, allogeneic BM transplantations may be performed and
TBI can be used to induce immunosuppression to eradicate malignant
cells and prevent donor BM rejection (20). TBI-induced damage is associated with
the type of irradiation, dose and time (58). The clinical choice of irradiation
can result in BM ablation and a more severe degree of
immunosuppression, which may induce treatment-related toxicity and
poor therapeutic effects (20).
Therefore, irradiation-induced damage on BM can be beneficial or
harmful depending on the application process. A previous study has
demonstrated that long-term BM suppression caused by irradiation is
primarily dependent on residual hematopoietic stem cells and BMSCs
for recovery (59). BMSCs have been
reported to be involved in the repair process of tumor tissue
following tumor resection (60) and
tumor cells secrete a variety of chemokines, including CCL5 and
CCL2 (61,62) to recruit BMSCs. Furthermore,
irradiation therapy on tumor tissues can cause damage to BMSCs in
surrounding tumor tissues (63).
The present study investigated the effect of radiation on mRNA and
protein expression of autophagy-related genes and the results
demonstrated that the mRNA and protein level of Beclin-1 were
significantly decreased in a dose-dependent manner, which indicated
that radiation may induce autophagy in BMSCs. Furthermore, LC3-II
and LC3-I expression levels were reduced in response to
irradiation; however, there was no significant difference between
the LC3-II/LC-I ratio. The mechanism underlying this phenomenon
remains unknown. Further investigations are required to elucidate
this mechanism. Additionally, the preseny study demonstrated that
irradiation upregulated the expression of circ-016901 and HIPK2 in
BMSCs and downregulated miR-1249-5p expression. Silencing of
circ-016901 attenuated the injury in irradiation-induced BMSCs by
regulating the miR-1249-5p/HIPK2 axis.
A recent study has reported that circ-016901 is
upregulated in BMSCs treated with irradiation (34). However, to the best of our
knowledge, no other studies have been reported concerning this
novel circRNA. In the present study, irradiation treatment
significantly increased the expression of circ-016901.
Additionally, circ-016901 silencing promoted proliferation and
autophagy, and inhibited apoptosis in irradiation-treated BMSCs.
Previous studies have indicated that circRNAs serve as competing
endogenous RNAs to adsorb miRNAs and regulate the expression of
target gene mRNA (64,65). Therefore, the current study focused
on the competing endogenous mechanism of circ-016901 that is
involved in radiation-induced injury of BMSCs. Online
bioinformatics analysis indicated that miR-1249-5p was a connecting
carrier for circ-016901 and HIPK2. Luciferase reporter assays
further verified that circ-016901 and HIPK2 could bind miR-1249-5p
in BMSCs. To investigate the role of miR-1249-5p in
irradiation-treated BMSCs, miR-1249-5p functional assays were
performed. The results demonstrated that miR-1249-5p overexpression
significantly promoted proliferation and autophagy, and inhibited
apoptosis; these results were similar to those obtained from
circ-016901 silencing. Notably, a previous study on colorectal
cancer cell lines reported that the protective role of miR-1249-5p
was contradicted with the inhibitory effect of miR-1249
overexpression (43), while the
positive regulatory role of miR-1249 on the proliferation in glioma
cell line (66) was similar to the
current results. These opposing regulatory functions of miR-1249
may be due to the complexity and differences in the physiological
regulatory networks of different cell lines or physiological or
pathological status.
Previous studies have demonstrated that HIPK2 may
represent a potential tumor suppressor that inhibits tumor growth
and enhance drug sensitivity via regulating certain key molecular
pathways including p53-independent apoptosis pathways and the
hypoxia inducible factor 1α (HIF-1α) pathway in tumor cells
(67,68). Research has reported that HIPK2
induces apoptosis in a dependent or non-dependent manner via the
p53 gene (69). Phosphorylation at
position 46 of p53 activates the expression of the negative
regulator p53, thereby directing cells for DNA repair or apoptosis
inhibition (53), while silencing
HIPK2 reduces apoptosis (70). The
current study revealed that miR-1249-5p overexpression
significantly downregulated the expression of HIPK2. Furthermore,
HIPK2 silencing promoted proliferation and autophagy, and inhibited
apoptosis, which mimicked the protective effects of miR-1249-5p
overexpression in irradiation-treated BMSCs. These results
confirmed that the miR-1249-5p/HIPK2 axis is involved in
circ-016901-mediated irradiation-induced injury of BMSCs. However,
the effects of circRNA-016901 on certain important markers for
osteoblast differentiation, including alkaline phosphatase (ALP),
collaged 1 (ConI), osteocalcin (OCN), osteopontin (OPN) and
Runt-related transcription factor 2 (RUNX2) were not examined.
Therefore, further studies are required to reveal the role of
circRNA-016901 in the regulation of these genes.
In summary, the present study demonstrated that
circ-016901 attenuated injury in irradiation-induced mouse BMSCs
via regulating the miR-1249-5p/HIPK2 axis. The
circRNA-016901/miR-1249-5p/HIPK2 axis may serve a crucial role in
protecting BMSCs from irradiation injury. These results indicated a
novel regulatory mechanism for BMSC radiation stress and further
elucidated the molecular mechanisms of radiobiological effects.
Future studies involving the role of circRNA-016901 in the
regulation of osteoblast differentiation markers (ALP, ConI, OCN,
OPN and RUNX2) will be conducted to investigate an effective
treatment for irradiation damage in BMSCs.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Hunan Natural
Science Foundation (grant no. 2018JJ3791), the National Natural
Science Foundation of China (grant nos. 81602801 and 81573091), the
Natural Science Foundation of Hunan Province (grant no.
2019JJ50909) and the Research Project of Hunan Health and Family
Planning Commission (grant no. B2017100).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WY, RG and XN analyzed and interpreted the data. DS,
RH and HD performed the experiments. XW wrote the manuscript and
interpreted the data. JZ contributed to the study design. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal studies were performed in accordance with the
Declaration of Helsinki and approved by the institutional Ethics
Committee of the Third Xiangya Hospital of Central South
University, Hunan, China (approval no. 2019-S534).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Berger JM, Singh P, Khrimian L, Morgan DA,
Chowdhury S, Arteaga-Solis E, Horvath TL, Domingos AI, Marsland AL,
Yadav VK, et al: Mediation of the Acute Stress Response by the
Skeleton. Cell Metab. 30:890–902.e8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hayashi M, Nakashima T, Yoshimura N,
Okamoto K, Tanaka S and Takayanagi H: Autoregulation of osteocyte
Sema3A orchestrates estrogen action and counteracts bone aging.
Cell Metab. 29:627–637.e5. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ren R, Ocampo A, Liu GH and Izpisua
Belmonte JC: Regulation of stem cell aging by metabolism and
epigenetics. Cell Metab. 26:460–474. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fan Y, Hanai JI, Le PT, Bi R, Maridas D,
DeMambro V, Figueroa CA, Kir S, Zhou X, Mannstadt M, et al:
Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell
Metab. 25:661–672. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stegen S, van Gastel N, Eelen G,
Ghesquière B, D'Anna F, Thienpont B, Goveia J, Torrekens S, Van
Looveren R, Luyten FP, et al: HIF-1α promotes glutamine-mediated
redox homeostasis and glycogen-dependent bioenergetics to support
postimplantation Bone Cell Survival. Cell Metab. 23:265–279.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li CJ, Xiao Y, Yang M, Su T, Sun X, Guo Q,
Huang Y and Luo XH: Long noncoding RNA Bmncr regulates mesenchymal
stem cell fate during skeletal aging. J Clin Invest. 128:5251–5266.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Li CJ, Cheng P, Liang MK, Chen YS, Lu Q,
Wang JY, Xia ZY, Zhou HD, Cao X, Xie H, et al: MicroRNA-188
regulates age-related switch between osteoblast and adipocyte
differentiation. J Clin Invest. 125:1509–1522. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Yu Y, Newman H, Shen L, Sharma D, Hu G,
Mirando AJ, Zhang H, Knudsen E, Zhang GF, Hilton MJ, et al:
Glutamine metabolism regulates proliferation and lineage allocation
in skeletal stem cells. Cell Metab. 29:966–978.e4. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kode A, Mosialou I, Manavalan SJ, Rathinam
CV, Friedman RA, Teruya-Feldstein J, Bhagat G, Berman E and
Kousteni S: FoxO1-dependent induction of acute myeloid leukemia by
osteoblasts in mice. Leukemia. 30:1–13. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Raaijmakers MHGP, Mukherjee S, Guo S,
Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F,
Hasserjian RP, Scadden EO, et al: Bone progenitor dysfunction
induces myelodysplasia and secondary leukaemia. Nature.
464:852–857. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Guipaud O, Jaillet C, Clément-Colmou K,
François A, Supiot S and Milliat F: The importance of the vascular
endothelial barrier in the immune-inflammatory response induced by
radiotherapy. Br J Radiol. 91:20170762. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee H, Li C, Zhang Y, Zhang D, Otterbein
LE and Jin Y: Caveolin-1 selectively regulates microRNA sorting
into microvesicles after noxious stimuli. J Exp Med. 216:2202–2220.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Singh PB, Pua HH, Happ HC, Schneider C,
von Moltke J, Locksley RM, Baumjohann D and Ansel KM: MicroRNA
regulation of type 2 innate lymphoid cell homeostasis and function
in allergic inflammation. J Exp Med. 214:3627–3643. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu Y, Wang N, Zhang S and Liang Q:
Autophagy protects bone marrow mesenchymal stem cells from
palmitate-induced apoptosis through the ROS-JNK/p38 MAPK signaling
pathways. Mol Med Rep. 18:1485–1494. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Brudno JN, Maric I, Hartman SD, Rose JJ,
Wang M, Lam N, Stetler-Stevenson M, Salem D, Yuan C, Pavletic S, et
al: T Cells genetically modified to express an anti-B-cell
maturation antigen chimeric antigen receptor cause remissions of
poor-prognosis relapsed multiple myeloma. J Clin Oncol.
36:2267–2280. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Turtle CJ, Hay KA, Hanafi LA, Li D,
Cherian S, Chen X, Wood B, Lozanski A, Byrd JC, Heimfeld S, et al:
Durable molecular remissions in chronic lymphocytic leukemia
treated with CD19-specific chimeric antigen receptor-modified T
cells after failure of ibrutinib. J Clin Oncol. 35:3010–3020.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang L, Yang F and Feng S: Allogeneic
hematopoietic stem-cell transplantation for myelofibrosis. Ther Adv
Hematol. 11:2040620720906002. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
García-García A, de Castillejo CL and
Méndez-Ferrer S: BMSCs and hematopoiesis. Immunol Lett.
168:129–135. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Damek-Poprawa M, Stefanik D, Levin LM and
Akintoye SO: Human bone marrow stromal cells display variable
anatomic site-dependent response and recovery from irradiation.
Arch Oral Biol. 55:358–364. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Paix A, Antoni D, Waissi W, Ledoux MP,
Bilger K, Fornecker L and Noel G: Total body irradiation in
allogeneic bone marrow transplantation conditioning regimens: A
review. Crit Rev Oncol Hematol. 123:138–148. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lucas D: The bone marrow microenvironment
for hematopoietic stem cells. Adv Exp Med Biol. 1041:5–18.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Le Y, Fraineau S, Chandran P, Sabloff M,
Brand M, Lavoie JR, Gagne R, Rosu-Myles M, Yauk CL, Richardson RB,
et al: Adipogenic mesenchymal stromal cells from bone marrow and
their hematopoietic supportive role: Towards understanding the
permissive marrow microenvironment in acute myeloid leukemia. Stem
Cell Rev Rep. 12:235–244. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu Z, Zhang Y, Xiao H, Yao Z, Zhang H,
Liu Q, Wu B, Nie D, Li Y, Pang Y, et al: Cotransplantation of bone
marrow-derived mesenchymal stem cells in haploidentical
hematopoietic stem cell transplantation in patients with severe
aplastic anemia: An interim summary for a multicenter phase II
trial results. Bone Marrow Transplant. 52:704–710. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rieger K, Marinets O, Fietz T, Körper S,
Sommer D, Mücke C, Reufi B, Blau WI, Thiel E and Knauf WU:
Mesenchymal stem cells remain of host origin even a long time after
allogeneic peripheral blood stem cell or bone marrow
transplantation. Exp Hematol. 33:605–611. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wu KH, Wu HP, Chan CK, Hwang SM, Peng CT
and Chao YH: The role of mesenchymal stem cells in hematopoietic
stem cell transplantation: From bench to bedsides. Cell Transplant.
22:723–729. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Herberg S, Shi X, Johnson MH, Hamrick MW,
Isales CM and Hill WD: Stromal cell-derived factor-1β mediates cell
survival through enhancing autophagy in bone marrow-derived
mesenchymal stem cells. PLoS One. 8(e58207)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
François S, Bensidhoum M, Mouiseddine M,
Mazurier C, Allenet B, Semont A, Frick J, Saché A, Bouchet S,
Thierry D, et al: Local irradiation not only induces homing of
human mesenchymal stem cells at exposed sites but promotes their
widespread engraftment to multiple organs: A study of their
quantitative distribution after irradiation damage. Stem Cells.
24:1020–1029. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mouiseddine M, François S, Semont A, Sache
A, Allenet B, Mathieu N, Frick J, Thierry D and Chapel A: Human
mesenchymal stem cells home specifically to radiation-injured
tissues in a non-obese diabetes/severe combined immunodeficiency
mouse model. Br J Radiol. 80:S49–S55. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Herberg S, Kondrikova G, Hussein KA,
Periyasamy-Thandavan S, Johnson MH, Elsalanty ME, Shi X, Hamrick
MW, Isales CM and Hill WD: Total body irradiation is permissive for
mesenchymal stem cell-mediated new bone formation following local
transplantation. Tissue Eng Part A. 20:3212–3227. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Floris G, Zhang L, Follesa P and Sun T:
Regulatory role of circular RNAs and neurological disorders. Mol
Neurobiol. 54:5156–5165. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jiang G, Ma Y, An T, Pan Y, Mo F, Zhao D,
Liu Y, Miao JN, Gu YJ, Wang Y, et al: Relationships of circular RNA
with diabetes and depression. Sci Rep. 7(7285)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P
and Wu M: CircRNA: Functions and properties of a novel potential
biomarker for cancer. Mol Cancer. 16(94)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang J, Jiang J, Huang R, Wang Y, Nie X
and Gui R: Circular RNA expression profiles are significantly
altered in mice bone marrow stromal cells after total body
irradiation. Leuk Res. 70:67–73. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Balaga O, Friedman Y and Linial M: Toward
a combinatorial nature of microRNA regulation in human cells.
Nucleic Acids Res. 40:9404–9416. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mousavi SR, Ahmadi A, Jamalkandi SA and
Salimian J: Involvement of microRNAs in physiological and
pathological processes in asthma. J Cell Physiol. 234:21547–21559.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Danka Mohammed CP, Park JS, Nam HG and Kim
K: MicroRNAs in brain aging. Mech Ageing Dev. 168:3–9.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ni J, Bucci J, Chang L, Malouf D, Graham P
and Li Y: Targeting MicroRNAs in prostate cancer radiotherapy.
Theranostics. 7:3243–3259. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lai EC: Micro RNAs are complementary to 3'
UTR sequence motifs that mediate negative post-transcriptional
regulation. Nat Genet. 30:363–364. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
41
|
Sayed D and Abdellatif M: MicroRNAs in
development and disease. Physiol Rev. 91:827–887. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu KX, Chen GP, Lin PL, Huang JC, Lin X,
Qi JC and Lin QC: Detection and analysis of apoptosis- and
autophagy-related miRNAs of mouse vascular endothelial cells in
chronic intermittent hypoxia model. Life Sci. 193:194–199.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen X, Zeng K, Xu M, Liu X, Hu X, Xu T,
He B, Pan Y, Sun H and Wang S: P53-induced miR-1249 inhibits tumor
growth, metastasis, and angiogenesis by targeting VEGFA and HMGA2.
Cell Death Dis. 10(131)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yan L, Li J, Wu Q and Chen L: Specific
miRNA expression profile in the blood serum of cardiac myxoma
patients. Oncol Lett. 16:4235–4242. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yoshii S, Hayashi Y, Iijima H, Inoue T,
Kimura K, Sakatani A, Nagai K, Fujinaga T, Hiyama S, Kodama T, et
al: Exosomal microRNAs derived from colon cancer cells promote
tumor progression by suppressing fibroblast TP53 expression. Cancer
Sci. 110:2396–2407. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Seshachalam VP, Sekar K and Hui KM:
Insights into the etiology-associated gene regulatory networks in
hepatocellular carcinoma from The Cancer Genome Atlas. J
Gastroenterol Hepatol. 33:2037–2047. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kulcheski FR, Christoff AP and Margis R:
Circular RNAs are miRNA sponges and can be used as a new class of
biomarker. J Biotechnol. 238:42–51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Rodriguez-Gil A, Ritter O, Hornung J,
Stekman H, Krüger M, Braun T, Kremmer E, Kracht M and Schmitz ML:
HIPK family kinases bind and regulate the function of the CCR4-NOT
complex. Mol Biol Cell. 27:1969–1980. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kuwano Y, Nishida K, Akaike Y, Kurokawa K,
Nishikawa T, Masuda K and Rokutan K: Homeodomain-interacting
protein kinase-2: A Critical regulator of the DNA damage response
and the epigenome. Int J Mol Sci. 17(1638)2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hashimoto K and Tsuji Y: Arsenic-induced
activation of the homeodomain-interacting protein kinase 2 (HIPK2)
to cAMP-response element binding protein (CREB) Axis. J Mol Biol.
429:64–78. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kwon MJ, Min SK, Seo J, Kim DH, Sung CO,
Lim MS, Cho J and Park HR: HIPK2 expression in progression of
cutaneous epithelial neoplasm. Int J Dermatol. 54:347–354.
2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zhang Z, Wen P, Li F, Yao C, Wang T, Liang
B, Yang Q, Ma L and He L: HIPK2 inhibits cell metastasis and
improves chemosensitivity in esophageal squamous cell carcinoma.
Exp Ther Med. 15:1113–1118. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Fan Y, Wang N, Chuang P and He JC: Role of
HIPK2 in kidney fibrosis. Kidney Int Suppl 2011. 4:97–101.
2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
D'Orazi G, Cecchinelli B, Bruno T, Manni
I, Higashimoto Y, Saito S, Gostissa M, Coen S, Marchetti A, Del Sal
G, et al: Homeodomain-interacting protein kinase-2 phosphorylates
p53 at Ser 46 and mediates apoptosis. Nat Cell Biol. 4:11–19.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
55
|
Moffitt AD, Brignolo LL, Ardeshir A and
Creamer-Hente MA: The role of emotional contagion in the distress
exhibited by grouped mice exposed to CO2. J Am Assoc Lab
Anim Sci. 58:430–437. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wagner EM: Monitoring gene expression:
Quantitative real-time rt-PCR. Methods Mol Biol. 1027:19–45.
2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wilke C, Holtan SG, Sharkey L, DeFor T,
Arora M, Premakanthan P, Yohe S, Vagge S, Zhou D, Holter
Chakrabarty JL, et al: Marrow damage and hematopoietic recovery
following allogeneic bone marrow transplantation for acute
leukemias: Effect of radiation dose and conditioning regimen.
Radiother Oncol. 118:65–71. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhou J, Pang H, Li W, Liu Q, Xu L, Liu Q
and Liu Y: Effects of Lycium barbarum polysaccharides on
apoptosis, cellular adhesion, and oxidative damage in bone marrow
mononuclear cells of mice exposed to ionizing radiation injury.
BioMed Res Int. 2016:1–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hang HL and Xia Q: Role of BMSCs in liver
regeneration and metastasis after hepatectomy. World J
Gastroenterol. 20:126–132. 2014.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Li Y, Zheng Y, Li T, Wang Q, Qian J, Lu Y,
Zhang M, Bi E, Yang M, Reu F, et al: Chemokines CCL2, 3, 14
stimulate macrophage bone marrow homing, proliferation, and
polarization in multiple myeloma. Oncotarget. 6:24218–24229.
2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Chen R, Lee WY-W, Zhang XH, Zhang JT, Lin
S, Xu LL, Huang B, Yang FY, Liu HL, Wang B, et al: Epigenetic
modification of the CCL5/CCR1/ERK Axis enhances glioma targeting in
dedifferentiation-reprogrammed BMSCs. Stem Cell Reports. 8:743–757.
2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zhang J, Qiu X, Xi K, Hu W, Pei H, Nie J,
Wang Z, Ding J, Shang P, Li B, et al: Therapeutic ionizing
radiation induced bone loss: A review of in vivo and in vitro
findings. Connect Tissue Res. 59:509–522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Rong D, Sun H, Li Z, Liu S, Dong C, Fu K,
Tang W and Cao H: An emerging function of circRNA-miRNAs-mRNA axis
in human diseases. Oncotarget. 8:73271–73281. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Li G, Huang M, Cai Y, Yang Y, Sun X and Ke
Y: Circ-U2AF1 promotes human glioma via derepressing
neuro-oncological ventral antigen 2 by sponging hsa-miR-7-5p. J
Cell Physiol. 234:9144–9155. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Fang B and Li G, Xu C, Hui Y and Li G:
MicroRNA miR-1249 downregulates adenomatous polyposis coli 2
expression and promotes glioma cells proliferation. Am J Transl
Res. 10:1324–1336. 2018.PubMed/NCBI
|
|
67
|
Lee S, Shang Y, Redmond SA, Urisman A,
Tang AA, Li KH, Burlingame AL, Pak RA, Jovičić A, Gitler AD, et al:
Activation of HIPK2 promotes ER stress-mediated neurodegeneration
in amyotrophic lateral sclerosis. Neuron. 91:41–55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Lin J, Zhang Q, Lu Y, Xue W, Xu Y, Zhu Y
and Hu X: Downregulation of HIPK2 increases resistance of bladder
cancer cell to cisplatin by regulating Wip1. PLoS One.
9(e98418)2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Mancini F, Pieroni L, Monteleone V, Lucà
R, Fici L, Luca E, Urbani A, Xiong S, Soddu S, Masetti R, et al:
MDM4/HIPK2/p53 cytoplasmic assembly uncovers coordinated repression
of molecules with anti-apoptotic activity during early DNA damage
response. Oncogene. 35:228–240. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Zhao YX, Zhang GY, Wang AY, Chen YH, Lin
DM and Li QF: Role of homeodomain-interacting protein kinase 2 in
the pathogenesis of tissue fibrosis in keloid-derived
keratinocytes. Ann Plast Surg. 79:546–551. 2017.PubMed/NCBI View Article : Google Scholar
|