Introduction
Osteoclast function is important in clinical
disorders of bone resorption, such as osteoarthritis, rheumatoid
arthritis and osteoporosis. Vasodilator-stimulated phosphoprotein
(VASP) has been demonstrated to be involved in several cell
processes, including differentiation and mobility (1). VASP has been reported to serve an
important role in cell migration and tumour metastasis (1). Previously, we demonstrated VASP
protein regulates osteosarcoma cell migration and metastasis,
potentially through interactions with Ras-related C3 botulinum
toxin substrate 1 (Rac1) (2). VASP
expression was significantly higher in patients with metastatic
osteosarcoma compared with patients with non-metastatic
osteosarcoma (2). VASP, a member of
the Ena/VASP family, links the cytoskeletal system to signal
transduction pathways and plays an essential role in cytoskeletal
dynamics. VASP, therefore regulates cell morphology, adhesion and
migration. In addition to its role in normal cell growth, embryonic
development and homeostasis, VASP plays an essential role in
numerous diseases, such as cancer metastasis, thrombosis,
arteriosclerosis and nephritis (2-5).
Notably, VASP has been shown to serve important roles in the
stimulation and inhibition of cell migration (2,4).
Osteoclasts are bone-resorbing cells that originate from
hematopoietic precursor cells. Osteoclasts require colony
stimulating factor and receptor activator of nuclear factor-κB
ligand (RANKL) for their survival, proliferation, differentiation
and activation. The binding of RANKL to its receptor RANK triggers
osteoclast precursors to differentiate into osteoclasts (6,7). Thus,
in the present study, the effects of VASP in osteoclast
differentiation and mobility were investigated, providing a basis
for exploring novel mechanisms of bone diseases. The molecular
details of the functional role of VASP in cell motility and
migration remain unknown. NF-κB signalling mediates RANK
ligand-induced osteoclastogenesis, and the inhibition of NF-κB has
been proposed as an effective approach to inhibiting osteoclast
formation and bone resorptive activity (8,9). The
transcription factor NF-κB consists of several subunits capable of
crossing the nuclear membrane, binding to specific promoters and
regulating various signalling pathways, which are essential for
normal cellular functions and development, including skeletal
development (8,9). The present study investigated the
effects of silencing VASP on the expression of αV-integrin and
tartrate-resistant acid phosphatase (TRAP), and lamellipodia
protrusion in murine macrophage RAW264.7 cells.
Materials and methods
Materials
Recombinant murine sRANKL (cat. no. 315-11C) was
purchased from PeproTech, Inc. The antibodies used for fluorescent
microscopy were as follows: Anti-VASP (cat. no. 13472-1-AP; 1:100;
ProteinTech Group, Inc.), Phalloidin (cat. no. P5282; 1:100;
Sigma-Aldrich; Merck KGaA), DAPI (1:1,000; cat. no. C1002; Beyotime
Institute of Biotechnology). Antibodies used for western blotting
experiments were anti-GAPDH (cat. no. 60004-1-Ig; 1:50,000;
ProteinTech Group, Inc.), anti-VASP (cat. no. 13472-1-AP; 1:1,000;
ProteinTech Group, Inc.), anti-TRAP (cat. no. sc-376875; 1:1,000;
Santa Cruz Biotechnology, Inc.), anti-αV integrin (cat. no.
10569-1-AP; 1:1,000; ProteinTech Group, Inc.), anti-IκBα (cat. no.
10268-1-AP; 1:1,000; ProteinTech Group, Inc.), anti P-IκBα (cat.
no. 13921-1; 1:1,000; Cayman Chemical Company), anti-NF-kB p65
(cat. no. ABP0167), anti-lamin A (cat. no. ABP0098), anti-nuclear
factor of activated T cells cytoplasmic 1 (NFATc1) (cat. no.
ABP53112) (all 1:1,000; Abbkine Scientific Co., Ltd.) and c-Fos
(cat. no. AF6489; 1:1,000; Beyotime Institute of Biotechnology).
Cy3-labeled goat anti-mouse was used as secondary antibody (cat.
no. A0521; 1:500; Beyotime Institute of Biotechnology).
Cell culture and transfection
The RAW 264.7 mouse monocyte macrophage cell line
was used as an osteoclast precursor. RAW 264.7 cells were obtained
from the American Type Culture Collection and maintained in α-MEM
(cat. no. PM150421; Procell Life Science & Technology Co.,
Ltd.) containing 10% FBS (cat. no. 10099-141; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (cat. no. ST488;
Beyotime Institute of Biotechnology) in a humidified atmosphere (5%
CO2, 37˚C). Cells were checked for mycoplasma
contamination on regular basis. After 3 days of cell growth, the
RAW 264.7 cells were treated with 50 ng/ml sRANKL for 5 days in
order to induce cell differentiation (i.e. osteoclastogenesis)
(10,11). sRANKL was dissolved in PBS and PBS
alone was used as the mock control.
For establishing stable clones of the VASP-siRNA,
scrambled and empty vector, 5x103 RAW 264.7 cells were
seeded per well in 6-well plates (2). After the cells were grown to 70-80%
confluence, transfection was performed using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.).
After preparation of the Lipofectamine 2000/siRNA mix according to
the manufacturer's protocol, 200 µl mix (5 µl Lipofectamine 2000
and 10 µl siRNA in MEM without FBS; total 200 µl) was added to each
well and the cells were incubated at 37˚C in a 5% CO2
incubator for 6 h. Stable clones were selected using blasticidin
(cat. no. ST018; Beyotime Institute of Biotechnology) at 5 µg/ml
for 3 weeks. VASP was silenced using specific siRNA (synthesized by
Guangzhou RiboBio Co., Ltd.) which are as follows: forward,
5'-GGGCUACUGUGAUGCUUUATT-3' and reverse,
3'-TTCCCGAUGACACUACGAAAU-5' and the scrambled sequence was used as
a negative control (forward, 5'-UUCUCCGAACGUGUCACGUTT-3' and
reverse, 3'-TTAAGAGGCUUGCACAGUGCA-5'). Prior to subsequent
experiments, cell were cultured in α-MEM containing 10% FBS for 2-6
h.
Isolation of RNA and RT-PCR
Total RNA was extracted from RAW 264.7 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. First-strand
complementary DNA (cDNA) was synthesized from the total RNA in a
final volume of 20 µl using Superscript II reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.) containing 1 µl
Oligo(dT)12-18, 1 µl dNTP Mix, 4 µl 5X First-Strand
Buffer and 1 µl reverse transcriptase. cDNA was amplified using
gene specific primers via PCR. The PCR conditions were as follows:
A holding stage of 94˚C for 3 min, followed by 40 cycles at 95˚C
for 30 sec, 60˚C for 30 sec and 72˚C for 30 sec. Primers used are
listed as follows: VASP forward, 5'-GGGCTACTGTGATGCTTTA-3' and
reverse 5'-GAATGATGGCACAGTTGATA-3 (174 bp); αV integrin forward,
5'-TTCTCGGTGGTCCTGGTAG-3' and reverse, 5'-ACATCTGCGTAATCATCCCC-3'
(370 bp); TRAP forward, 5'-CCCAGCCCTTACTACCGTTT-3' and reverse,
5'-TGCTTTTTGAGCCAGGACAG-3' (176 bp); and GAPDH forward,
5'-ATGGGTGTGAACCACGAGA-3' and reverse, 5'-CAGGGATGATGTTCTGGGCA-3'
(229 bp). Relative gene expression was calculated using the
standard 2-ΔΔCq method (12). Data are presented as the fold-change
in gene expression normalized to the level of GAPDH.
Microscopy
Cells were seeded onto glass coverslips in 24-well
plates at concentration of 5x103 cells/well in medium
(α-MEM and 10% FBS) with 50 ng/ml sRANKL for 5 days with or without
knockdown of VASP. Following incubation, coverslips were washed
three times with PBS. Cells were fixed with 4% formaldehyde for 15
min and then washed with PBS thrice at room temperature. Before
blocking with 5% BSA (cat. no. SW3015; Beijing Solarbio Science
& Technology Co., Ltd.) for 30 min cells were immersed in 0.5%
Triton X-100 for 20 min and washed thrice with PBS at room
temperature. VASP antibody (1:100) was added and the cells were
incubated overnight at 4˚C. The next day, the cells were incubated
with goat anti-mouse polyclonal secondary antibody for 1 h at room
temperature. After washing the cells with PBS, Phalloidin (5 µg/ml)
was added for F-actin staining and the cells were incubated for 1 h
at room temperature. Subsequently, cells were washed with PBS and
DAPI (5 µg/ml) was added to stain the nuclei for 5 min.
Representative images were captured using a C2+ confocal microscope
system (Nikon Corporation) at x400 magnification.
Trypan blue exclusion assay (viability
assay)
RANKL-treated RAW264.7 cells were harvested and
suspended with 0.4% trypan blue solution (cat. no. ST798; Beyotime
Institute of Biotechnology) for 3 min at room temperature. The
cells were counted using a haemocytometer under an inverted
microscope (CKX53; Olympus Corporation) and cells that were
observed to exclude the dye were considered viable, as described
previously (13).
TRAP staining
TRAP staining was performed to determine the
pre-osteoclasts (RAW264.7 cells) and multinuclear mature
osteoclasts using commercially available kit (Sigma-Aldrich; Merck
KGaA, 387A). RAW 264.7 cells (6x103 cells/well) were
plated into 24-well plates and allowed to attach overnight, and 50
ng/ml RANKL was added the next day. RAW 264.7 cells were allowed to
differentiate for different numbers of days. After incubation, the
RANKL-treated RAW 264.7 cells were fixed with fixative solution (25
ml citrate solution, 65 ml acetone and 8 ml of 37% formaldehyde) at
room temperature for 30 sec and TRAP staining solution was added
and incubated at 37˚C for 1 h in the dark. The morphology was
observed using a Leica DMI6000 B microscope (Leica Microsystems
GmbH) at x200 magnification. The number of TRAP-positive
multinucleated cells was counted in 20 fields per treatment and the
average number of TRAP-positive multinucleated cells in a
triplicate experiment are shown in the results.
Western blotting
Cell lysates were prepared in
radioimmunoprecipitation assay (RIPA) buffer with a protease and
phosphatase cocktail inhibitor (Thermo Fisher Scientific, Inc.).
Cellular debris was cleared from lysates by centrifugation at
14,000 x g for 20 min at 4˚C, and the protein concentration was
determined using a BCA protein assay (Pierce; Thermo Fisher
Scientific, Inc.). Equal amounts of cell lysates containing 30 µg
protein were separated on an 12% gel using SDS-PAGE, transferred to
a PVDF membrane (Bio-Rad Laboratories, Inc.), blocked with 5% BSA
(cat. no. SW3015; Beijing Solarbio Science & Technology Co.,
Ltd.) for 2 h at room temperature, and then visualized with
enhanced chemiluminescence substrate (Pierce; Thermo Fisher
Scientific, Inc.) as previously described (14,15).
GAPDH was used as a reference protein and lamin was used as a
reference for nuclear p65. Quantification of western blotting was
performed using ImageJ software (National Institutes of
Health).
Cell adhesion assays
RAW 264.7 cells were cultured on a 96-well plate at
density of 5x104 cells/well for 3 h. Unbound cells were
removed by washing with PBS prior to the addition of 50 µl/well
hexosaminidase substrate (3.75 mM 4-Nitrophenyl
N-acetyl-β-D-glucosaminide, 0.25% Triton X-100, 0.05 M citrate
buffer, pH 5.0). After 2 h, 75 µl/well development buffer (5 mM
EDTA, 50 mM glycine, pH 10.4) was added and plates were read at 405
nm as previously described (2,16).
Statistical analysis
Statistical analysis was performed using a
two-tailed unpaired Student's t-test or ANOVA with Tukey's post hoc
test, and was based on a minimum of three replicates using Prism 5
for Mac statistical software (GraphPad Software, Inc.). P<0.05
was considered to indicate a statistically significant
difference.
Results
RANKL treatment increases osteoclast
differentiation
RANKL treatment helps to induce cell differentiation
(i.e. osteoclastogenesis) (10,17).
Fig. 1 demonstrates that the RANKL
treatment significantly increased the gene and protein expression
of VASP, αV integrin and TRAP in RAW 264.7 cells over 7 days as
determined by RT-PCR (Fig. 1A and
B) and western blot analysis
(Fig. 1B and C), respectively, when compared to basal
levels (day 0). However, no significant differences in the
expression of the three genes were observed during the 7 days in
the negative control group (Fig.
S1). Furthermore, the effect of RANKL treatment on osteoclast
differentiation and maturation was explored using a TRAP assay.
TRAP is an early enzyme marker of osteoclastogenesis, which is
readily detectable in mononuclear pre-osteoclasts, through to
mature osteoclasts (18). Fig. 1E and F shows the number of TRAP-positive
multinucleated osteoclasts differentiated from RAW 264.7 cells in
the presence of RANKL for a period of 7 days.
Knockdown of VASP downregulates the
expression of osteoclast-specific genes
VASP has been reported to play an important role in
cell behaviour, including in cell differentiation (1,2). To
further elucidate the role of VASP in osteoclast differentiation,
VASP expression was knocked down in RAW 264.7 cells using si-RNA
post-RANKL treatment. Fig. 2
demonstrates a successful VASP-knockdown in the RAW cells as
determined at the transcriptional (RT-PCR) and translation (western
blotting) level when compared with the mock group.
Next, the effect of VASP-knockdown on the
osteoclast-specific genes in differentiated RAW 264.7 cells was
determined. A significant decrease in the gene (Fig. 3A-D) (compared with the mock group)
and protein (compared with the siNC group) (Fig. 3E-F) expression of αV-integrin and
TRAP was observed in the RANKL-treated VASP-knockdown cells.
Furthermore, a significant decrease in multinucleated cell
formation was observed in VASP-knockdown cells as determined by the
TRAP staining (P<0.05; Fig. 4A
and B). A Trypan blue exclusion
assay (viability assay) was performed as a control before
proceeding to the TARP staining to rule out the possibility of
apoptosis or cell death. No significant differences in the
viability of cells were observed following VASP-knockdown (Fig. S2). These results demonstrate the
role of VASP in the osteoclast differentiation and its effect on
osteoclast-specific genes. Future studies should explore the
underlying mechanism behind the low level of cell differentiation
following VASP silencing.
 | Figure 3Effect of VASP silencing. Gene
expression of (A) VASP, (B) αV-integrin and (C) TRAP after using
siRNA as measured by RT-PCR. (D) Representative gel images of
RT-PCR for VASP, αV-integrin, TRAP and GAPDH. Protein expression of
(E) VASP, (F) αV-integrin and (G) TRAP after using si-RNA as
measured by western blotting. (H) Representative western blotting
for VASP, αV-integrin, TRAP and GAPDH. GAPDH was used as the
loading control. Data are presented as the mean ± standard
deviation (three repeats). ***P<0.001 compared with
mock. VASP, vasodilator-stimulated phosphoprotein; TRAP,
tartrate-resistant acid phosphatase; NC, negative control; si,
small interfering. |
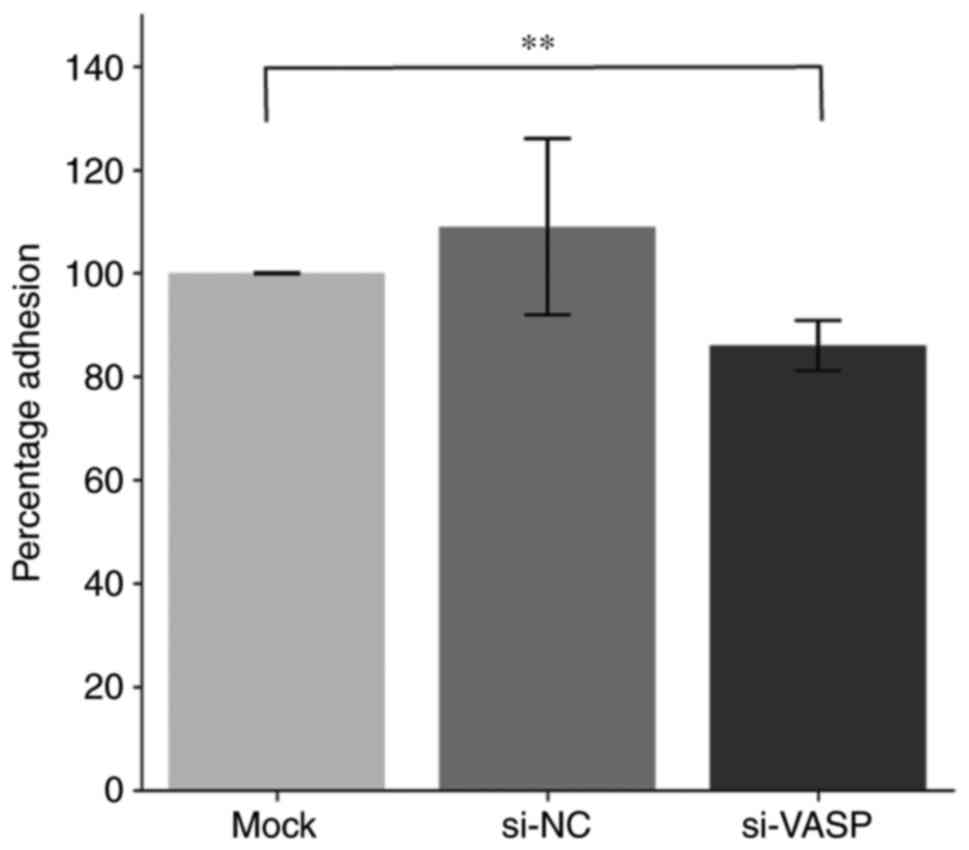
VASP-knockdown inhibits lamellipodia
protrusion and cell adhesion of osteoclasts
VASP protein acts as an actin anti-capping protein
and can therefore regulate the formation of membrane protrusions,
such as lamellipodia and filopodia, which consequently affects cell
motility and tumour metastasis (19,20).
The effect of VASP-knockdown on the morphological changes of
lamellipodia protrusion and actin fibbers was determined by
microscopy in differentiated murine macrophage RAW 264.7 cells. A
decrease in the length of F-actin fibers and lamellipodia
protrusion in VASP-knockdown cells compared with their controls was
observed (Fig. 5). Additionally,
VASP-knockdown significantly reduced the percentage of RAW 264.7
cell adhesion compared with the mock control group (Fig. 6).
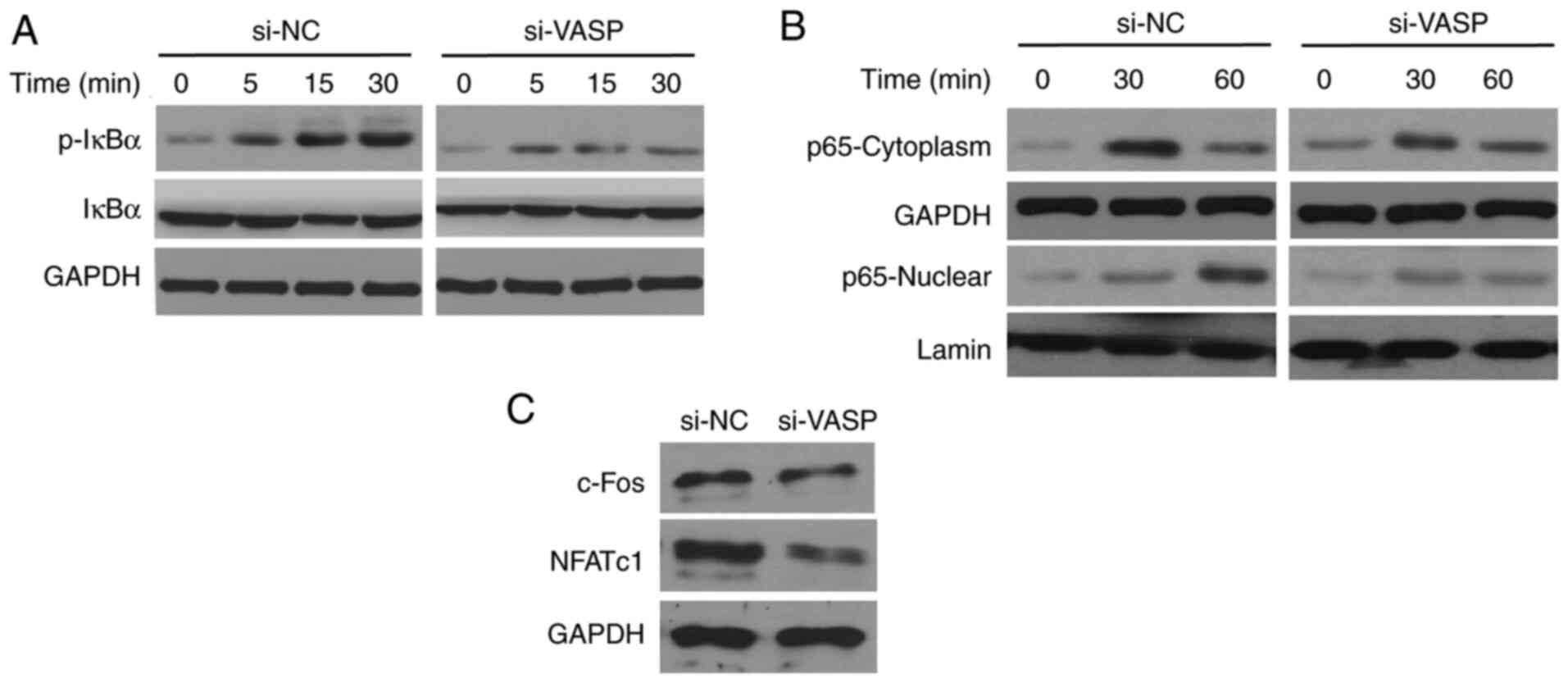
VASP-knockdown reduces the activation
of NF-κB signaling
The effects of VASP-knockdown on NF-κB signalling
were determined. RANKL, a known activator of the NF-κB pathway
triggers a rapid increase and mobilization of NF-κB components, and
IκB kinase (IKK) complex formation (7). RANKL treatment increased the
phosphorylation of IκBα in a time-dependent manner in RAW 264.7
cells (Fig. 7). Notably, the levels
of RANKL-induced IκBα phosphorylation were low in the
VASP-knockdown cells compared with the scrambled control cells.
Additionally, RANKL-induced nuclear translocation of p65 was
significantly reduced in the cells treated with si-VASP. In
addition, the expression of NF-κB downstream signalling factors,
including c-Fos and NFATc1, was also inhibited in VASP-siRNA cells
compared with scrambled control cells. These results confirm the
involvement of NF-κB signalling in the modulation of osteoclast
differentiation by VASP.
Discussion
Osteoclasts play an essential role in bone
homeostasis as characterized by their bone resorptive activity.
RANKL, an indispensable cytokine for osteoclastogenesis, activates
a series of intracellular signal pathways once it binds to its
receptor, which in turn initiates monocyte/macrophage
differentiation into multinucleated osteoclasts in vitro
(7,21-23).
The feasibility to generate osteoclast-type cells in vitro
makes it easy for researchers to investigate the molecular
mechanisms of this process (7,21-23).
In the present study, RANKL-differentiated RAW 276.4 cells
significantly increased the expression of VASP, TRAP and
αV-integrin. These results are consistent with the previous
findings, whereby TRAP mRNA expression has been demonstrated to be
upregulated by RANKL treatment in different experimental settings
(24). Increased TRAP expression
has been used as a marker of bone absorption of bone metastases in
cancer (25).
VASP plays an important role in cellular activities
that depend on cytoskeletal rearrangement. A positive correlation
between VASP expression, and pathological stage and metastasis
indicates its importance in various pathophysiology (2,5,7,26).
Our previous work demonstrated a significant increase in VASP
expression in samples from patients with metastatic osteosarcoma
compared with patients with non-metastatic osteosarcoma (2). The data from the VASP-knockdown
experiment in the present study demonstrated the importance of VASP
in the regulation of osteoclast-specific genes in differentiated
murine osteoclasts.
Osteoclasts are large, multinucleated cells that
derive from the monocyte lineage, and express signalling molecules,
such as integrins, paxillin, vinculin, talin, protein kinases and
actin-associated molecules (27,28).
Although mature osteoclasts express numerous integrins, it is
considered that integrins of the β1, β2 and αv families are the
major adhesion proteins (27,29).
Integrins are highly induced in differentiating and mature
osteoclasts, and bind to extracellular matrix components.
VASP-knockdown significantly reduced the gene and protein
expression of the αV-integrin in murine osteoclasts in the current
study. These results further indicate the importance of VASP in
osteosarcoma metastasis.
The importance of VASP proteins in facilitating
actin-filament and membrane protrusions is well-established
(19). However, the cell motility
affected by VASP is not completely established and appears to be
cell type-dependent. VASP-knockdown decreased filipodia formation
and length of F-actin fibers when compared with the control in the
current study. Day 5 was chosen to observe F-actin fibers and
lamellipodia protrusion using a confocal microscope, because a
significant increase in the expression of osteoclastogenesis genes
at day 5 post RANKL treatment has been observed in the present
study and other previous studies (11,30,31).
These data further support VASP as a positive regulator of
osteoclast cell migration. Overexpression of VASP in wild-type NIH
3T3 cells results in metaplasia to cancer cells (32,33).
VASP-expressing Mg-63 osteosarcoma cells have high migration
capacity compared with reduced VASP-expressing Saos-2 cells
(2), which corroborates the results
of the present study.
RANKL signalling is considered the main target of
anti-resorptive agents that suppress osteoclast activation and bone
loss. RANKL-induced osteoclast differentiation activates NF-κB and
NFATc1. The activation of NF-κB and NFATc1 is mediated by adaptors,
including TRAF6 and IKK, that leads to osteoclast differentiation
(34). To understand the possible
mechanisms by which VASP modulates osteoclast differentiation, the
effect of VASP on the NF-κB activation was investigated in RAW
264.7 mouse monocyte macrophages. Post-translational modification
of NF-κB subfamily proteins is critical in the modulation of NF-κB
activation, especially, phosphorylation of p65 and IκB kinase to
induce osteoclastogenesis (34,35).
Osteoclastogenic genes, such as TRAP, are also activated via this
pathway (36). Furthermore, the
results of the present study demonstrated that the number of
TRAP-positive osteoclasts decreased significantly with silencing of
VASP. Additionally, RANKL treatment increased NF-κB expression and
phosphorylation of the p65 and Iκk in RAW264.7 macrophages.
Pre-treatment with si-VASP significantly inhibited the
RANKL-induced expression of NF-κB complex subunits. Consequently,
these results demonstrate the importance of NF-κB signaling in the
modulation of osteoclast differentiation by VASP in
RANKL-stimulated macrophages. In addition to the NF-κB signalling,
we have previously demonstrated the role of the c-Fos/NFATc1
pathway in osteoclast development and arrest osteoclastogenesis
(16).
In the present study, a decrease in the
RANKL-induced c-Fos and NFATc1 expression was observed in
VASP-knockdown cells. Taken together, the present findings
demonstrate that silencing of VASP inhibits RANKL-induced
osteoclast differentiation in vitro, and prevents activation
of pivotal transcription factors, such as NF-κB, c-Fos and NFATc1.
Caution should be extrapolated to interpret the results, as it is
unclear whether these changes are directly caused by VASP by
regulating the expression of effected proteins or indirectly as a
product of upstream or downstream genes in the VASP signalling e.g.
cyclic nucleotide-dependent kinases PKA and PKG (2,4,36) of
VASP silencing. However, bearing in mind the diversity of the
intracellular and intercellular signalling networks that are built
around osteoclast maturation and differentiation, targeting VASP
more specifically might be promising for the treatment or
prevention of osteoclast-related diseases. Therefore, further
investigations into the precise mechanisms by which VASP modulates
the osteoclastogenesis are required.
Supplementary Material
RAW 264.7 cells treated with PBS
(negative control) had no significant change in the expression of
genes as determined by RT-PCR. Data are presented as the mean ±
standard deviation (three repeats). VASP, vasodilator-stimulated
phosphoprotein; TRAP, tartrate-resistant acid phosphatase.
Trypan blue exclusion assay (viability
assay). Viability of RAW 264.7 was determined before proceeding to
TARP staining in different treatment groups. No significant change
observed among different treatments were observed. Data are
presented as the mean ± standard deviation (three repeats). VASP,
vasodilator-stimulated phosphoprotein; NC, negative control; si,
small interfering.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science Foundation of
Hubei Provincial Hospital of Traditional Chinese Medicine (grant
nos. 20190619 and 20191011) and the Hubei Provincial Natural
Science Foundation of China (grant nos. 2016CFB363 and
2016CFB187).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HH performed the cell culture, the transfection and
the TRAP-staining. CL and HZ performed the PCR and western
blotting. YH conceived and designed the research study. GW
performed the statistical analysis, and analysed and interpreted
the data. HH and GW wrote the manuscripts and all authors read and
approved it.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rachubik P and Piwkowska A: The role of
vasodilator-stimulated phosphoprotein in podocyte functioning. Cell
Biol Int. 43:1092–1101. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu G, Wei L, Yu A, Zhang M, Qi B, Su K, Hu
X and Wang J: Vasodilator-stimulated phosphoprotein regulates
osteosarcoma cell migration. Oncol Rep. 26:1609–1615.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li K, Ma YB, Tian YH, Xu XL, Gao Y, He YQ,
Pan WT, Zhang JW, He CJ and Wei L: Silencing lncRNA SNHG6
suppresses proliferation and invasion of breast cancer cells
through miR-26a/VASP axis. Pathol Res Pract.
215(152575)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Aszódi A, Pfeifer A, Ahmad M, Glauner M,
Zhou XH, Ny L, Andersson KE, Kehrel B, Offermanns S and Fässler R:
The vasodilator-stimulated phosphoprotein (VASP) is involved in
cGMP- and cAMP-mediated inhibition of agonist-induced platelet
aggregation, but is dispensable for smooth muscle function. EMBO J.
18:37–48. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bonello L, Camoin-Jau L, Armero S, Com O,
Arques S, Burignat-Bonello C, Giacomoni MP, Bonello R, Collet F,
Rossi P, et al: Tailored clopidogrel loading dose according to
platelet reactivity monitoring to prevent acute and subacute stent
thrombosis. Am J Cardiol. 103:5–10. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Park JH, Lee NK and Lee SY: Current
understanding of RANK signaling in osteoclast differentiation and
maturation. Mol Cells. 40:706–713. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
van Dam PA, Verhoeven Y, Trinh XB, Wouters
A, Lardon F, Prenen H, Smits E, Baldewijns M and Lammens M:
RANK/RANKL signaling inhibition may improve the effectiveness of
checkpoint blockade in cancer treatment. Crit Rev Oncol Hematol.
133:85–91. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cheng AM, Rizzo-DeLeon N, Wilson CL, Lee
WJ, Tateya S, Clowes AW, Schwartz MW and Kim F:
Vasodilator-stimulated phosphoprotein protects against vascular
inflammation and insulin resistance. Am J Physiol Endocrinol Metab.
307:E571–E579. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zeng XZ, He LG, Wang S, Wang K, Zhang YY,
Tao L, Li XJ and Liu SW: Aconine inhibits RANKL-induced osteoclast
differentiation in RAW264.7 cells by suppressing NF-κB and NFATc1
activation and DC-STAMP expression. Acta Pharmacol Sin. 37:255–263.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Brito C, Stavroullakis AT, Ferreira AC, Li
K, Oliveira T, Nogueira-Filho G and Prakki A: Extract of acai-berry
inhibits osteoclast differentiation and activity. Arch Oral Biol.
68:29–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee SH, Kim JK and Jang HD: Genistein
inhibits osteoclastic differentiation of RAW 264.7 cells via
regulation of ROS production and scavenging. Int J Mol Sci.
15:10605–10621. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Oliveira V, Mahajan N, Bates ML, Tripathi
C, Kim KQ, Zaher HS, Maggi LB Jr and Tomasson MH: The snoRNA target
of t(4;14) in multiple myeloma regulates ribosome biogenesis. FASEB
Bioadv. 1:404–414. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gang W, Tanjun W, Yong H, Jiajun Q, Yi Z
and Hao H: Inhibition of miR-9 decreases osteosarcoma cell
proliferation. Bosn J Basic Med Sci. 20:218–225. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mahajan N, Hoover B, Rajendram M, Shi HY,
Kawasaki K, Weibel DB and Zhang M: Maspin binds to cardiolipin in
mitochondria and triggers apoptosis. FASEB J. 33:6354–6364.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huang H, Chang EJ, Ryu J, Lee ZH, Lee Y
and Kim HH: Induction of c-Fos and NFATc1 during RANKL-stimulated
osteoclast differentiation is mediated by the p38 signaling
pathway. Biochem Biophys Res Commun. 351:99–105. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen Y, Di Grappa MA, Molyneux SD, McKee
TD, Waterhouse P, Penninger JM and Khokha R: RANKL blockade
prevents and treats aggressive osteosarcomas. Sci Transl Med.
7(317ra197)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhao H, Gu J, Dai N, Gao Q, Wang D, Song
R, Liu W, Yuan Y, Bian J, Liu X and Liu Z: Osteoprotegerin exposure
at different stages of osteoclastogenesis differentially affects
osteoclast formation and function. Cytotechnology. 68:1325–1335.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Applewhite DA, Barzik M, Kojima SI,
Svitkina TM, Gertler FB and Borisy GG: Ena/VASP proteins have an
anti-capping independent function in filopodia formation. Mol Biol
Cell. 18:2579–2591. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bear JE and Gertler FB: Ena/VASP: Towards
resolving a pointed controversy at the barbed end. J Cell Sci.
122:1947–1953. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yu J, Yun H, Shin B, Kim Y, Park ES, Choi
S, Yu J, Amarasekara DS, Kim S, Inoue JI, et al: Interaction of
tumor necrosis factor receptor-associated factor 6 (TRAF6) and Vav3
in the receptor activator of nuclear factor κB (RANK) signaling
complex enhances osteoclastogenesis. J Biol Chem. 291:20643–20660.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Amarasekara DS, Yun H, Kim S, Lee N, Kim H
and Rho J: Regulation of osteoclast differentiation by cytokine
networks. Immune Netw. 18(e8)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Feng X and Teitelbaum SL: Osteoclasts: New
insights. Bone Res. 1:11–26. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wittrant Y, Theoleyre S, Couillaud S,
Dunstan C, Heymann D and Rédini F: Regulation of osteoclast
protease expression by RANKL. Biochem Biophys Res Commun.
310:774–778. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Honig A, Rieger L, Kapp M, Krockenberger
M, Eck M, Dietl J and Kämmerer U: Increased tartrate-resistant acid
phosphatase (TRAP) expression in malignant breast, ovarian and
melanoma tissue: An investigational study. BMC Cancer.
6(199)2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu Z, Wang Y, Dou C, Xu M, Sun L, Wang L,
Yao B, Li Q, Yang W, Tu K and Liu Q: Hypoxia-induced up-regulation
of VASP promotes invasiveness and metastasis of hepatocellular
carcinoma. Theranostics. 8:4649–4663. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schmidt S, Nakchbandi I, Ruppert R,
Kawelke N, Hess MW, Pfaller K, Jurdic P, Fässler R and Moser M:
Kindlin-3-mediated signaling from multiple integrin classes is
required for osteoclast-mediated bone resorption. J Cell Biol.
192:883–897. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Linder S and Kopp P: Podosomes at a
glance. J Cell Sci. 118:2079–2082. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Helfrich MH, Nesbitt SA, Lakkakorpi PT,
Barnes MJ, Bodary SC, Shankar G, Mason WT, Mendrick DL, Väänänen HK
and Horton MA: Beta 1 integrins and osteoclast function:
Involvement in collagen recognition and bone resorption. Bone.
19:317–328. 1996.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Feng MX, Hong JX, Wang Q, Fan YY, Yuan CT,
Lei XH, Zhu M, Qin A, Chen HX and Hong D: Dihydroartemisinin
prevents breast cancer-induced osteolysis via inhibiting both
breast caner cells and osteoclasts. Sci Rep.
6(19074)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen Z, Andreev D, Oeser K, Krljanac B,
Hueber A, Kleyer A, Voehringer D, Schett G and Bozec A: Th2 and
eosinophil responses suppress inflammatory arthritis. Nat Commun.
7(11596)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tian Y, Xu L, He Y, Xu X, Li K, Ma Y, Gao
Y, Wei D and Wei L: Knockdown of RAC1 and VASP gene expression
inhibits breast cancer cell migration. Oncol Lett. 16:2151–2160.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu K, Li L, Nisson PE, Gruber C, Jessee J
and Cohen SN: Reversible tumorigenesis induced by deficiency of
vasodilator-stimulated phosphoprotein. Mol Cell Biol. 19:3696–3703.
1999.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Abu-Amer Y: NF-κB signaling and bone
resorption. Osteoporos Int. 24:2377–2386. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mussbacher M, Salzmann M, Brostjan C,
Hoesel B, Schoergenhofer C, Datler H, Hohensinner P, Basílio J,
Petzelbauer P, Assinger A and Schmid JA: Cell type-specific roles
of NF-κB linking inflammation and thrombosis. Front Immunol.
10(85)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wada T, Nakashima T, Hiroshi N and
Penninger JM: RANKL-RANK signaling in osteoclastogenesis and bone
disease. Trends Mol Med. 12:17–25. 2006.PubMed/NCBI View Article : Google Scholar
|