Case report
A 77-year-old male non-smoker, without significant
personal pathological history, presented to the Second Department
of Internal Medicine of the ‘Sf. Spiridon’ Emergency Clinical
Hospital, Iasi, Romania, complaining of chronic pruritus associated
with skin rash for 12 months and significant weight loss;
approximately 6 kg over a 2 month-period. The patient reported that
the itch was not alleviated by any medication prescribed by the
allergy specialist; several courses of antihistamine, antimycotic
and antibiotic schemes resulted in worsening symptoms. The patient
attributed the unintentional weight loss to a special diet which he
had been following for 2 months in order to manage his
pruritus.
On initial physical examination, the patient's good
general status was observed; he was perfectly conscious and had no
fever. His weight was 59 kg (versus current weight of 65 kg), with
an ideal body weight (IDW) equal to 71 kg and body mass index (BMI)
equal to 18.41 kg/m2. The patient experienced a slight
loss of subcutaneous fat and muscle mass: apparent ribs, with a
less pronounced, slight depression between them and with the
clavicle bone region visible.
The skin examination revealed a few erythematous
papules which were confluent in plaques and vaguely circumscribed;
the lesions were itchy showing scaly skin with hematic crusts
(Fig. 1).
The external chest was normal without lifts, heaves
or thrills. The point of maximal impulse was not visible and was
palpated in the 5th intercostal space at the midclavicular line.
The heart rate and rhythm were normal, i.e., no murmurs, gallops,
or rubs were auscultated. On pulmonary examination he presented no
signs of lung fluid; the pulmonary murmur was normal. The abdominal
examination revealed no significant abnormalities and normal bowel
movement. The urogenital examination showed no dysuria, no
nocturia, no change in frequency, no dribbling, and no signs of
incontinence. Testicular examination showed no pain and no
testicular abnormality.
The blood work performed showed a hemoglobin of 11.1
g/dl confirming iron deficiency anemia, eosinophilia and negative
tumor markers (carbohydrate antigen 19-9, α fetoprotein,
carcinoembryonic antigen, cyfra 21-1). The abdominal ultrasound
showed bilateral kidney stones. The chest X-ray revealed no
pulmonary formations. Due to the fact that the patient had
hypoanabolic syndrome and anemia, investigations continued with
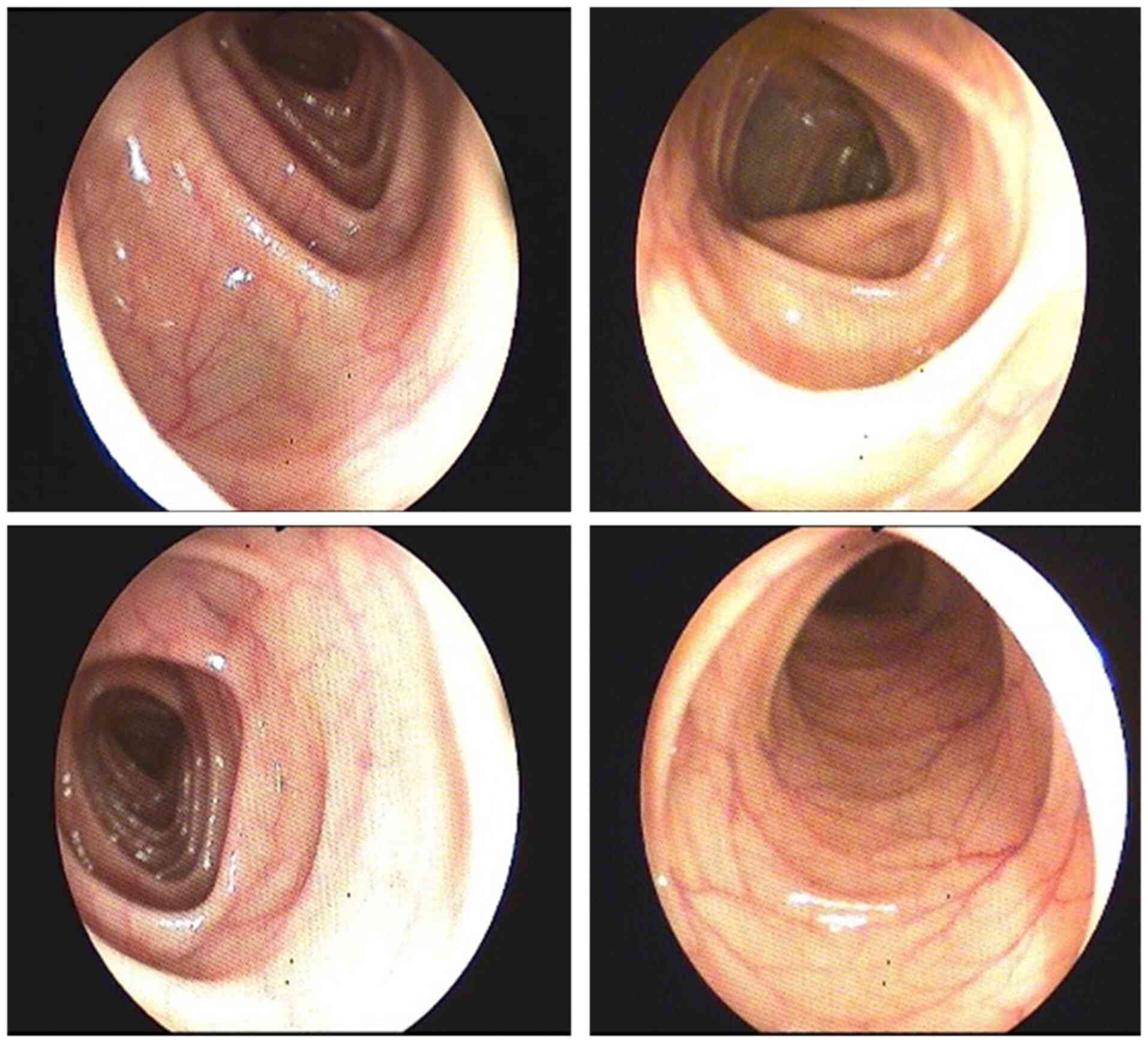
upper GI endoscopy which revealed gastroesophageal reflux (Fig. 2). Colonoscopy showed enlarged
haustral folds (Fig. 3) and a few
diverticular filling defects. There were no signs of tumors,
inflammation or bleeding.
The patient was consulted in the Dermatology Clinic
and the following diagnostic suspicions were raised: chronic
eczema, herpetiform dermatitis, and chronic prurigo. Skin biopsy
was performed and treatment was started. The patient received 4
mg/day of chlorphenamine and hydrocortison cream was applied 3
times per day.
The skin biopsy showed hyperkeratosis with ortho-
and parakeratosis, hypergranulosis and inflammatory infiltrations,
mainly limphoplasmocitary with perivascular distribution and few
eosinophils and mast cells (Fig.
4).
The patient's status declined within a week, leading
to systemic steroid being added to the medication. Considering that
the patient's progress was unfavorable in the subsequent days, and
the small intestine was not investigated, an abdominal and pelvic
computed tomography (CT) scan was performed showing a tumoral mass
located in the small bowel on the terminal ileum (Fig. 5).
The patient was transferred to the 2nd Surgery
Department of the ‘Sf. Spiridon’ Emergency Clinical Hospital, Iasi,
Romania, where right hemicolectomy with ileotransverse anastomosis
was performed. The biopsy examination from the resected tumor
fragment was classified according to the World Health Organization
Classification and Suggested Grading of Neuroendocrine Neoplasms of
the Digestive System classification-NET G2 (Fig. 6) (1). Microscopically, the tumor cells had
round or oval nuclei with ‘salt and pepper’ chromatin and
eosinophilic granular cytoplasm. The tumor nests were arranged in
trabecular, insular, or sheet-like patterns. Glandular-like
structures or palisading of the peripheral cell layers were
occasionally evident (1).
The patient gradually improved clinically after the
surgery and his prognosis was favorable on discharge. The skin
lesions healed within two weeks. The hemoglobin levels improved and
were normal shortly after the surgical intervention. Following
surgery, the patient received adjuvant chemotherapy for 6 months
with cisplatin and etoposide. The patient reported side effects
which were managed using symptom release medication. At the
follow-up appointments, the patient status remained favorable and
the blood works were within normal range.
Discussion
One of the symptoms that can decrease the life of
quality is pruritus (synonym: itch). A major impact on the quality
of life can occur owing to different sensations perceived on the
skin. Pruritus is an unpleasant sensory perception that gives an
intense sensation of scratching (2,3). Itch
can occur using hematogenic or neurogenic mediators via multiple
pathways, mainly located in the central nervous system. Pruritus
may have a different origin being determined in the central nervous
system via haematogenic or neurogenic mediators. In recent years,
particular interest has been shown in the pathogenesis of pruritus,
as well as in the nerves, receptors and mediators involved, leading
to a better understanding thereof (4). Chronic pruritus is an itchy sensation
that lasts for more than 6 weeks (5). It has been associated with neoplastic
diseases and especially with lymphoproliferative diseases.
Paraneoplastic itching has the following characteristics: i) it can
appear even before the neoplastic evidence; ii) it is not caused by
an invasive mass and iii) after the neoplasm is remitted the
itching vanishes (6).
Malignancies can be accompanied by paraneoplastic
symptoms or syndromes, which may be caused by an indirect effect of
the malignancy and can represent the first sign of cancer (7). Pruritus appears as a sign at the
beginning of the illness and is accentuated, particularly at night.
The intensity of itching can reach a peak; thus, the patients with
malignant tumors can associate real skin problems including
excoriations, hyperpigmentation, lichenification or prurigo
nodules. Paraneoplastic pruritus is most often associated with
Hodgkin's lymphoma (the reported prevalence is 30%) (8), and other lymphomas, including leukemia
and polycythemia vera (9); however,
studies show that neuroendocrine tumors (NETs) associate itch as
well.
NETs are defined as heterogeneous tumors that
originate in the cells with secretory function of the
neuroendocrine system (10). In
recent decades, in the US and around the world, the number of
patients with NETs has increased considerably (11). In adults, NETs have an average
diagnosis of 66 years of age with a higher incidence for women. In
children, the incidence is approximately 0.995 cases per 100.000 in
patients under 20 years of age. Non-specific symptoms of NETs lead
to a low degree of diagnosis (12).
Small bowel (SB)-NETs occur more often during the sixth and seventh
decade of life. In 1867, Langhans was the first to describe a
neuroendocrine tumor in the small intestine, a polypoid tumor in
the small bowel identified at an autopsy. Research continued and in
1988, Lubarsch reported two cases of patients in whom numerous
small tumors of the ileum were identified (13). The description of the first
carcinoid syndrome in a patient who experienced diarrhea and
dyspnea aggravated by food was made by Ransom, who at an autopsy
identified that the patient had diffuse hepatic metastases and a
distal ileal mass (14). However,
most patients with NETs already have metastases at the time of
diagnosis (15). Secondary
metastatic lesions were found in approximately 21% of
population-based studies during diagnosis confirmation (11). On the other hand, percentages as
high as 56-69% have been reported in retrospective chart reviews
(16).
SB-NETs have a slow growth and cause metastases in
the lymph nodes and the liver. The mortality caused by this tumor
is in 80% of cases due to liver failure and in 16% due to bowel
obstruction. The small size of SB-NETs that does not express a
specific symptomatology determines a late diagnosis in the case of
most patients. The majority of SB-NETs are identified at an
advanced stage when the tumors cause symptoms specific to local
complication. Enterochromaffin (EC) cells are responsible for
5-hydroxytryptamine (5-HT) secretion and are most often involved in
NETs in the small intestine. 5-HT stored in EC cells is released
under the action of various stimuli: mechanical stimulation, and
nutrients and chemical stimulators, such as acetylcholine.
Hypersecretion of serotonin from the small intestine NETs causes
numerous symptoms, such as fatigue, diarrhea, flushes,
bronchoconstriction, and feeling of squeezing or pressure in the
chest. These symptoms summarize the carcinoid syndrome that is most
common in advanced metastatic stages. The characteristic flush of
NETs can lead to tumor localization. Cyanotic flushing associated
with a burning sensation lasting no more than 1 min is associated
with midgut tumors. Tumors with anterior location cause generalized
pruritus (17).
CT is the imaging scan that can guide the diagnosis
of NETs in the small intestine (18). CT detection sensitivity for NETs in
the small bowel is from 7 to 38%. The sensitivity can reach to 82%
if the presence of mesenteric lymphadenopathy/fibrosis is
interpreted (19).
Circulating biomarker determination is considered
useful to aid in diagnosis; however, it is not a mandatory
determination for establishing a NETs diagnosis. Attempts have been
made to measure some biomarkers, such as chromogranin A,
pancreastatin, neurokinin A, neuron-specific enolase,
pre-progastrin, pancreatic polypeptide, serotonin, and urinary or
plasma 5-hydroxyindoleacetic acid; however, these biomarkers cannot
provide data on tumor location. The differentiating usefulness
between functional and non-functional tumors has been described by
experts especially for patients with non-specific symptoms in which
a carcinoid syndrome, induced by neuroendocrine tumors, is
suspected. No consensus has been reached on the value of plasma
biomarkers and tumor grade. Measurements of circulating biomarkers
cannot differentiate low-level malignancy from high-grade disease
(20).
Patients with NETs have a range of therapeutic
options that must be adapted to the stage and disease evolution
degree, such as somatostatin receptor agonist blockade, targeted
radionuclides, immunotherapy (interferon), cytotoxic chemotherapy,
rationally designed targeted drugs, external radiation,
interventional radiological approaches, and surgery (either for
cure or palliative debulking) (21,22).
In conclusion, diagnosing NET G2 was possible due to
the atypic paraneoplastic sign: chronic pruritus. While there are
not many studies suggesting a clear bond between neoplasia and
pruritus, it is worth discussing and researching this vast
interdisciplinary domain. This case study highlights the
association between itch and malignancy, and presents an atypical
way of NETs presentation while tumor markers remained negative and
unreliable.
Acknowledgements
The authors thank Dr Karina Bulavschi from the
Regional Institute of Oncology, Iasi, Romania, for support in
collecting patient data and Iulian Bârsan for technical
support.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LȘ, AC, VȘ, AS, DV, CP, EDG carried out the patient
investigation and ORP, REH, AEC, CB, OS data curation and
interpretation. CDL performed the surgical intervention. GV, CL,
LGV carried out the writing and original draft preparation and
revised it for important intellectual content. GV, GP, GD, MC
performed the literature data review and LȘ, VȘ, AC finally
reviewed the manuscript. AC and VȘ confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript. All authors contributed equally to this work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient signed informed written consent about
hospitalization, diagnostic and treatment interventions, and future
data publication.
Competing interests
The authors declare no competing interests.
References
|
1
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO Classification of Tumours of the Digestive System.
4th Edition, Volume 3, 2010.
|
|
2
|
Patel T and Yosipovitch G: Therapy of
pruritus. Expert Opin Pharmacother. 11:1673–1682. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pogatzki-Zahn E, Marziniak M, Schneider G,
Luger TA and Stander S: Chronic pruritus: Targets, mechanisms and
future therapies. Drug News Perspect. 21:541–551. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Grundmann S and Ständer S: Chronic
pruritus: Clinics and treatment. Ann Dermatol. 23:1–11.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rajagopalan M, Saraswat A, Godse K,
Shankar DS, Kandhari S, Shenoi SD, Tahiliani S and Zawar VV:
Diagnosis and management of chronic pruritus: An expert consensus
review. Indian J Dermatol. 62:7–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yosipovitch G: Chronic pruritus: A
paraneoplastic sign. Dermatol Ther. 23:590–596. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Beigi M, Häberle M, Gschwendtner A, Baum U
and Weisshaar E: Generalized chronic itch as a first sign of
malignancy resembling paraneoplastic sensomotoric neuropathy. Acta
Derm Venereol. 98:526–527. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Villafranca JJ, Siles MG, Casanova M,
Goitia BT and Dominguez AR: Paraneoplastic pruritus presenting with
Hodgkin's lymphoma: A case report. J Med Case Rep.
8(300)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Alpsoy E: Paraneoplastic pruritus and
paraneoplastic erythroderma. Türkderm. 47 (Suppl 1):S65–S68.
2013.
|
|
10
|
Allan B, Davis J, Perez E, Lew J and Sola
J: Malignant neuroendocrine tumors: Incidence and outcomes in
pediatric patients. Eur J Pediatr Surg. 23:394–399. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JM, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Khanna G, O'Dorisio SM, Menda Y, Kirby P,
Kao S and Sato Y: Gastroenteropancreatic neuroendocrine tumors in
children and young adults. Pediatr Radiol. 38:251–259; quiz 358-9.
2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Scott AT and Howe JR: Management of small
bowel neuroendocrine tumors. J Oncol Pract. 14:471–482.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ransom WB: A case of primary carcinoma of
the ileum. Lancet. 136:1020–1023. 1890.
|
|
15
|
Hallet J, Law CH, Cukier M, Saskin R, Liu
N and Singh S: Exploring the rising incidence of neuroendocrine
tumors: A population-based analysis of epidemiology, metastatic
presentation, and outcomes. Cancer. 121:589–597. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Raphael MJ, Chan DL, Law C and Singh S:
Principles of diagnosis and management of neuroendocrine tumours.
CMAJ. 189:E398–E404. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Forero Molina MA, Garcia E, Gonzalez-Devia
D, Garcia-Duperly R and Vera A: A 17-year-old male with a small
bowel neuroendocrine tumor: Flushing differential diagnosis. World
Allergy Organ J. 10(30)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Maxwell JE and Howe JR: Imaging in
neuroendocrine tumors: An update for the clinician. Int J Endocr
Oncol. 2:159–168. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Keck KJ, Maxwell JE, Menda Y, Bellizzi A,
Dillon J, O'Dorisio TM and Howe JR: Identification of primary
tumors in patients presenting with metastatic
gastroenteropancreatic neuroendocrine tumors. Surgery. 161:272–279.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Oberg K, Modlin IM, De Herder W, Pavel M,
Klimstra D, Friling A, Metz DC, Heaney A, Kwekkeboom D, Strosberg
J, et al: Consensus on biomarkers for neuroendocrine tumour
disease. Lancet Oncol. 16:e435–e446. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pavel M: Translation of molecular pathways
into clinical trials of neuroendocrine tumors. Neuroendocrinology.
97:99–112. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Levy AD and Sobin LH: From the archives of
the AFIP: Gastrointestinal carcinoids: Imaging features with
clinicopathologic comparison. Radiographics. 27:237–257.
2007.PubMed/NCBI View Article : Google Scholar
|