Introduction
Since December 2019, coronavirus disease 2019
(COVID-19) caused by severe acute respiratory syndrome
coronavirus-2 (SARS-CoV-2) has spread worldwide, seriously
threatening human life and health. Although SARS-CoV-2 infection
may be asymptomatic, ~15% of clinically diagnosed cases have a
severe course of the disease (1,2). The
infection may also begin with flu-like symptoms. Indeed, nearly 2
or 3 of every 4 subjects that have positive PCR throat swab results
remain without symptoms and only 10% of symptomatic patients
develop dyspnea, interstitial pneumonia, acute respiratory distress
syndrome and/or multiorgan dysfunction (3). Fever, headache, myalgia, fatigue,
rhinorrhea, cough, mild dyspnea, sore throat and conjunctivitis are
common symptoms of the disease (4,5),
which are seen in other respiratory conditions as well. COVID-19
vaccines have become the most important preventive measure to
control the COVID-19 pandemic. At present, there are three
inactivated vaccines approved for administration in China, which
are produced by Sinopharm China Biological Beijing Institute of
Biological Products Co., Ltd., Wuhan Institute of Biological
Products Co., Ltd. and Beijing Kexing Zhongwei Biotechnology Co.,
Ltd., one adenoviral vector vaccine, which is produced by Kangxino
Biological Co., Ltd., and one recombinant novel coronavirus vaccine
(CHO cell), which is produced by Anhui Longke Ma Biopharmaceutical
Co., Ltd. With mass vaccination and subsequent follow-up,
vaccine-related adverse reactions have emerged in clinical
practice. In the present study, a case of immune thrombocytopenic
purpura (ITP) induced by a COVID-19 vaccine (Vero Cells; Beijing
Kexing Zhongwei Biotechnology Co., Ltd.) was reported.
Case report
A previously healthy 78-year-old female had received
the first and second doses of the COVID-19 vaccine in early August
and September 2021, respectively, 4 weeks apart. The patient was
admitted to the hospital at 30 days after the second vaccination,
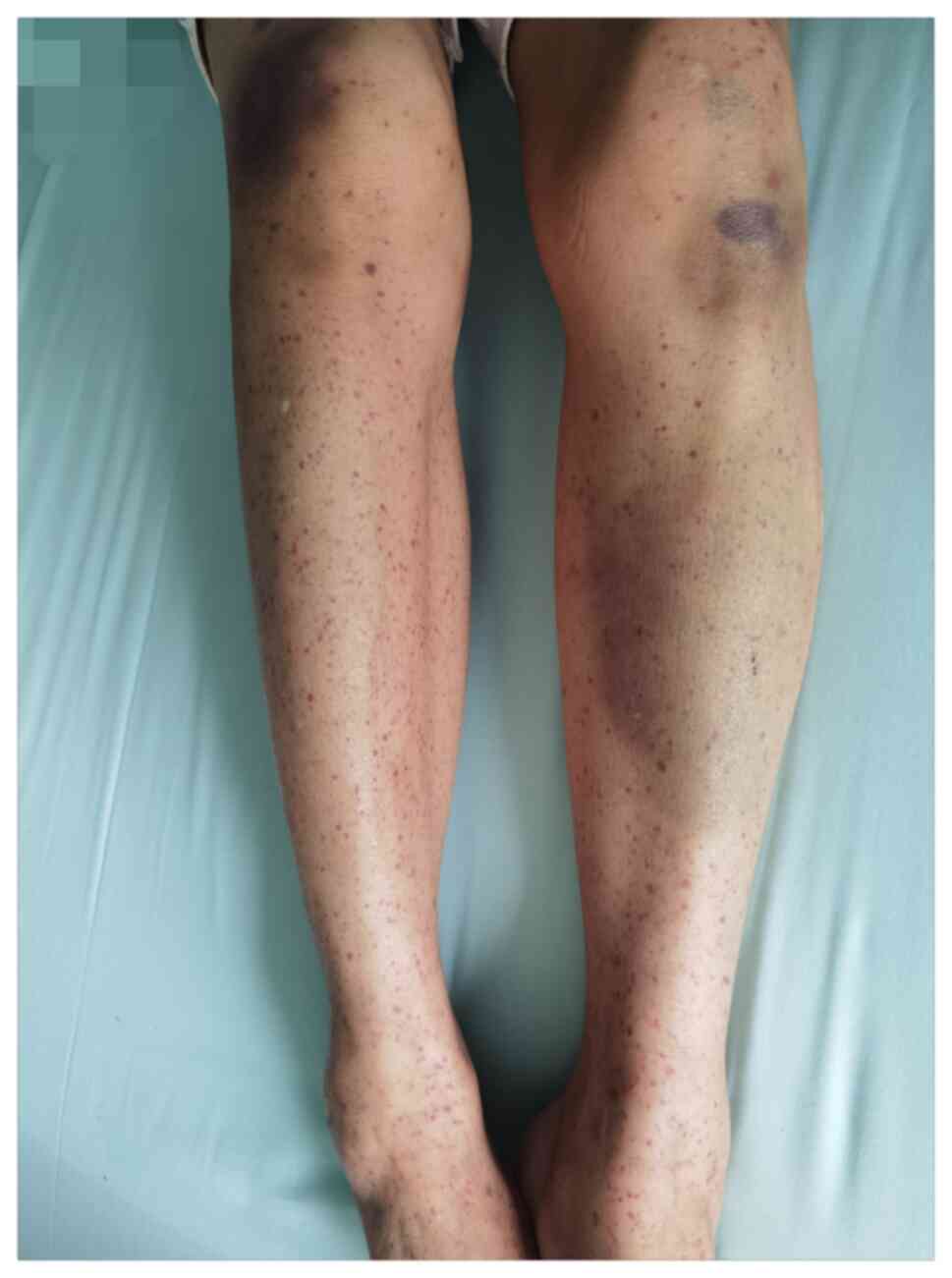
due to ‘oral bleeding for 2 days and scattered hemorrhagic spots on
the limbs for 1 day’. The patient had oral bleeding after eating
hard food at 28 days after the second vaccination. In the morning
of the next day, the patient had dark red blood clots in the mouth
and continuous oral bleeding with scattered hemorrhagic spots on
the limbs. The patient denied any other adverse reactions after the
first dose of vaccination and developed oral bleeding with
scattered hemorrhagic spots on the limbs 1 month after the second
dose of vaccination (Fig. 1). The
patient presented at the Department of Emergency of the 940th
Hospital of Joint Logistics Support Force of the Chinese People's
Liberation Army (Lanzhou, China) 30 days after the second
vaccination, and blood routine analysis indicated a white blood
cell count (WBC) of 6.27x109/l (reference interval,
4-10x109/l), hemoglobin (Hb) of 144 g/l (reference
interval, 110-150 g/l) and a platelet (PLT) count of
1x109/l (reference interval, 100-300x109/l).
The patient did not take any medications and dietary supplements 2
months prior to hospitalization. In addition, the patient had no
medical history of thrombocytopenia prior to vaccination and blood
routine analysis 1 week prior to vaccination indicated a PLT count
>100x109/l. The patient was finally admitted to the
Department of Hematology. The blood routine reexamination 2 days
after hospitalization indicated the following: WBC,
8.14x109/l; Hb, 133 g/l; and PLT, 1x109/l.
After admission, the patient was negative for novel coronavirus by
PCR, and HIV, hepatitis C virus (HCV), HBV and H. pylori
were also negative. At the same time, no abnormality of blood
coagulation was detected and all parameters of biochemistry,
immunity and autoantibody parameters were normal. Bone marrow
cytomorphology showed thrombocytopenia and no platelet-producing
megakaryocytes were observed. The patient was definitely diagnosed
with ITP due to significant bleeding symptoms, such as oral
bleeding and scattered hemorrhagic spots on the limbs. Prednisone
acetate 1 mg/kg, recombinant human thrombopoietin and intravenous
human immunoglobulin (IVIG) 0.4 g/kg were given to increase
platelets, and carbazochrome sodium sulfonate to reduce capillary
permeability and prevent bleeding. At 1 week after the treatment
was started, the patient's PLT began to increase, and 9 days later,
it returned to a normal level (Fig.
2); the treatment was adjusted to oral herombopag on day 10.
The patient tolerated the treatment and is currently on maintenance
therapy with the oral thrombopoietin receptor agonist herombopag as
an outpatient. The patient was followed up 4 months after
discharge, and the PLT was still >100x109/l.
Discussion
COVID-19 has become a global pandemic, but treatment
options remain limited. Up to now, vaccination has been the main
strategy to prevent transmission and reduce disease severity. In
the initial clinical trials of vaccines, no significant adverse
events other than rare allergic reactions were reported. However,
with the follow-up observations after massive vaccination, ITP
induced by COVID-19 vaccines has attracted the attention of
investigators.
Physical examination of patients with immune
thrombocytopenia revealed signs including bleeding from mucous
membranes and skin. Petechiae, purpuric rashes, gingival bleeding
and hemorrhagic bullae may manifest in those patients. The case of
the present study suffered from gingival hemorrhage and skin
rashes. The pathogenesis of ITP involves autoantibodies produced by
B cells, which target PLT membrane glycoproteins, particularly
GpIIb/IIIa, and the production of anti-PLT antibodies results in T
cell-mediated PLT destruction and impaired megakaryocyte function.
The most common predisposing factors for ITP include environmental
factors (e.g., infections, drugs and malignancies), genetic
predisposition and viral infections (6-9).
At the same time, there is evidence that ITP may develop after
various vaccinations (10-14).
There is a direct causal relationship between ITP and the
measles-mumps-rubella vaccine (15). Although a causal relationship has
not been established in numerous cases, ITP has been reported after
multiple immunizations, including live varicella vaccine, human
papilloma vaccine, Haemophilus influenzae vaccine, hepatitis
B vaccine, poliomyelitis vaccine and diphtheria tetanus pertussis
vaccine (16). It has also been
reported in the literature that ITP occurred following influenza
vaccination; although autoantibodies to platelets could not be
detected, the diagnosis of ITP was precise (17). Alternatively, ITP may also be
induced by other components of the vaccine, such as yeast proteins,
adjuvants and preservatives or diluents, which have been implicated
in adjuvant-induced autoimmune/inflammatory syndromes (12,16,18,19).
To date, these immune mechanisms have not been fully elucidated.
Certain phenomena that may explain this autoimmunity reaction
include molecular mimicry, cryptic antigen expression and epitome
spreading. Molecular mimicry is well explained in viruses including
HIV, HCV and varicella zoster virus (VZV), as well as in H.
pylori. However, the sequence homology between SARS-CoV-2 and
platelets has yet to be identified (20). In addition, it has been reported
that inactivated vaccines against COVID-19 infection may trigger
immunity against platelets and may lead to recurrence of immune
thrombocytopenia in these patients (21). In the present case, the patient's
tests were negative for novel coronavirus by PCR, H. pylori,
and HBV, HCV and HIV after admission. However, relevant tests for
systemic lupus erythematosus, antiphospholipid syndrome,
cytomegalovirus and VZV should be performed in similar cases
afterwards in subsequent clinical work, as soon as the patients are
admitted; positivity is less likely but it is reasonable to
test.
ITP is one of the adverse events of SARS-CoV-2
vaccines and the majority of ITP patients after vaccination have
exhibited good responses after treatment with steroid hormones and
IVIG (13,14). In a retrospective study (22), the occurrence of ITP after mRNA
COVID-19 vaccination was included in the vaccine adverse event
reporting system and the results indicated that 15 cases of
thrombocytopenia occurred with 18,841,309 doses of Pfizer
Biotechnology COVID-19 vaccine given and 13 cases of
thrombocytopenia occurred with 16,260,102 doses of Moderna COVID-19
vaccine given. Of the above 28 patients, all but one case occurred
after the first vaccination. The Japanese Society of Hematology
recently published a report on immune-mediated acute exacerbations
of ITP after SARS-CoV-2 mRNA vaccination (23). Furthermore, the Massachusetts
General Hospital ITP Center performed a prospective study of all 52
patients with ITP who received the COVID-19 vaccine on January 1,
2021(24). The PLT counts were
determined 1-7 days prior to vaccination and 3-14 days after
vaccination, and the degree of thrombocytopenia and bleeding after
vaccination were assessed. The results indicated that 6/52 (12%)
patients had severe worsening of thrombocytopenia, accompanied by
worsening of bleeding symptoms. The aggravation of thrombocytopenia
usually occurred 2-5 days after vaccination and patients responded
to IVIG and steroid hormones. In addition, there is increasing
concern about the development of newly diagnosed ITP after
SARS-CoV-2 vaccination. It has been reported that 2 patients
developed severe thrombocytopenia accompanied by bleeding tendency
4 and 14 days after mRNA vaccination (22). PLT returned to normal after PLT
transfusion or treatment with IVIG and steroids.
Vaccines are a major weapon against SARS-CoV-2
throughout the world. Their efficacy and safety have been
demonstrated by rigorous clinical trials, and the present report
cannot question their safety in terms of possible adverse effects.
However, it is still necessary to monitor PLT counts prior to and
after vaccination, so as to determine the true incidence of
thrombocytopenia after COVID-19 vaccination (25). The management of vaccination in
patients with existing ITP is complex and still requires further
study and discussion. The opinion of the Platelet Disorder Support
Association Medical Advisory Committee is that in most patients,
the benefits of vaccination outweigh the risk of deterioration in
patients with ITP (26).
Currently, for patients with ITP, it appears reasonable to obtain a
baseline count prior to vaccination and then obtain additional PLT
measurements after vaccination according to the patient's clinical
presentation and treatment history. Active treatment according to
ITP is appropriate for patients who develop severe thrombocytopenia
shortly after vaccination in the absence of other possible causes.
Whether a second dose of vaccine is required for patients who
develop thrombocytopenia or whether a different vaccine is needed
requires further investigation. In the present case, ITP occurred
after vaccination with a novel coronavirus inactivated vaccine, and
it has also been previously indicated that there was the
development of ITP and a decrease in PLT counts in healthy subjects
after mRNA vaccination (27).
However, the differences between mRNA and inactivated vaccines in
triggering the pathogenesis of ITP still require further study. The
aim of the present study was to raise the awareness of medical
staff regarding this disease and to increase the vigilance of the
general public. At the same time, an effective method to manage
this type of adverse reaction to COVID-19 vaccine was provided.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by a grant from the Gansu
Province Innovation Base and Talent Plan (Gansu Province Leukemia
Clinical Research Center; grant no. 21JR7RA015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL analyzed data and wrote the manuscript. FX, HT
and RS collected and provided data on the treatment of the case
presented, and performed cytomorphological analysis of bone marrow
aspirates. TW and HB analyzed data, compiled diagnostic data and
contributed to the writing of the manuscript. TW and HB read and
approved the final manuscript. WL, TW and HB confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gulali A: A comprehensive review on
rational and effective treatment strategies against an invisible
enemy; SARS Cov-2 infection. Exp Biomed Res. 3:293–311. 2020.
|
|
2
|
Wu Z and McGoogan JM: Characteristics of
and important lessons from the coronavirus disease 2019 (COVID-19)
outbreak in China: Summary of a report of 72 314 cases from the
chinese center for disease control and prevention. JAMA.
323:1239–1242. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lavezzo E, Franchin E, Ciavarella C,
Cuomo-Dannenburg G, Barzon L, Del Vecchio C, Rossi L, Manganelli R,
Loregian A, Navarin N, et al: Suppression of a SARS-CoV-2 outbreak
in the Italian municipality of Vo'. Nature. 584:425–429.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen N, Zhou M, Dong X, Qu J, Gong F, Han
Y, Qiu Y, Wang J, Liu Y, Wei Y, et al: Epidemiological and clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia in
Wuhan, China: A descriptive study. Lancet. 395:507–513.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H,
Wu Y, Zhang L, Yu Z, Fang M, et al: Clinical course and outcomes of
critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China:
A single-centered, retrospective, observational study. Lancet
Respir Med. 8:475–481. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ali E, Al-Maharmeh Q, Rozi WM, Habib MB
and Yassin M: Immune thrombocytopenia purpura flare post COVID-19
vaccine. Ann Med Surg (Lond). 75(103164)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bennett C, Chambers LM, Son J and Goje O:
Newly diagnosed immune thrombocytopenia in a pregnant patient after
coronavirus disease 2019 vaccination. J Obstet Gynaecol Res.
47:4077–4080. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zufferey A, Kapur R and Semple JW:
Pathogenesis and therapeutic mechanisms in immune thrombocytopenia
(ITP). J Clin Med. 6(16)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang J: Clinical research progress of
viral infection and primary immune thrombocytopenia. Clin Med
Practice. 28:463–465. 2019.
|
|
10
|
Zhong Z, Su T, Zhang S, Chen Q, Ma R, Li
Q, Li J and Dong S: Association between vaccination and
thrombocytopenic purpura: Analysis of 13 cases. Chinese J Dis
Control. 25:1014–1019. 2021.
|
|
11
|
Koch M, Fuld S, Middeke JM, Fantana J, von
Bonin S and Beyer-Westendorf J: Secondary immune thrombocytopenia
(ITP) associated with ChAdOx1 Covid-19 vaccination-a case report.
TH Open. 5:e315–e318. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
David P and Shoenfeld Y: ITP following
vaccination. Int J Infect Dis. 99:243–244. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jasaraj RB, Shrestha DB, Gaire S and
Kassem M: Immune thrombocytopenic purpura following Pfizer-BioNTech
COVID-19 vaccine in an elderly female. Cureus.
13(e16871)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
King ER and Towner E: A case of immune
thrombocytopenia after BNT162b2 mRNA COVID-19 vaccination. Am J
Case Rep. 22(e931478)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cecinati V, Principi N, Brescia L,
Giordano P and Esposito S: Vaccine administration and the
development of immune thrombocytopenic purpura in children. Hum
Vaccin Immunother. 9:1158–1162. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Saudagar V, Patil S, Goh S and Pothiawala
S: Vigilance regarding immune thrombocytopenic purpura after
COVID-19 vaccine. Ir J Med Sci. 191:919–920. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gulali A, Rabia A, Zuhal MA and Haluk S:
Immune thrombocytopenia; following seasonal flu vaccine and
non-steroidal anti-inflammatory drug use. Professional Med J.
23:630–633. 2016.
|
|
18
|
Pellegrino P, Clementi E and Radice S: On
vaccine's adjuvants and autoimmunity: Current evidence and future
perspectives. Autoimmun Rev. 14:880–888. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nagasaki J, Manabe M, Ido K, Ichihara H,
Aoyama Y, Ohta T, Furukawa Y and Mugitani A: Postinfluenza
vaccination idiopathic thrombocytopenic purpura in three elderly
patients. Case Rep Hematol. 2016(7913092)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bhattacharjee S and Banerjee M: Immune
thrombocytopenia secondary to COVID-19: A systematic review. SN
Compr Clin Med. 2:2048–2058. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jawed M, Khalid A, Rubin M, Shafiq R and
Cemalovic N: Acute immune thrombocytopenia (ITP) following COVID-19
vaccination in a patient with previously stable ITP. Open Forum
Infect Dis. 8(ofab343)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Welsh KJ, Baumblatt J, Chege W, Goud R and
Nair N: Thrombocytopenia including immune thrombocytopenia after
receipt of mRNA COVID-19 vaccines reported to the vaccine adverse
event reporting system (VAERS). Vaccine. 39:3329–3332.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hagihara M, Uchida T, Inoue M, Ohara S and
Imai Y: Severe thrombocytopenia after COVID-19 mRNA vaccination.
Rinsho Ketsueki. 62:1684–1687. 2021.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
24
|
Vaira LA, Podda L, Doneddu P, Careddu MG,
Fozza C and De Riu G: Secondary thrombocytopenia after SARS-CoV-2
vaccine: Report of a case of hemorrhage and hematoma after minor
oral surgery. J Stomatol Oral Maxillofac Surg. 123:95–97.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kuter DJ: Exacerbation of immune
thrombocytopenia following COVID-19 vaccination. Br J Haematol.
195:365–370. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee EJ, Cines DB, Gernsheimer T, Kessler
C, Michel M, Tarantino MD, Semple JW, Arnold DM, Godeau B, Lambert
MP and Bussel JB: Thrombocytopenia following Pfizer and Moderna
SARS-CoV-2 vaccination. Am J Hematol. 96:534–537. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Visser C, Swinkels M, van Werkhoven ED,
Croles FN, Noordzij-Nooteboom HS, Eefting M, Last-Koopmans SM,
Idink C, Westerweel PE, Santbergen B, et al: COVID-19 vaccination
in patients with immune thrombocytopenia. Blood Adv. 6:1637–1644.
2022.PubMed/NCBI View Article : Google Scholar
|