Introduction
Benign fibrous histiocytoma is a type of mesenchymal
tumor that was first reported by Stout and Lattes (1) in 1967; it mostly occurs on the skin
of the extremities, while bony involvement is uncommon, with
<100 reported cases to date (2-5).
According to the 2020 World Health Organization (WHO)
classification of tumors of bone, BFH is categorized into the same
disease entity as non-ossifying fibroma (3). Most bony BFHs occur in the pelvic
bone, femur and tibia, which share identical histological
characteristics with cutaneous BFH (5-8).
However, mandibular involvement of BFH is rare and only 11 cases
have been reported to date (5,7,9-16).
The present study reported a case of mandibular multiple BFH
treated at the Affiliated Hospital of Stomatology, Sun Yat-Sen
University (Guangzhou, China), who was followed up for 46 months.
Furthermore, a literature search concerning mandible BHF was
performed in Medline (www.medline.com) with the keywords ‘Benign fibrous
histiocytoma’ and ‘Mandible’ to summarize the clinicopathological
features, as well as points regarding the diagnosis and treatment
of mandible BFH.
Case report
In November 2017, a 42-year-old female with slowly
progressive swelling of the bilateral mandible and slight facial
asymmetry over a period of 4 months was referred to the Department
of Oral and Maxillofacial Surgery of the Affiliated Hospital of
Stomatology, Sun Yat-Sen University (Guangzhou, China). The patient
denied any history of dental pain or pus discharge. The first
molars of the bilateral mandible underwent root canal treatments in
an outpatient setting one month prior to presentation. However, the
swelling did not subside significantly. The mandibular molars were
finally extracted in an outpatient setting due to unsatisfactory
therapeutic outcomes. Thereafter, the patient consulted our
department for further treatment. Physical examination revealed
hard but non-tender swelling involving the area from the second
premolar to the first molar of the bilateral mandible, covered by
normal mucosa, and the loss of buccal vestibular depth was noticed.
The first molar of the bilateral mandible was missing and the tooth
extraction wound exhibited no obvious abnormality. Palpation
examination of regional lymph nodes was negative.
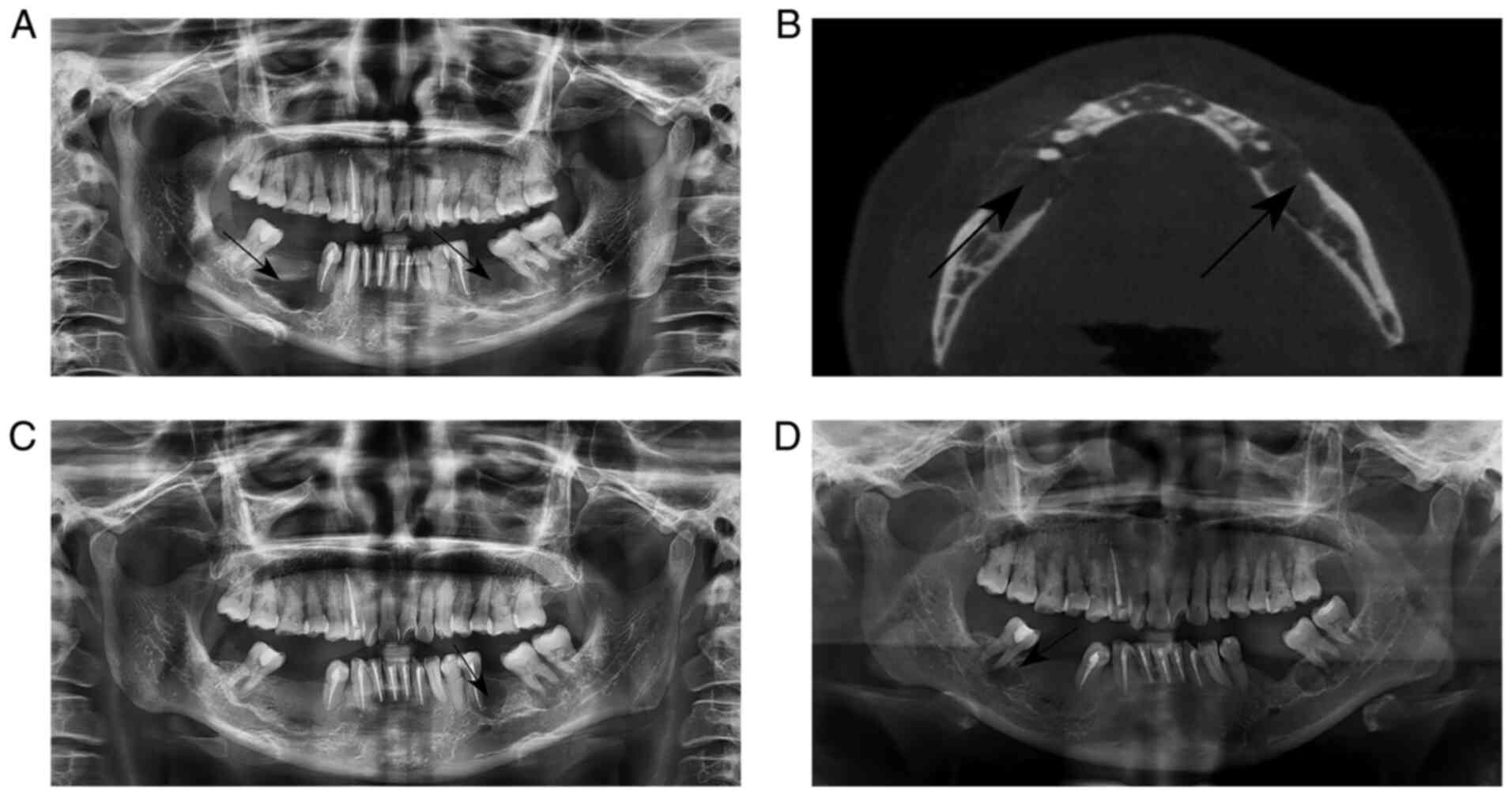
Panoramic radiograph revealed two diffuse,
well-defined multilocular osteolytic radiolucency areas from the
second premolar to the first molar of the bilateral mandible, which
were surrounded by sclerotic rims (Fig. 1A). In cone-beam computed tomography
(CBCT) images, expansion of the buccal cortex and even certain
cortical defects of the involved region were observed (Fig. 1B).
Under general anesthesia via naso-tracheal
intubation, the patient was treated by transoral curettage and
discharged from the hospital 7 days after the surgery. After 16
months, the patient felt gingival swelling and discomfort in the
left posterior area. Intraoral examination indicated swelling in
the region of the lower left first premolar to the left second
premolar region of the mandible. A panoramic radiograph revealed a
rounded hypodense image of the apical root of the patient's lower
left first premolar (Fig. 1C). The
patient underwent surgical curettage of the lesion and mandibular
left first premolar extraction. At 46 months after the first
surgery, routine follow-up examination revealed gingival swelling
in the area from the right lower first molar to the second molar
and the panoramic radiograph revealed a low-density image of the
apical part of the right lower second molar (Fig. 1D). The patient underwent surgical
curettage of the lesion again.
Generally, the tumors in the first surgical
resection were partly congestion, well-encapsulated and consisted
of solid masses (Fig. 2), in which
the maximum diameter of the left tumor (Fig. 2A) and the right tumor (Fig. 2B) were about 3.5 and 4.0 cm,
respectively. The patient was ask to revisit 1 month, 3 months,
half a year and annually thereafter. Histological evaluation with
hematoxylin-eosin staining (the paraffin sections were dyed with
hematoxylin for 5 min and the stained in eosin dye solution for 2
min at the room temperature of 25˚C) revealed that the tumors in
the first surgical resection consisted of diffused spindle-shaped
cells, which were arranged in a storiform pattern and surrounded by
a large amount of collagen fibers. A small number of mitoses were
noted but no nuclear atypia (Fig.
3A). Furthermore, on immunohistochemical examination (17,18),
tumor cells were strongly positive for CD68 (Fig. 3B) and XIIIa factor (FXIIIa)
(Fig. 3C), weakly positive for
alpha smooth muscle actin (α-SMA) (Fig. 3D) and desmin (Fig. 3E), and negative for CD34 (Fig. 3F). The monoclonal antibodies used
were all from Thermo Fisher Scientific. The catalogue number of
CD68, FXIIIa, α-SMA, desmin and CD34 were 14-0688-82, PA5-102931,
701457, MA1-06401 and 14-0341-82. The dilution ratios all were
1:200. The diagnosis of bilateral mandible BFH was made. The
pathological examination results of the latter two surgically
removed tumors were the same as the first surgically removed
tumor.
Discussion
Fibrous histiocytoma represents a group of diverse
tumors, with both fibroblast and histiocytic differentiation, which
was first described by Stout and Lattes (1) in 1967 (19,20).
In the criteria of histological classification for bony tumors
proposed by the WHO, 2nd edition, BFH was used to describe those
independent tumors different from the connective tissue ones
(2,13,21).
Cutaneous BFH involvements are mostly seen but bony involvement of
BFH is uncommon (4,21-24),
and mandible BFH is rare, with only 11 reported cases in the past
40 years (Table I).
 | Table ISummary of clinical data of reported
cases of mandible BFH from 1980 to 2021. |
Table I
Summary of clinical data of reported
cases of mandible BFH from 1980 to 2021.
| Author, year | Age/ gender | Site | Syndrome | Duration | Surgical
approach | Follow-up | Recurrence | (Refs.) |
|---|
| Pattamparambath et
al, 2016 | 51/F | R, P | Swelling | 15 Y | SM | NA | NA | (16) |
| Vyloppilli et
al, 2015 | 46/F | L, P | Swelling and
pain | 3 Mo | SM | NA | NA | (15) |
| Shoor et al,
2015 | 30/F | L, P | Swelling and
pain | 1 Y | SM | 2 Y | None | (14) |
| Ou et al,
2012 | 31/M | L, P | Swelling and
pain | 1 Mo | SM | 58 Mo | None | (13) |
| Tanaka et
al, 2011 | 80/M | R, P | Swelling and
pain | 2Y | TC | 6 Mo | Yes | (12) |
| Katagiri et
al, 2008 | 48/M | R, CP | No symptom | NA | TC | NA | NA | (11) |
| Kishino et
al, 2005 | 49/F | L, P, CP | Swelling and
pain | NA | SM | 35 Mo | None | (10) |
| Heo et al,
2004 | 42/M | L, P | Swelling | 8 Y | SM | 12 Mo | None | (9) |
| Ertaş et al,
2003 | 32/M | A | Swelling | NA | TC | NA | NA | (7) |
| Remagen et
al, 1986 | 17/M | L, P | Swelling | 3 Y | NA | 4 Mo | None | (5) |
| White and Makar,
1986 | 29/F | L, P | Swelling | NA | TC | 24 Mo | None | (4) |
| Wang et al,
2022 (1st time) | 42/F | B, P | Swelling | 4 Mo | TC | 16 Mo | Yes | Present study |
| Wang et al,
2022 (2nd time) | 44/F | L, A | Swelling | NA | TC | 22 Mo | Yes | Present study |
| Wang et al,
2022 (3rd time) | 46/F | R, A | Swelling | NA | TC | NA | NA | Present study |
The etiology of mandible BFH remains to be fully
established. According to certain experts, the tumor cells are
derived from fibroblasts (24),
while others assume that the tumor originates from the dendritic
cells based on the evidence that the tumor is strongly positive for
FXIIIa (20-23);
furthermore, inflammation, viral infections and injuries may be
major causes of bony BFH (25,26).
Mandible BFH shares similar histopathological
features with cutaneous BFH, which are characterized by whirlpooled
or radially arranged fibrous tissue, heterogeneous polynuclear
giant cells, foamy cells, hemosiderin deposition and infiltration
of a small amount of chronic inflammatory cells (13,16,27,28).
Immunohistochemical staining helps clarify the composition of the
tumor, which is positive for CD68, vimentin, α-1-antitrypsin and
α-1-antichymotrypsin, but negative for CD34, S-100 protein,
epithelial membrane antigen, α-SMA and cytokeratin (21,29-31).
In the present case, immunohistochemical staining of the tumor
indicated strong positivity for FXIIIa and CD68, weak positivity
for desmin, partial positivity for SMA and negativity for CD34,
indicating that the tumors consisted of avascular fibrous tissue
and muscle tissue. According to the clinical characteristics of
mandible BFH, the differentiation of BFH from giant cell tumor
(GCT) and aneurysmal bone cyst (ABC) is required, which share an
identical clinical manifestation of swelling (32,33).
GCT consists of osteoclast-like multinucleated giant cells,
scattered in the background of mononuclear stromal cells, which is
strongly positive for CD14, human leukocyte antigen-DR, α-SMA and
CD33 (32,34). ABC has an appearance similar to
soap bubbles on macroscopic examination, which is comprised of
numerous blood sinuses of different sizes, with a large number of
blood cells (33,35).
In addition to the patient in the present case
report, 11 cases of mandible BFH have been reported over the past
four decades (Table I). The
average age at onset was 41.3 years (range, 17-80 years). The
incidence of mandible BFH was slightly higher in males than in
females and unilateral lesions were more frequently described (10
cases, 90.9%), with a slight tendency in the left region of the
mandible (63.6%). According to the reported cases, two mandible
BFHs occurred in the condylar process (10,11).
Most patients presented with swelling of the involved area
(5,9,16)
and certain patients had complaints of pain (5 cases, 45.4%). Only
one patient had a history of pus discharge (10) (Table
I). Cell marker studies have suggested a fibroblastic origin
for BFH (36). Walther et
al (37) and Płaszczyca et
al (38) considered that the
development of BFH may be associated with abnormalities in the
protein kinase C gene. However, there are no studies demonstrating
that recurrence of BFH is associated with a certain pathological
condition (39). Mandible BFH
appears as a unilocular or multilocular radiolucency with an
irregular margin and at times a sclerotic rim in panoramic
radiography images (5,9,10).
CBCT is helpful in determining the continuity of the mandible
cortex, the axial section indicates a well-defined osteolytic
lesion with the buccal cortex expansion and in certain cases, the
continuity of the cortical bone was even interrupted (5,9,12).
The surgical approaches were described in 10 cases.
Clinically, the most common surgical methods for mandible BFH are
segmental mandibulectomy and transoral curettage. Segmental
mandibulectomy accounted for 54.5% of all of the cases included in
the present literature review and transoral curettage was performed
for the remaining patients. Follow-up data were included in 7
cases, which suggested that the prognosis of mandible BFH was good
overall, with only one recurrence and transformation into the
malignant form (12). According to
the results of the present review, the choice of surgical method
for mandible BFH is mainly based on the size of the mass and also
the general condition of the patient. Transoral curettage is
suggested when the CBCT scan indicates that the maximum diameter of
the BFH lesion is <5.0 cm, while segmental mandibulectomy is
advisable when it exceeds 5.0 cm (7,9,13).
However, Heo et al (9)
performed segmental mandibulectomy for the tumor with a maximum
diameter of <5.0 cm. Tanaka et al (12) and Ertaş et al (7) chose to perform transoral curettage
for masses with a maximum diameter of >5.0 cm. In 6 cases of
segmental mandibulectomy of BFH, there was no recurrence during the
follow-up period. However, in 4 cases of transoral curettage, one
was confirmed to have recurrence within the follow-up period, and
in the case of the present study, recurrences of the BFH occurred
16 and 46 months after the first transoral curettage. Transoral
curettage helps to maintain the continuity of the mandible, the
occlusion relationship and outlook of the surgery, but it brings
the risk of BFH recurrence. While segmental mandibulectomy is able
to remove the tumor completely and reduce the chance of tumor
recurrence, it has more complex operative procedures and a longer
period of recovery after the surgery. Furthermore, the financial
burden on patients and the difficulties of reconstructing the
occlusion relationship after segmental mandibulectomy cannot be
underestimated. The recurrence rate of mandible BFH is relatively
low, with only one recurrence of the 11 previous cases (9%). Still,
it is noteworthy that the recurrence of the tumor may transform
into its malignant form (12,36).
However, to the best of our knowledge, metastasis of BFH has never
been reported. Regular follow-up of patients with mandible BFH is
advisable after transoral curettage due to the potential recurrence
and malignant transformation of the tumor. In conclusion, mandible
BHF is rare and the diagnosis of mandible BFH should only be made
based on evidence of histopathological and immunohistochemical
staining. The appropriate surgical methods were selected based on
the general condition of the patients and the size of the mass.
Regular follow-up is advisable to detect the recurrence of the
tumor after transoral curettage.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QT was responsible for the study conception; YW was
responsible for the design of the study and drafting of the
manuscript; YH and WC were responsible for reviewing the literature
and collecting relevant data. WC and QT were responsible for
revising the manuscript for important intellectual content. QT and
YW confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Informed written consent was obtained from the
patient for publication of this report and any images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stout AP and Lattes R: Atlas of Tumor
Pathology: Tumors of the Soft Tissues. Armed Forces Institute of
Pathology, Washington, DC, pp38-52, 1967.
|
|
2
|
Nielsen GP and Kyriakos M: Non-ossifying
fibroma/benign fibrous histiocytoma of bone. In: World Health
Organization Classification of Tumours of Soft Tissue and Bone.
International Agency for Research on Cancer, Lyon, 2013.
|
|
3
|
Choi JH and Ro JY: The 2020 WHO
classification of tumors of bone: An updated review. Adv Anat
Pathol. 28:119–138. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
White RD and Makar J Jr: Xanthofibroma of
the mandible. J Oral Maxillofac Surg. 44:1010–1014. 1986.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Remagen W, Nidecker A and Prein J: Case
report 359: Gigantic benign fibrous histiocytoma (nonossifying
fibroma). Skeletal Radiol. 15:251–253. 1986.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li X, Meng Z, Li D, Tan J and Song X:
Benign fibrous histiocytoma of a rib. Clin Nucl Med, 2014.
39:837–841. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ertaş U, Büyükkurt MC and Ciçek Y: Benign
fibrous histiocytoma: Report of case. J Contemp Dent Pract.
4:74–79. 2003.PubMed/NCBI
|
|
8
|
Grohs JG, Nicolakis M, Kainberger F, Lang
S and Kotz R: Benign fibrous histiocytoma of bone: A report of ten
cases and review of literature. Wien Klin Wochenschr. 114:56–63.
2002.PubMed/NCBI
|
|
9
|
Heo MS, Cho HJ, Kwon KJ, Lee SS and Choi
SC: Benign fibrous histiocytoma in the mandible. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 97:276–280. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kishino M, Murakami S, Toyosawa S,
Nakatani A, Ogawa Y, Ishida T and Ijuhin N: Benign fibrous
histiocytoma of the mandible. J Oral Pathol Med. 34:190–192.
2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Katagiri W, Nakazawa M and Kishino M:
Benign fibrous histiocytoma in the condylar process of the
mandible: Case report. Br J Oral Maxillofac Surg. 46:e1–e2.
2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tanaka T, Kobayashi T and Iino M:
Transformation of benign fibrous histiocytoma into malignant
fibrous histiocytoma in the mandible: Case report. J Oral
Maxillofac Surg. 69:e285–e290. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ou DM, Zheng GS, Liao GQ, Su YX, Liu HC
and Liang YJ: Clinical and pathologic characteristics and surgical
management of benign fibrous histiocytoma of the mandible: A case
report. J Oral Maxillofac Surg. 70:2719–2723. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shoor H, Pai KM, Shergill AK and Kamath
AT: Benign fibrous histiocytoma: A rare case involving jaw bone.
Contemp Clin Dent. 6 (Suppl 1):S266–S268. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vyloppilli S, Joseph B, Manoj Kumar KP,
Kurian SD, Anirudhan A and Kumar N: Benign spindle cell tumour of
mandible and points of modification in reconstruction with
nonvascularised iliac crest graft. J Maxillofac Oral Surg. 15
(Suppl 2):S262–S265. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pattamparambath M, Sathyabhama S, Khatri
R, Varma S and Narayanan NM: Benign fibrous histiocytoma of
mandible: A case report and updated review. J Clin Diagn Res.
10:ZD24–ZD26. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Maclean A, Bunni E, Makrydima S,
Withington A, Kamal AM, Valentijn AJ and Hapangama DK: Fallopian
tube epithelial cells express androgen receptor and have a distinct
hormonal responsiveness when compared with endometrial epithelium.
Hum Reprod. 35:2097–2106. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dogan S, Vasudevaraja V, Xu B, Serrano J,
Ptashkin RN, Jung HJ, Chiang S, Jungbluth AA, Cohen MA, Ganly I, et
al: DNA methylation-based classification of sinonasal
undifferentiated carcinoma. Mod Pathol. 32:1447–1459.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mohanty A, Mishra P, Kumar H and Panda A:
A rare presentation of benign fibrous histiocytoma in the maxilla.
J Oral Maxillofac Pathol. 24 (Suppl 1):S73–S76. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Prasanna Kumar D, Umesh Rathi T and Jain
V: Benign fibrous histiocytoma: A rare case report and literature
review. J Maxillofac Oral Surg. 15:116–120. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bielamowicz S, Dauer MS, Chang B and
Zimmerman MC: Noncutaneous benign fibrous histiocytoma of the head
and neck. Otolaryngol Head Neck Surg. 113:140–146. 1995.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Saluja H, Kasat VO, Rudagi BM, Dehane V,
Kalburge JV and Nikam A: Benign fibrous histiocytoma of the
maxilla: A case report and review of literature. Indian J Dent Res.
25:115–118. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nam KH, Park SW and Yun SK: A
clinicohistopathological analysis of cutaneous fibrous
histiocytomas of the finger. Indian J Dermatol. 65:401–405.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Luzar B and Calonje E: Cutaneous
fibrohistiocytic tumours-an update. Histopathology. 56:148–165.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Giovani P, Patrikidou A, Ntomouchtsis A,
Meditskou S, Thuau H and Vahtsevanos K: Benign fibrous histiocytoma
of the buccal mucosa: Case report and literature review. Case Rep
Med. 2010(306148)2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Priya NS, Rao K, Umadevi HS and Smitha T:
Benign fibrous histiocytoma of the tongue. Indian J Dent Res.
24:635–638. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang J: Fibrohistiocytic tumor of skin.
Zhonghua Bing Li Xue Za Zhi. 42:134–137. 2013.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
28
|
Franchi A and Santucci M: Tenascin
expression in cutaneous fibrohistiocytic tumors.
Immunohistochemical investigation of 24 cases. Am J Dermatopathol.
18:454–459. 1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Anand N, Kaur R, Saxena S and Bhardwaj N:
Benign fibrous histiocytoma of the lower lip. J Oral Maxillofac
Pathol. 24 (Suppl 1):S97–S100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sood MA, Nair S, Nilakantan BA and Malik
A: Benign fibrous histiocytoma of larynx. Med J Armed Forces India.
73:97–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mentzel T: Fibrohistiocytic tumors of the
skin: A heterogeneous group of superficially located mesenchymal
neoplasms. Pathologe. 36:79–88. 2015.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
32
|
Bahbah S, Harti KE and Wady WE: Giant cell
tumor of the maxilla: An unusual neoplasm. Pan Afr Med J.
36(342)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Urs AB, Augustine J and Chawla H:
Aneurysmal bone cyst of the jaws: Clinicopathological study. J
Maxillofac Oral Surg. 13:458–463. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Park SR, Chung SM, Lim JY and Choi EC:
Giant cell tumor of the mandible. Clin Exp Otorhinolaryngol.
5:49–52. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Motamedi MH, Behroozian A, Azizi T,
Nazhvani AD, Motahary P and Lotfi A: Assessment of 120
maxillofacial aneurysmal bone cysts: A nationwide quest to
understand this enigma. J Oral Maxillofac Surg. 72:1523–1530.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Stiller D and Bahn H: Fibronectin in
relation to growth patterns of fibrohistiocytic tumours-an
immunohistochemical study of benign and malignant fibrous
histiocytomas. Acta Histochem. 82:95–108. 1987.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Walther C, Hofvander J, Nilsson J,
Magnusson L, Domanski HA, Gisselsson D, Tayebwa J, Doyle LA,
Fletcher CD and Mertens F: Gene fusion detection in formalin-fixed
paraffin-embedded benign fibrous histiocytomas using fluorescence
in situ hybridization and RNA sequencing. Lab Invest. 95:1071–1076.
2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Płaszczyca A, Nilsson J, Magnusson L,
Brosjö O, Larsson O, Vult von Steyern F, Domanski HA, Lilljebjörn
H, Fioretos T, Tayebwa J, et al: Fusions involving protein kinase C
and membrane-associated proteins in benign fibrous histiocytoma.
Int J Biochem Cell Biol. 53:475–481. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kirschnick LB, Schuch LF, Silveira FM, Só
BB, Martins MAT, Lopes MA, Vargas PA, Santos-Silva AR, Carrard VC,
Vasconcelos ACU, et al: Benign fibrous histiocytoma of the oral and
maxillofacial region: A systematic review. Oral Surg Oral Med Oral
Pathol Oral Radiol. 133:e43–e56. 2022.PubMed/NCBI View Article : Google Scholar
|