Henoch-Schonlein purpura (HSP) is an immunoglobulin
(Ig)A-mediated relapsing vasculitis that invades small arteries and
capillaries of the skin and other organs, such as gastrointestinal
tract and kidneys. It primarily manifests as cutaneous purpura,
joint swelling, digestive symptoms (mainly abdominal pain) and
kidney injury, which can be fatal (1,2).
Based on clinical features, HSP is classified into five categories:
Simple, arthritis, abdominal, renal and mixed types (3). HSP frequently occurs in children,
affecting 3-27 children per 100,000 population; but there has been
an increasing number of incidents in adults who possibly suffer
more serious systemic issues due to age (4). To the best of our knowledge, the
pathogenesis of HSP has not been completely elucidated; however,
immune imbalance and oxidative stress (OS) that induce IgA-mediated
vascular damage may be involved (5,6). It
is also hypothesized that toll-like receptor (TLR)4/myeloid
differentiation primary response gene 88 (MyD88)/nuclear factor-κB
(NF-κB) signaling is involved (7).
Various treatments have been applied to HSP, including
anti-inflammatory drugs, anti-histamine, vitamin C, calcium,
corticosteroids, cytotoxic drugs and immunosuppressants, as well as
blood-activating and stasis-resolving medicines (8-10).
However, these therapies exhibit poor efficacy and do not stop
recurrence, research has shown that relapses occur in about 25% of
patients and that children over age 8 or those with kidney-based
disease are more prone to relapse (11); furthermore, most approaches to HSP
are unsuitable for long-term application owing to high expenditures
and adverse side effects, e.g. long-term and large-scale use of
glucocorticoids could cause infections and Cushing's syndrome;
long-term use of cytotoxic drugs that could result in
gastrointestinal reactions, hepatotoxicity, bone marrow
suppression, reproductive toxicity, cardiotoxicity (12). Thus, a novel and effective cure for
HSP is required.
Proanthocyanidins are a class of natural
polyphenolic compounds that consist of epicatechin and catechin.
Proanthocyanidins are obtained from seeds, vegetables, fruits and
plants. Previous studies have demonstrated that proanthocyanidins
are beneficial to human health due to few side effects and multiple
biological activities, such as immunoregulation, antioxidation,
anti-inflammation, anti-angiogenesis, anti-proliferation and
anti-tumor effects (13-17).
Growing evidence from clinical and experimental studies indicates
that proanthocyanidins are predominantly safe and effective against
diseases, such as inflammatory disorders, OS-associated diseases,
vascular disorders and immune complaints. Proanthocyanidins
function by regulating immune balance, attenuating OS damage and
promoting vascular endothelial integrity (14,16,18,19).
Additionally, proanthocyanidins modulate the abnormal metabolism of
the body to control metabolic complaints and neoplastic disorders
through suppressing lipid peroxidation (LPO) and inflammatory
responses (20-22).
However, reports scarcely focus on the possible efficacy of
proanthocyanidins in the treatment of HSP. Given that HSP is an
immune-mediated and OS-induced vasculitis and proanthocyanidins
possess immunomodulatory and antioxidative properties, the present
study hypothesizes that proanthocyanidins could be a treatment for
HSP. Therefore, the evidence that suggests the potential efficacy
of proanthocyanidins in treating HSP is reviewed and discussed in
the present study.
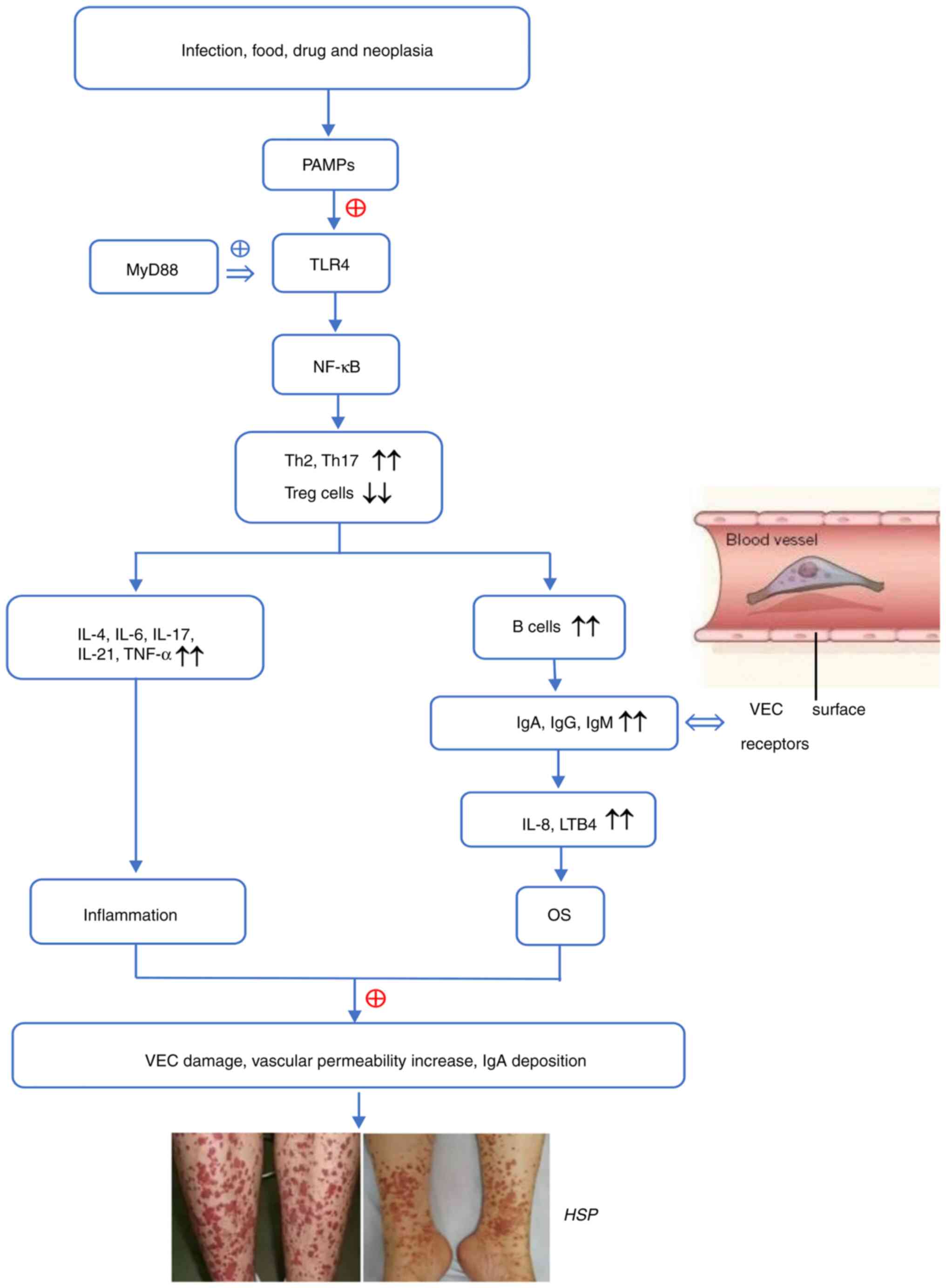
As a multi-factorial disease, the etiology of HSP is
diverse and complex and includes microbial infection, foods, drugs
and malignant tumors. Reports suggest that immune imbalance and OS
that induce IgA-mediated vessel injury are involved in the
pathogenesis of HSP (23,24). The abnormal activation of TLRs
(primarily TLR4) and the downstream pathways, are implicated in
this process (25-29).
Immune imbalance, especially T helper 2 (Th2) and
Th17 overactivity, facilitates secretion of numerous chemokines and
inflammatory cytokines (such as TNF-α, IL-1, IL-4, IL-6, IL-17 and
cell adhesion factors) and promotes the migration of leukocytes to
inflammatory sites, thus aggravating vascular inflammation and
exacerbating HSP (28,30). Donadio et al (27) demonstrated that overactivation of
TLRs (primarily TLR4) and elevated levels of adaptive immunity
appear in patients with HSP; these promote an imbalance of the T
cells and a release of inflammatory cytokines. As the downstream
molecule of TLRs, NF-κB is required for the development of T cells
and is involved in the activation of CD4+ T cells, particularly Th2
and Th17 cells (31). TLRs
initially promote NF-κB translocation and activation by recruiting
MyD88; TLRs combined with MyD88 employ the IL-1 receptor-associated
kinase 1 (IRAK1) and IRAK4 to induce the activation of the
transforming growth factor-β-activated kinase (TAK) complex, which
causes autophosphorylation of TAK1. TAK1 promotes phosphorylation
of IκBα by degrading the inhibitor κ-B kinase, which facilitates
dissociation of IκBα from NF-κB and the production of the NF-κB
complex (a dimer of p50 and p65) (32). The activated NF-κB then promotes T
cells to produce activation-associated molecules, thereby
activating and increasing naive T cells. Stimulated naive T cells
differentiate into the effector subpopulations, such as Th1, Th2
and Th17. NF-κB, by combining with enhancer sites of IL-4 and
simultaneously binding with NF of activated T cells, stimulates
IL-4 that then further promotes Th2 development and activation. By
contrast, NF-κB facilitate the growth and development of Th17 cells
through enhances the secretion of Th17 directive signals such as
IL-17a, IL-6, TNF-α and TGF-β, and stimulating the expression of
major transcription factors of Th17 (RORγt and RORγ) alongside
c-Rel of the Rel family. In addition, activated NF-κB suppresses
the function of regulatory T (Treg) cells, followed by initiation
of immune imbalance and the inflammatory response (29,33-36).
Due to activation of TLRs, a number of aberrant alterations emerge
in patients with HSP, including a high-proportion of Th17 cells,
Th1/Th2 cell imbalance and Th2-dominant immune response, as well as
decreased IFN-γ and IL-4 release. These alterations contribute to
production of pro-inflammatory/inflammatory factors and adhesion
molecules, recruitment of immune/inflammatory cells and
amplification of the immune/inflammatory response (28,37,38).
Consequently, overactivated T cells in turn release inflammatory
factors, especially Th2-released cytokines, to accelerate the
proliferation and differentiation of B cells that synthesize and
secrete antibodies (IgA, IgG and IgM). Igs bind to corresponding
receptors and activate complement components 3/4 to trigger OS and
initiate inflammation, which leads to vessel wall destruction and
HSP (39).
Decreased Treg cells aggravates HSP. Based on the
origin and differentiation, Treg cells are classified into three
subtypes: Thymus-derived (t), also termed natural Treg cells;
peripheral (p) and inducible (i). The tTregs are generated in
thymus, while pTregs and iTregs are induced in peripheral sites
(40,41). It is generally considered that an
immune and inflammation imbalance weakens the function of Treg
cells, which promotes inflammatory reactions and exacerbates the
disease (34,42). Levels of CD4+CD25+Foxp3+ Treg cells
markedly decrease in patients with HSP, whereas Th17 cell levels
markedly increase; this imbalance of Th17/Treg promotes Treg cell
dysfunction in HSP, amplifying inflammatory responses and
destroying immune defense (35).
Conversely, stable expression of Foxp3 induces Treg cells to
produce anti-inflammatory factor IL-10, which inhibits the
inflammatory response and maintains immune homeostasis.
Furthermore, there is a decrease of follicular Treg cells in blood
of pediatric patients with HSP, along with an increase in
follicular Th cells; this imbalance leads to the overactivation of
B cells, which then stimulate IgA production and inflammation
(43).
As an antigen-antibody reaction, the inflammatory
process mediated by IgA is the pathology of HSP. B cell-secreted
IgA antibodies induce chemokine IL-8/leukotriene B4 (LTB4)
production and promote chemotaxis/aggregation of neutrophils, which
stimulates the inflammatory response and causes damage to
endothelial cells (ECs) (44,45).
Excessive numbers of IgA antibody interact with FcαRI (a
prototypical IgA receptor) and bind to vascular ECs (VECs), thereby
attracting neutrophils to migrate to the blood vessel wall in the
presence of IL-8, in addition to eliciting ROS production and OS
occurrence and membrane LPO) (46,47).
OS describes an imbalance where production of
oxidative species exceeds the capacity of antioxidant defense
systems. This then leads to macromolecular damage and redox
homeostatic disorder. Free radical reaction is a widely recognized
phenomenon that is greatly involved in macromolecular damage
(48-51).
The overproduction of oxygen radicals or oxides, including ROS
[such as superoxide anion (O2-), hydrogen peroxide (H2O2), hydroxyl
radical and singlet oxygen] and reactive nitrogen species [such as
nitric oxide (NO), peroxynitrite anion and nitrite ion] (52,53),
disturbs the equilibrium of the oxidant/antioxidant system. This
imbalance creates OS that causes damage to the skin and VECs. As
the primary contributor to OS, ROS production is triggered by
exogenous factors [such as ultraviolet (UV) radiation, heavy metal
ions, ozone, drugs or toxins] and endogenous factors including
mitochondrial electron transport chain, membrane-bound NADPH
oxidase isoforms 1-5 and endoplasmic reticulum. NADPH oxidases in
particular are the primary source of ROS (53,54).
The electrons generated by NADPH are transferred by an NADPH enzyme
onto molecular oxygen that is reduced to O2•-. Superoxide dismutase
(SOD) catalyzes O2-conversion into H2O2, and more ROS are formed
(55-57).
Increasing evidence has supported OS as a promoter
in the progression of vascular disorder, and in particular HSP
(58-60).
It has been revealed that OS often occurs in HSP, presenting as
high levels of ROS and malondialdehyde (MDA) and low SOD levels and
total antioxidant capacity. Serum MDA is an indicator of LPO, which
increases in the active phase of HSP (massive purpura is seen on
the skin and some patients experience abdominal and joint pain) and
indirectly signifies the degree of VEC injury in patients with HSP
(5,61,62).
The dysfunction or damage of ECs impacts on the integrity of the
vascular wall and the permeability of the vessel, which is
attributed to the amount of OS (63-65).
As a result, inflammatory cells adhere to the vessel wall to
exacerbate damage of the vascular endothelium and deposit IgA at
the vascular wall; these in turn promote the production of
chemokines, accumulation/infiltration of inflammatory cells and
injury of the vessel to exacerbate OS, thereby forming a positive
feedback loop (66-68).
Previous studies have reported higher levels of MDA in the serum of
patients with HSP compared with levels in healthy individuals; MDA
levels are highest in patients with renal or gastrointestinal
involvement (21-23).
Similar reports have indicated that the damage of ECs and
aggravation of vascular inflammation frequently occur in the
production of ROS from OS, while neutrophils in patients with HSP
positively mediate oxygen radical production (61,69).
Superfluous ROS destroy the antioxidant system and trigger OS,
whereas the impaired antioxidant defense system aggravates the
persistence of OS. This stimulates the release of diverse
inflammatory factors and inflammatory reactions around the blood
vessels. IL-1β, activated by TLRs, contributes to the HSP
inflammatory reaction and is regulated by a biphasic redox response
(70-72).
The excess ROS and attenuated antioxidant defense stimulates the
secretion of IL-1β, while IL-1β and cytokines involved in the
inflammatory cascade response enhance the deposition of IgA on ECs
(71,73). Conversely, damaged VECs and
activated neutrophils produce large amounts of ROS, triggering the
pathological and clinical manifestations of HSP (5,60,74).
The aforementioned processes are regulated by TLRs
and downstream signaling pathways. Activating numerous inflammatory
signals such as MyD88 and NF-κB, TLRs promote release of
inflammatory mediators and the formation of immune complexes along
with the overactivation of Th2/Th17 and the reduction of Treg cell
populations and functional activity.
TLRs, belonging to a family of pattern recognition
receptors, functionally recognize molecules that are associated
with infection/tissue damage-associated molecules to trigger innate
immunity and inflammation. The activation of TLRs promotes immune
dysregulation, inflammatory responses and OS, thereby promoting
vascular skin disorder (such as HSP) by stimulating TLR-mediated
signaling pathways. TLRs comprise 13 members (TLR1-TLR13) (75,76).
In all TLR members, the extracellular region is responsible for
identifying the pathogen-associated molecular patterns (PAMPs) of
the disease-causing microorganism, while the cytoplasmic region
induces cellular immunological response (77). TLR4 localization on the cell
surface is important in immune inflammatory disorders; it
specifically recognizes the lipopolysaccharide (LPS) of pathogens
and stimulates immune inflammatory reactions by activating adaptive
immunity-associated genes, such as MyD88(78).
TLR4-mediated signaling pathways in immune responses
primarily contain two categories: MyD88- and TIR-domain-containing
adapter-inducing interferon-β (TRIF)-dependent pathway. Trough
interacting with the MyD88, TRIF or TLR4 pathway that promotes
downstream signaling molecule (NF-κB or activator protein-1)
participation in immune inflammation (79). By stimulating MyD88, TLR4 activates
NF-κB, thereby triggering signal transduction cascades and
activating immune cells. MyD88 is an adaptor molecule recruited by
almost all TLRs (TLR3-TLR13) (80,81).
MyD88 has two domains, namely the TIR domain that interacts with
the cognate domain in the cytoplasmic tails of TLRs and the death
domain that binds to the corresponding domain of IRAK4. In the
MyD88-dependent pathway, the signal pathway is exclusively
activated by TLR4; signals are transmitted from TLR4 to MyD88, then
to IRAK4; in the presence of phosphorylated IRAK1, IRAK4 activates
TNF receptor-associated factor 6 and promotes NF-κB-dependent gene
expression (82). NF-κB is
activated after binding with TAK1 complex; activated NF-κB
up-regulates the expression of pro-inflammatory factors (such as
TNF-α and IL-1β) and increases the functional activities of NADPH
oxidase and mitochondria. Both NADPH oxidase and mitochondria could
promote the production of ROS, which touch off OS and inflammation,
further contributing abnormal activation of Th17/Th2 cells,
arresting Treg cell function and resulting in immune disturbance
(25,28,83-85).
Stimulated Th17 and Th2 cells generate chemokines and inflammatory
cytokines to trigger the migration of neutrophils to inflammatory
sites, thereby exacerbating vascular inflammation and facilitating
HSP initiation (28,30).
HSP is caused by factors such as infection, drugs or
tumors, which cause TLRs recognize PAMPs to activate the innate
immunity. By binding to MyD88, TLR4 triggers signal transduction
and stimulates the NF-κB signaling pathway, which promotes Th1/Th2
imbalance and release of pro-inflammatory mediators (25,74,83).
These enhance B cell proliferation and differentiation, which
contributes to IgA production and deposition on the vascular wall,
as well as vascular endothelial damage (25,38).
As a result, inflammatory cytokines are attracted to elicit ROS
production and OS. Increased ROS in turn promotes TLR4 expression
and the interplay between TLR4 and ROS stimulates the
immuno-inflammatory response (47,68).
The aforementioned molecular events cause further VEC injury and
vascular permeability, ultimately leading to histopathological
changes and clinical manifestations of HSP. The possible
pathogenesis of HSP is presented in Fig. 1.
Proanthocyanidins, a type of polyphenolic compound,
are present in leaves, flowers, seeds, stems and roots, including
common fruits and plants, such as grape, cranberry, black currant,
pomegranate, cocoa and medlar (86). Proanthocyanidins comprise
epicatechin, catechin, epigallocatechin or gallocatechin subunits
connected by C4-C6 and C4-C8 bonds. Depending on the degree of
polymerization, proanthocyanidins are primarily classified into two
isoforms, namely oligomer proanthocyanidin (OPC) that have 2-5
monomers and polymer proanthocyanidin (PPC) that possess >5
monomers (87). Based on low
molecular weight, OPCs exert prominent bioactivities such as immune
modulatory, antioxidant, anti-inflammatory, antibacterial,
anticarcinogenic, vascular protective and anti-hyperglycemic
effects, which enable their application in clinical treatment
(88-93).
The bioactivity of PPCs is weaker due to the long carbon chain but
increased by degeneration (94).
Owing to their powerful immunomodulatory and antioxidant effects,
proanthocyanidins are used to control immune-inflammatory and
OS-associated disorder (95).
Previous studies have indicated that
proanthocyanidins improving immune/inflammatory response and
promoting lymphocyte transformation (96,97).
Studies have confirmed that proanthocyanidins could modulate the
balance of immune cells (especially T cells) and inflammatory cells
to alleviate the immune/inflammatory reaction (96-99).
Park et al (98)
demonstrated in vitro and in vivo that
proanthocyanidins notably increase gene transcription and protein
levels of IFN-γ and decrease expression of IL-6. This results in
activation of Th1 cells and inactivation of Th2 cells and a balance
in humoral and cellular immunity. Furthermore, research on a murine
model of auto-immune arthritis revealed that proanthocyanidins are
effective in alleviating mouse arthritis symptoms by suppressing
proliferation and differentiation of Th17 cells, increasing the
activity and quantity of Treg cells and preventing secretion of
various inflammatory cytokines, such as IL-17, IL-6, IL-21, IL-22
and TNF-α (96,99). Jhun et al (97) demonstrated that obesity and
arthritis of both diet-induced obese mice and rheumatoid
arthritis-like mice are relieved by proanthocyanidin treatment due
to its ability to increase populations of the Treg cells and
decrease the populations of the Th17 cells. Additionally,
proanthocyanidins effectively prevent ovalbumin-induced allergic
reactions by not only decreasing the release of inflammatory
factors by regulating Th-derived cytokine expression (such as IL-4,
IL-5 and IL-13), but also by inhibiting Ig (IgG1 and IgE) synthesis
(100).
Furthermore, proanthocyanidins prevent OS, restore
normal immune function and enhance antioxidant capacity by
regulating multiple pathways, eliminating ROS/MDA, maintaining the
balance of immune cells and inflammatory factors and upregulating
detoxication enzyme and antioxidants (101-103).
Kim et al (104)
demonstrated that grape seed-derived proanthocyanidins effectively
relieve joint swelling and the histopathological damage in a
collagen-induced murine arthritis model through decreasing the
levels of TLR4, MyD88 and NF-κB and inhibiting the TLR4/MyD88/NF-κB
signaling pathway. Additionally, similar report has demonstrated
that proanthocyanidins protectors against cisplatin-induced
inflammatory and oxidative damage in the liver by suppressing
TLR4/NF-κB (105). The
proanthocyanidins not only downregulate gene expression of TLR4 and
NF-κB and decrease levels of inflammatory factors such as IL-1β,
IL-6 and TNF-α, but also decrease the production of OS-associated
mediators (such as ROS and MDA) and upregulate antioxidants [such
as heme oxygenase-1 (HO-1), glutathione (GSH), SOD and catalase]
(105). Proanthocyanidins
minimize the formation of MDA and its metabolites by inhibiting the
LPO process (106);
proanthocyanidins could protect the function of ECs and minimize
risk of vascular complications in diabetes by decreasing LPO and
inhibiting OS (107). Outcomes
from numerous studies have demonstrated that proanthocyanidins
mitigates photo-oxidative damage to prevent UV-induced melanoma and
other types of skin cancer by regulating T cells and inhibiting
MAPK/NF-κB signaling pathways (19,108,109). Furthermore, proanthocyanidins
decrease LPS-stimulated inflammation and OS by inhibiting
inflammatory cytokines (TNF-α and IL-6) and controlling MAPK/NF-κB
signaling pathways (102,110,111). Jia et al (112) demonstrated that suppression of
NF-κB by proanthocyanidins facilitates protect human lens
epithelial B-3 cells from oxidative damage and inflammatory injury.
Proanthocyanidins could reduce H2O2-induced OS damage
and delay cataracts occurrence by inhibiting activation of NF-κB
and MAPK pathways. In a previous study, proanthocyanidins were
demonstrated to inhibit production of iNOS, which is key to lower
NO levels, indicating the antioxidant ability of proanthocyanidins
(110). Proanthocyanidins exerted
a protective effect in the animal model of gingivitis with
porphyromonas gingivalis infection via reducing the release of
IL-1β, IL-6, ROS, NO and lipid peroxide, thereby inhibiting
alveolar bone loss (91,113). Due to their potent antioxidant
and anti-inflammatory capacities, proanthocyanidins enhance the
function of VECs and maintain blood pressure stability by
inhibiting inflammatory cytokine production (74). Proanthocyanidins protect VECs from
OS damage through inhibiting NADPH oxidase activity and scavenging
excess ROS (114,115). Pinna et al (116) demonstrated that proanthocyanidins
prevent vascular endothelial damage in diabetic rats by serving as
a strong ROS scavenger. Proanthocyanidins similarly attenuate OS
and inflammatory responses by eliminating ROS and inhibiting NF-κB
gene expression, thus decreasing capillary permeability and
maintaining vascular homeostasis (117).
A number of clinical trials has verified that
proanthocyanidins are a pharmacologically safe and effective agent
without negative effects and are suitable for a range of
populations, including pregnant people (118,119). Proanthocyanidins reliably relieve
symptoms of patients that suffered from urinary tract infections or
gingivitis (91,120). However, an in vivo study
in female rats, reported that high-dose proanthocyanidin (>432
mg/kg) could cause delayed gastric emptying and pica behavior
(121). At present, to the best
of our knowledge, extensive research of proanthocyanidins has been
performed in various disorders but reports regarding HSP are rare
(91,105,109,122). Fig.
2 presents the molecular structure and multi-bioactivity of
proanthocyanidins as well as their application in different types
of disease.
As immune imbalance and OS-induced IgA mediated
vessel injury are implicated in HSP and proanthocyanidins have
immunoregulatory, antioxidant and anti-inflammatory activity, it is
hypothesized that proanthocyanidins may be beneficial to patients
with HSP. HSP is a recurrent, immune-mediated vasculitis that
invades arterioles and capillaries, typically featuring IgA
deposition along vascular walls (23,45,46,123); immune imbalance and OS are
responsible for HSP pathogenesis and involve signals from TLR4 and
its downstream pathways MyD88/NF-κB (5,23,46,61,62);
high proportions of Th2/Th1 as well as Th17/Treg and Th2 and Th17
overactivation are associated with increased ROS and redox
imbalance in lesions and blood samples from patients with HSP;
furthermore, Th2/Th17 promotion of Ig (primarily IgA) production
and deposition are frequently observed in patients with HSP. The
aforementioned factors stimulate and maintain the progression of
HSP and, therefore may serve as potential targets for treatment of
HSP (26,27,62,124). The aforementioned events are
mediated by the TLR4/MyD88/NF-κB signaling pathway and a number of
studies demonstrated high levels of TLR4 in patients with HSP
accompanied by an increase in MyD88 and NF-κB, followed by release
of inflammatory factors (such as IL-4, IL-6, IL-8, IL-17, IL-21 and
LTB4) (5,25-29).
Proanthocyanidins mitigate inflammatory and oxidative damage in
inflammation/OS-associated disorder by inhibiting TLR4/MyD88/NF-κB
pathways and inflammatory factors (IL-1β, IL-6 and TNF-α),
scavenging NO, ROS and MDA and increasing release of HO-1, GSH, SOD
and catalase (18,89,102,104,105,125). Proanthocyanidins improve vascular
endothelial function, decrease vascular permeability and prevent
vascular damage by inhibiting release of inflammatory factors,
eliminating ROS and reducing the production of Igs and deposition
of immune complexes (92,116,117,126). Thus, proanthocyanidins are
currently applied in immune-mediated and vascular disorders to
improving vascular endothelial function in hypertensive patients
(92). The aforementioned
hypothetical mechanisms of proanthocyanidins in treating HSP are
based on inactivation of the TLR4/MyD88/NF-κB pathways, inhibition
of OS, suppression of inflammation and balance of immunity
(Fig. 3).
As the most common form of systemic vasculitis in
children, annual incidence rate is up to ~27/100,000(127). HSP presents as a skin impairment;
multi-systemic involvement, which can be life threatening, is a
research focus. Despite conventional medications to control HSP,
such as anti-inflammatory drugs, corticosteroids, cytotoxic drugs
and immunosuppressants (8,127,128). These measures are unsuitable for
long-term use due to the high cost, transient efficacy and severe
adverse effects, like long-term and large-scale use of
glucocorticoids could cause infections and Cushing's syndrome;
long-term use of cytotoxic drugs that could result in
gastrointestinal reactions, hepatotoxicity, bone marrow
suppression, reproductive toxicity, cardiotoxicity (12). Proanthocyanidins, as natural
extracts from plants, have widespread sources and diverse
bioactivity with minimal side effects and can be used by pregnant
people (118). Therefore,
proanthocyanidins are preferable options for treatment of HSP based
on their immunoregulatory, antioxidant and anti-inflammatory
properties and may prevent OS-induced damage and modulate immune
balance by scavenging ROS, maintaining Th1/Th2 and Th17/Treg
balance and decreasing the secretion of inflammatory factors by the
inhibition of TLR4/MyD88/NF-κB signaling pathways (99,102,104). In addition, a number of factors
may affect the efficacy of proanthocyanidins in treatment of
patients with HSP, such as dose of medication, the duration of drug
intervention, the quality of the experimental models (Models with
insignificant pathological manifestations or molecular biological
alterations could affect the judgement of the efficacy of
proanthocyanidins), the physical status of patients and the
presence of underlying pathological conditions.
Future research should evaluate the effects of
proanthocyanidins on HSP-associated clinical manifestations,
histopathological or immunopathological alterations, cytological
and serological changes such as cutaneous or systemic symptoms, VEC
swelling, fibrin degeneration/necrosis, inflammatory cell
infiltration, IgA deposition along vascular walls, T/B cell
quantities and inflammatory/OS-associated indicator levels, in the
presence or absence of proanthocyanidins. The present study assumes
that proanthocyanidins would be beneficial for HSP. Therefore, to
verify this hypothesis and reveal the effectiveness of
proanthocyanidins in the treatment of patients with HSP, animal
experiments in vivo should be performed to evaluate the
effects of proanthocyanidins in well-established HSP-like rats
models (129). The present review
describes the successful construction of HSP rat models based on
the model research of Li et al (130). In the aforementioned study,
Sprague Dawley rats were intraperitoneally injected with ovalbumin
(OVA) emulsified solution (1:1 OVA solution mixed with Freund's
Complete Adjuvant) once/week for 3 weeks. After 3 weeks, rats were
injected with 1 ml 10 mg/ml OVA saline via the tail vein, and
intradermal injection of 1 ml 0.3% saline to stimulate
hypersensitivity type III; finally, a rat model of HSP was
successfully built. The preliminary results indicated the skin of
the abdomen and tail of the rats visually presented with scattered
petechiae and ecchymosis; histopathology of the skin revealed
subcutaneous hemorrhage and inflammatory infiltration; renal
histopathology revealed mesangial cell and matrix proliferation and
immunofluorescence of renal tissue exhibited granular deposition of
IgA immune complexes in the mesangial area (Fig. 4) (129). Additionally, in vitro
studies using HSP-like cell models should be performed to
investigate the mechanism of proanthocyanidins in HSP. Furthermore,
updated biological techniques should be used to determine the
associated parameters. In addition, randomized placebo-controlled
clinical trials may establish a scientific basis for further
research and clinical application of proanthocyanidins.
HSP, an IgA-mediated vasculitis, commonly affects
children, affects the skin and systemic organs and threatens life.
The etiology and pathogenesis of HSP are unclear but currently it
is hypothesized to be a multifactorial disorder involving immune
imbalance, OS, infection, genes, foods, drugs and complex cytokine
networks (23,27,124). Immune dysregulation and OS that
induce IgA-mediated vascular injury are responsible for HSP and
involve abnormal activation of TLR4/MyD88/NF-κB signaling. Thus,
targeting these areas could lead to a treatment for patients with
HSP. Treatments, such as targeting key inflammatory mediators,
pathways or cells, have exhibited positive effects on HSP (5,131).
Typically, corticosteroids have greater efficacy on severe
cutaneous and visceral involvement such as gastrointestinal or
kidney impairment by suppressing T/B cell activation, antibody
production and inflammatory factor release (132-134);
for example, patients with severe purpura nephritis are treated
with methylprednisolone pulse therapy that effectively reduces
kidney pathological injury and shortens treatment course (135). However, glucocorticoids merely
ease the symptoms, while excessive use of glucocorticoids may lead
to gastrointestinal bleeding or electrolyte disturbance (136,137). Furthermore, because of
susceptibility to resistance to glucocorticoid treatment alone, the
combination of glucocorticoids with an immunosuppressant tends to
be more effective and safer, especially for patients with severe
HSP nephritis (137,138); immunosuppressive agents such as
mycophenolate mofetil act against purpura nephritis via selectively
suppressing T/B lymphocytes (139). Additionally, plasma exchange has
a notable effect on patients with recurrent or acute HSP by
improving renal function and clearing immune complexes (140). Despite these treatments having
efficacy against HSP, adverse reactions, short-term efficacy and
high cost prevent widespread use. Therefore, safe, effective and
cheaper treatments are needed for HSP. The present study explained
the pathogenesis of HSP and applications of proanthocyanidins in
clinical practice. Proanthocyanidins have potential as therapy of
HSP, although factors, such as the dose of drug, the duration of
drug intervention, quality of experimental models and patient's
physical condition may affect proanthocyanidins for HSP treatment.
Thus, the aforementioned factors should be investigated in future
research. Additionally, studies in vitro and in vivo
should be performed to verify the signaling pathways in HSP and to
identify improvements in diagnostic biomarkers following
proanthocyanidin treatment.
The authors would like to thank Professor Xia Xiong
and Professor Yongqiong Deng (Department of Neurobiology, Southwest
Medical University, Luzhou, China) for their valuable
suggestions.
Funding: The present study was supported by the Healthcare for
Cadre Research Project of Sichuan Province (grant no. 2020-1501)
supported by the Sichuan Provincial Government.
Not applicable.
QD revised the manuscript. MG conceived the study.
XL and YX wrote the manuscript. YX, JZ and DX performed the
literature review. Data authentication is not applicable. All
authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Wang K, Sun X, Cao Y, Dai L, Sun F, Yu P
and Dong L: Risk factors for renal involvement and severe kidney
disease in 2731 Chinese children with Henoch-Schönlein purpura: A
retrospective study. Medicine (Baltimore).
97(e12520)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chimenz R, Cannavò L, Spinuzza A, Fede C,
Cucinotta U, Pensabene L, Betta P, Gitto E, Concolino D and Cuppari
C: Unusual presentation of Henoch-Schönlein purpura. J Biol Regul
Homeost Agents. 33 (5 Suppl 1):S69–S74. 2019.PubMed/NCBI
|
|
3
|
Gómez S, Pérez M, Pellegrini M, Isern E,
Quintana C, Artacho P, Bertolini M, Pomerantz B and Gadda N:
Henoch-Schonlein purpura in pediatrics: Ten years of experience at
a moderate risk office of a general hospital. Arch Argent Pediatr.
118:31–37. 2020.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
4
|
Yang HR: What we know about
Henoch-Schönlein purpura in children up to date? J Korean Med Sci.
33(e199)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhu Y, Dong Y, Wu L and Deng F: Changes of
inflammatory mediators and oxidative stress indicators in children
with Henoch-Schönlein purpura and clinical effects of hemoperfusion
in the treatment of severe Henoch-Schönlein purpura with
gastrointestinal involvement in children. BMC Pediatr.
19(409)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang N, Guo PJ, Liu PL, Yang HR, Xiao J,
Li XP, Huang JB and Zheng YZ: Comparison of age-based clinical and
abnormal immune parameters in patients with Henoch-Schönlein
purpura. Zhonghua Xue Ye Xue Za Zhi. 38:60–64. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
7
|
Niknam N, Ha L and Gautam-Goyal P: Adult
onset immunoglobulin A vasculitis (Henoch-Schonlein purpura) with
alveolar hemorrhage. IDCases. 12:47–48. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tan J, Tang Y, Zhong Z, Yan S, Tan L,
Tarun P and Qin W: The efficacy and safety of immunosuppressive
agents plus steroids compared with steroids alone in the treatment
of Henoch-Schönlein purpura nephritis: A meta-analysis. Int Urol
Nephrol. 51:975–985. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Roman C, Dima B, Muyshont L, Schurmans T
and Gilliaux O: Indications and efficiency of dapsone in IgA
vasculitis (Henoch-Schonlein purpura): Case series and a review of
the literature. Eur J Pediatr. 178:1275–1281. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vonend C, Rifkin SI, Baliga RS and
Weinstein SS: Henoch-Schönlein purpura and recurrent renal failure.
Ren Fail. 32:888–891. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Oni L and Sampath S: Childhood IgA
vasculitis (Henoch Schonlein Purpura)-advances and knowledge gaps.
Front Pediatr. 7(257)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fan L, Yan H, Zhen X, Wu X, Hao J, Hou L
and Han L: Safety and efficacy evaluation of traditional chinese
medicine (Qingre-Lishi-Yishen Formula) based on treatment of
regular glucocorticoid combined with cyclophosphamide pulse in
children suffered from moderately Severe Henoch-Schonlein Purpura
nephritis with nephrotic proteinuria. Evid Based Complement
Alternat Med. 2020(3920735)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nichols JA and Katiyar SK: Skin
photoprotection by natural polyphenols: Anti-inflammatory,
antioxidant and DNA repair mechanisms. Arch Dermatol Res.
302:71–83. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tong H, Song X, Sun X, Sun G and Du F:
Immunomodulatory and antitumor activities of grape seed
proanthocyanidins. J Agric Food Chem. 59:11543–1157.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang S, Yang N, Liu Y, Hu L, Zhao J, Gao
J, Li Y, Li C, Zhang X and Huang T: Grape seed proanthocyanidins
inhibit angiogenesis via the downregulation of both vascular
endothelial growth factor and angiopoietin signaling. Nutr Res.
32:530–536. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wen W, Lu J, Zhang K and Chen S: Grape
seed extract inhibits angiogenesis via suppression of the vascular
endothelial growth factor receptor signaling pathway. Cancer Prev
Res (Phila). 1:554–561. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu L, Huang Z, Qin P, Yao Y, Meng X, Zou
J, Zhu K and Ren G: Chemical characterization of a procyanidin-rich
extract from sorghum bran and its effect on oxidative stress and
tumor inhibition in vivo. J Agric Food Chem. 59:8609–8615.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang L, Xian D, Xiong X, Lai R, Song J and
Zhong J: Proanthocyanidins against oxidative stress: From molecular
mechanisms to clinical applications. Biomed Res Int.
2018(8584136)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mantena SK and Katiyar SK: Grape seed
proanthocyanidins inhibit UV-radiation-induced oxidative stress and
activation of MAPK and NF-kappaB signaling in human epidermal
keratinocytes. Free Radic Biol Med. 40:1603–1614. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Koudoufio M, Feldman F, Ahmarani L, Delvin
E, Spahis S, Desjardins Y and Levy E: Intestinal protection by
proanthocyanidins involves anti-oxidative and anti-inflammatory
actions in association with an improvement of insulin sensitivity,
lipid and glucose homeostasis. Sci Rep. 11(3878)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Baldivia DDS, Leite DF, Castro DTH, Campos
JF, Santos UPD, Paredes-Gamero EJ, Carollo CA, Silva DB, de Picoli
Souza K and Dos Santos EL: Evaluation of in vitro antioxidant and
anticancer properties of the aqueous extract from the stem bark of
stryphnodendron adstringens. Int J Mol Sci. 19(2432)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Alsharairi NA: Insights into the
mechanisms of action of proanthocyanidins and anthocyanins in the
treatment of nicotine-induced non-small cell lung cancer. Int J Mol
Sci. 23(7905)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lau KK, Suzuki H, Novak J and Wyatt RJ:
Pathogenesis of Henoch-Schönlein purpura nephritis. Pediatr
Nephrol. 25:19–26. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Keskin N, Civilibal M, Elevli M, Koldas M,
Duru NS and Ozturk H: Elevated plasma advanced oxidation protein
products in children with Henoch-Schonlein purpura. Pediatr
Nephrol. 26:1989–1993. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xu H, Jiang G, Shen H, Li W, Mao J and Pan
Y: Association of TLR4 gene polymorphisms with childhood
Henoch-Schönlein purpura in a Chinese population. Rheumatol Int.
37:1909–1915. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Canpinar H, Ozaltin F, Bilginer Y,
Bakkaloğlu A and Ozen S: Toll-like receptors 2 and 4 cell surface
expression reflects endotoxin tolerance in Henoch-Schönlein
purpura. Turk J Pediatr. 52:22–27. 2010.PubMed/NCBI
|
|
27
|
Donadio ME, Loiacono E, Peruzzi L, Amore
A, Camilla R, Chiale F, Vergano L, Boido A, Conrieri M, Bianciotto
M, et al: Toll-like receptors, immunoproteasome and regulatory T
cells in children with Henoch-Schönlein purpura and primary IgA
nephropathy. Pediatr Nephrol. 29:1545–1551. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chang H, Zhang QY, Lin Y, Cheng N and
Zhang SQ: Correlation of TLR2 and TLR4 expressions in peripheral
blood mononuclear cells to Th1- and Th2-type immune responses in
children with Henoch-Schönlein Purpura. Int J Clin Exp Med.
8:13532–13539. 2015.PubMed/NCBI
|
|
29
|
Chang H, Yu DS, Liu XQ, Zhang QY, Cheng N,
Zhang SQ and Qu ZH: Clinical significance of TLR3 and TLR4 in
peripheral blood mononuclear cells from children with
Henoch-Schönlein purpura nephritis. Exp Ther Med. 7:1703–1707.
2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhou L, Xu F, Chang C, Tao Y, Song L and
Li X: Interleukin-17-producing CD4+ T lymphocytes are increased in
patients with primary immune thrombocytopenia. Blood Coagul
Fibrinolysis. 27:301–307. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Barnabei L, Laplantine E, Mbongo W,
Rieux-Laucat F and Weil R: NF-κB: At the borders of autoimmunity
and inflammation. Front Immunol. 12(716469)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yu H, Lin L, Zhang Z, Zhang H and Hu H:
Targeting NF-κB pathway for the therapy of diseases: Mechanism and
clinical study. Signal Transduct Target Ther. 5(209)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Oh H and Ghosh S: NF-κB: Roles and
regulation in different CD4(+) T-cell subsets. Immunol Rev.
252:41–51. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pandiyan P and Zhu J: Origin and functions
of pro-inflammatory cytokine producing Foxp3+ regulatory T cells.
Cytokine. 76:13–24. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chang H, Lin Y, Lei K, Wang F, Zhang QQ
and Zhang QY: Role of hypomethylation of suppressor of cytokine
signaling in T helper 17 cell/regulatory T cell imbalance in
children with Henoch-Schönlein purpura. Zhongguo Dang Dai Er Ke Za
Zhi. 21:38–44. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
36
|
Li-Weber M, Giaisi M, Baumann S, Pálfi K
and Krammer PH: NF-kappa B synergizes with NF-AT and NF-IL6 in
activation of the IL-4 gene in T cells. Eur J Immunol.
34:1111–1118. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li YY, Li CR, Wang GB, Yang J and Zu Y:
Investigation of the change in CD4+ T cell subset in children with
Henoch-Schonlein purpura. Rheumatol Int. 32:3785–3792.
2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yang J, Li CR, Zu Y, Wang GB and Li YB:
Role of regulatory T cells in pathogenesis of Henoch-Schonlein
purpura in children. Zhonghua Er Ke Za Zhi. 44:411–414.
2006.PubMed/NCBI(In Chinese).
|
|
39
|
Gujer C, Sundling C, Seder RA, Karlsson
Hedestam GB and Loré K: Human and rhesus plasmacytoid dendritic
cell and B-cell responses to Toll-like receptor stimulation.
Immunology. 134:257–269. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ohue Y and Nishikawa H: Regulatory T
(Treg) cells in cancer: Can Treg cells be a new therapeutic target?
Cancer Sci. 110:2080–2089. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Adeegbe DO and Nishikawa H: Natural and
induced T regulatory cells in cancer. Front Immunol.
4(190)2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Weng L, Cao X, Han L, Zhao H, Qiu S, Yan
Y, Wang X, Chen X, Zheng W, Xu X, et al: Association of increased
Treg and Th17 with pathogenesis of moyamoya disease. Sci Rep.
7(3071)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang CM, Luo Y, Wang YC and Sheng GY:
Roles of follicular helper T cells and follicular regulatory T
cells in pathogenesis of Henoch-Schönlein purpura in children.
Zhongguo Dang Dai Er Ke Za Zhi. 17:1084–1087. 2015.PubMed/NCBI(In Chinese).
|
|
44
|
Yang YH, Lai HJ, Huang CM, Wang LC, Lin YT
and Chiang BL: Sera from children with active Henoch-Schönlein
purpura can enhance the production of interleukin 8 by human
umbilical venous endothelial cells. Ann Rheum Dis. 63:1511–1513.
2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yang YH, Huang YH, Lin YL, Wang LC, Chuang
YH, Yu HH, Lin YT and Chiang BL: Circulating IgA from acute stage
of childhood Henoch-Schönlein purpura can enhance endothelial
interleukin (IL)-8 production through MEK/ERK signalling pathway.
Clin Exp Immunol. 144:247–253. 2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Heineke MH, Ballering AV, Jamin A, Ben
Mkaddem S, Monteiro RC and Van Egmond M: New insights in the
pathogenesis of immunoglobulin A vasculitis (Henoch-Schönlein
purpura). Autoimmun Rev. 16:1246–1253. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wu JJ, Zhu YT and Hu YM: Mechanism of
feedback regulation of neutrophil inflammation in Henoch-Schönlein
purpura. Eur Rev Med Pharmacol Sci. 20:4277–4285. 2016.PubMed/NCBI
|
|
48
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Cesselli D, Aleksova A, Sponga S,
Cervellin C, Di Loreto C, Tell G and Beltrami AP: Cardiac cell
senescence and redox signaling. Front Cardiovasc Med.
4(38)2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ciccarese F, Raimondi V, Sharova E,
Silic-Benussi M and Ciminale V: Nanoparticles as tools to target
redox homeostasis in cancer cells. Antioxidants (Basel).
9(211)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Nakai K and Tsuruta D: What are reactive
oxygen species, free radicals, and oxidative stress in skin
diseases? Int J Mol Sci. 22(10799)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Sharifi-Rad M, Anil Kumar NV, Zucca P,
Varoni EM, Dini L, Panzarini E, Rajkovic J, Tsouh Fokou PV, Azzini
E, Peluso I, et al: Lifestyle, oxidative stress, and antioxidants:
Back and forth in the pathophysiology of chronic diseases. Front
Physiol. 11(694)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Li Y and Pagano PJ: Microvascular NADPH
oxidase in health and disease. Free Radic Biol Med. 109:33–47.
2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Schröder K: NADPH oxidases: Current
aspects and tools. Redox Biol. 34(101512)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Murphy MP: How mitochondria produce
reactive oxygen species. Biochem J. 417:1–13. 2009.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Arazi H, Eghbali E and Suzuki K: Creatine
supplementation, physical exercise and oxidative stress markers: A
review of the mechanisms and effectiveness. Nutrients.
13(869)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kim YW, West XZ and Byzova TV:
Inflammation and oxidative stress in angiogenesis and vascular
disease. J Mol Med (Berl). 91:323–328. 2013.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Salazar G: NADPH Oxidases and mitochondria
in vascular senescence. Int J Mol Sci. 19(1327)2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhu Y, Dong Y, Xu DL, Jiang JY, Wu L, Ke
RJ, Fang SH and Peng Y: Clinical effect and mechanism of
hemoperfusion in treatment of children with severe abdominal
Henoch-Schönlein purpura. Zhongguo Dang Dai Er Ke Za Zhi.
20:378–382. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
61
|
Gurses D, Parlaz N, Bor-Kucukatay M,
Kucukatay V and Erken G: Evaluation of oxidative stress and
erythrocyte properties in children with henoch-shoenlein purpura.
Iran J Pediatr. 24:166–172. 2014.PubMed/NCBI
|
|
62
|
Ece A, Kelekçi S, Kocamaz H, Hekimoğlu A,
Balik H, Yolbaş I and Erel O: Antioxidant enzyme activities, lipid
peroxidation, and total antioxidant status in children with
Henoch-Schönlein purpura. Clin Rheumatol. 27:163–169.
2008.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Higashi Y, Maruhashi T, Noma K and Kihara
Y: Oxidative stress and endothelial dysfunction: Clinical evidence
and therapeutic implications. Trends Cardiovasc Med. 24:165–169.
2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Mai J, Virtue A, Shen J, Wang H and Yang
XF: An evolving new paradigm: Endothelial cells-conditional innate
immune cells. J Hematol Oncol. 6(61)2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Hou X, Yang S and Yin J: Blocking the
REDD1/TXNIP axis ameliorates LPS-induced vascular endothelial cell
injury through repressing oxidative stress and apoptosis. Am J
Physiol Cell Physiol. 316:C104–C110. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wu H, Wen Y, Yue C, Li X and Gao R: Serum
TNF-α level is associated with disease severity in adult patients
with immunoglobulin a vasculitis nephritis. Dis Markers.
2020(5514145)2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Yuan P, Bo Y, Ming G, Wen-Jun F, Qin Z and
Bo H: Apoptosis of human umbilical vein endothelial cells (HUVEC)
induced by IgA1 isolated from Henoch-Schonlein purpura children.
Asian Pac J Allergy Immunol. 32:34–38. 2014.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Montezano AC and Touyz RM: Reactive oxygen
species and endothelial function-role of nitric oxide synthase
uncoupling and Nox family nicotinamide adenine dinucleotide
phosphate oxidases. Basic Clin Pharmacol Toxicol. 110:87–94.
2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Elalfy MS, Elhenawy YI, Deifalla S, Hegazy
M, Sabra A and Abdelaziz Y: Oxidant/antioxidant status in children
and adolescents with immune thrombocytopenia (ITP) and the role of
an adjuvant antioxidant therapy. Pediatr Blood Cancer. 62:830–837.
2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Carta S, Semino C, Sitia R and Rubartelli
A: Dysregulated IL-1β secretion in autoinflammatory diseases: A
matter of stress? Front Immunol. 8(345)2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Noval Rivas M, Wakita D, Franklin MK,
Carvalho TT, Abolhesn A, Gomez AC, Fishbein MC, Chen S, Lehman TJ,
Sato K, et al: Intestinal permeability and IgA provoke immune
vasculitis linked to cardiovascular inflammation. Immunity.
51:508–521.e6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Sugino H, Sawada Y and Nakamura M: IgA
Vasculitis: Etiology, treatment, biomarkers and epigenetic changes.
Int J Mol Sci. 22(7538)2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Lavieri R, Piccioli P, Carta S, Delfino L,
Castellani P and Rubartelli A: TLR costimulation causes oxidative
stress with unbalance of proinflammatory and anti-inflammatory
cytokine production. J Immunol. 192:5373–5381. 2014.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Gong L, Lei Y, Liu Y, Tan F, Li S, Wang X,
Xu M, Cai W, Du B, Xu F, et al: Vaccarin prevents ox-LDL-induced
HUVEC EndMT, inflammation and apoptosis by suppressing ROS/p38 MAPK
signaling. Am J Transl Res. 11:2140–2154. 2019.PubMed/NCBI
|
|
75
|
Duan T, Du Y, Xing C, Wang HY and Wang RF:
Toll-like receptor signaling and its role in cell-mediated
immunity. Front Immunol. 13(812774)2022.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Aluri J, Cooper MA and Schuettpelz LG:
Toll-Like receptor signaling in the establishment and function of
the immune system. Cells. 10(1374)2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Vijay K: Toll-like receptors in immunity
and inflammatory diseases: Past, present, and future. Int
Immunopharmacol. 59:391–412. 2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Kuzmich NN, Sivak KV, Chubarev VN, Porozov
YB, Savateeva-Lyubimova TN and Peri F: TLR4 signaling pathway
modulators as potential therapeutics in inflammation and sepsis.
Vaccines (Basel). 5(34)2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Yao C, Oh JH, Lee DH, Bae JS, Jin CL, Park
CH and Chung JH: Toll-like receptor family members in skin
fibroblasts are functional and have a higher expression compared to
skin keratinocytes. Int J Mol Med. 35:1443–1450. 2015.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Krishnan JS, KSelvarajoo K, Tsuchiya M,
Lee G and Choi S: Toll-like receptor signal transduction. Exp Mol
Med. 39:421–438. 2007.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Rong Z, Huang Y, Cai H, Chen M, Wang H,
Liu G, Zhang Z and Wu J: Gut microbiota disorders promote
inflammation and aggravate spinal cord injury through the
TLR4/MyD88 signaling pathway. Front Nutr. 8(702659)2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Yamamoto M, Sato S, Hemmi H, Uematsu S,
Hoshino K, Kaisho T, Takeuchi O, Takeda K and Akira S: TRAM is
specifically involved in the Toll-like receptor 4-mediated
MyD88-independent signaling pathway. Nat Immunol. 4:1144–1150.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
83
|
Mills KH: TLR-dependent T cell activation
in autoimmunity. Nat Rev Immunol. 11:807–822. 2011.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Park HS, Jung HY, Park EY, Kim J, Lee WJ
and Bae YS: Cutting edge: Direct interaction of TLR4 with NAD(P)H
oxidase 4 isozyme is essential for lipopolysaccharide-induced
production of reactive oxygen species and activation of NF-kappa B.
J Immunol. 173:3589–3593. 2004.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Gill R, Tsung A and Billiar T: Linking
oxidative stress to inflammation: Toll-like receptors. Free Radic
Biol Med. 48:1121–1132. 2010.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Rauf A, Imran M, Abu-Izneid T,
Iahtisham-Ul-Haq Patel S, Pan X, Naz S, Sanches Silva A, Saeed F
and Rasul Suleria HA: Proanthocyanidins: A comprehensive review.
Biomed Pharmacother. 116(108999)2019.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Bensa M, Glavnik V and Vovk I: Leaves of
Invasive Plants-Japanese, bohemian and giant knotweed-the promising
new nource of Flavan-3-ols and proanthocyanidins. Plants (Basel).
9(118)2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
González-Quilen C, Rodríguez-Gallego E,
Beltrán-Debón R, Pinent M, Ardévol A, Blay MT and Terra X:
Health-promoting properties of proanthocyanidins for intestinal
dysfunction. Nutrients. 12(130)2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Li S, Xu M, Niu Q, Xu S, Ding Y, Yan Y,
Guo S and Li F: Efficacy of procyanidins against in vivo cellular
oxidative damage: A systematic review and meta-analysis. PLoS One.
10(e0139455)2015.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Ravindranathan P, Pasham D, Balaji U,
Cardenas J, Gu J, Toden S and Goel A: Mechanistic insights into
anticancer properties of oligomeric proanthocyanidins from grape
seeds in colorectal cancer. Carcinogenesis. 39:767–777.
2018.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Nawrot-Hadzik I, Matkowski A,
Kubasiewicz-Ross P and Hadzik J: Proanthocyanidins and Flavan-3-ols
in the prevention and treatment of periodontitis-immunomodulatory
effects, animal and clinical Studies. Nutrients.
13(239)2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Odai T, Terauchi M, Kato K, Hirose A and
Miyasaka N: Effects of grape seed proanthocyanidin extract on
vascular endothelial function in participants with prehypertension:
A randomized, double-blind, placebo-controlled study. Nutrients.
11(2844)2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Zhang R, Kang X, Liu L, Wang X, Li H, Zhu
J, Cao Y and Zhu H: Gut microbiota modulation by plant polyphenols
in koi carp (Cyprinus carpio L.). Front Microbiol.
13(977292)2022.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Suo H, Tian R, Li J, Zhang S, Cui Y, Li L
and Sun B: Compositional characterization study on
high-molecular-mass polymeric polyphenols in red wines by chemical
degradation. Food Res Int. 123:440–449. 2019.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Yuan L, Wang Q, Zhang S and Zhang L:
Correlation between serum inflammatory factors TNF-α, IL-8, IL-10
and Henoch-Schonlein purpura with renal function impairment. Exp
Ther Med. 15:3924–3928. 2018.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Park MK, Park JS, Cho ML, Oh HJ, Heo YJ,
Woo YJ, Heo YM, Park MJ, Park HS, Park SH, et al: Grape seed
proanthocyanidin extract (GSPE) differentially regulates Foxp3(+)
regulatory and IL-17(+) pathogenic T cell in autoimmune arthritis.
Immunol Lett. 135:50–58. 2011.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Jhun JY, Moon SJ, Yoon BY, Byun JK, Kim
EK, Yang EJ, Ju JH, Hong YS, Min JK, Park SH, et al: Grape seed
proanthocyanidin extract-mediated regulation of STAT3 proteins
contributes to Treg differentiation and attenuates inflammation in
a murine model of obesity-associated arthritis. PLoS One.
8(e78843)2013.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Park IJ, Cha SY, Kang M, So YS, Go HG, Mun
SP, Ryu KS and Jang HK: Effect of proanthocyanidin-rich extract
from Pinus radiata bark on immune response of
specific-pathogen-free White Leghorn chickens. Poult Sci.
90:977–982. 2011.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Min HK, Kim SM, Baek SY, Woo JW, Park JS,
Cho ML, Lee J, Kwok SK, Kim SW and Park SH: Anthocyanin extracted
from black soybean seed coats prevents autoimmune arthritis by
suppressing the development of Th17 cells and synthesis of
proinflammatory cytokines by such cells, via inhibition of NF-κB.
PLoS One. 10(e0138201)2015.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Takano F, Takata T, Yoshihara A, Nakamura
Y, Arima Y and Ohta T: Aqueous extract of peanut skin and its main
constituent procyanidin A1 suppress serum IgE and IgG1 levels in
mice-immunized with ovalbumin. Biol Pharm Bull. 30:922–927.
2007.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Long M, Yang SH, Han JX, Li P, Zhang Y,
Dong S, Chen X, Guo J, Wang J and He JB: The protective effect of
grape-seed proanthocyanidin extract on oxidative damage induced by
zearalenone in kunming mice liver. Int J Mol Sci.
17(808)2016.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Han S, Gao H, Chen S, Wang Q, Li X, Du LJ,
Li J, Luo YY, Li JX, Zhao LC, et al: Procyanidin A1 Alleviates
Inflammatory Response induced by LPS through NF-κB, MAPK, and
Nrf2/HO-1 Pathways in RAW264.7 cells. Sci Rep.
9(15087)2019.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Katiyar SK: Proanthocyanidins from grape
seeds inhibit UV-radiation-induced immune suppression in mice:
Detection and analysis of molecular and cellular targets. Photochem
Photobiol. 91:156–162. 2015.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Kim SH, Bang J, Son CN, Baek WK and Kim
JM: Grape seed proanthocyanidin extract ameliorates murine
autoimmune arthritis through regulation of TLR4/MyD88/NF-κB
signaling pathway. Korean J Intern Med. 33:612–621. 2018.PubMed/NCBI View Article : Google Scholar
|
|
105
|
El-Shitany NA and Eid B: Proanthocyanidin
protects against cisplatin-induced oxidative liver damage through
inhibition of inflammation and NF-κβ/TLR-4 pathway. Environ
Toxicol. 32:1952–1963. 2017.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Mittal A, Elmets CA and Katiyar SK:
Dietary feeding of proanthocyanidins from grape seeds prevents
photocarcinogenesis in SKH-1 hairless mice: Relationship to
decreased fat and lipid peroxidation. Carcinogenesis. 24:1379–1388.
2003.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Rodríguez RM, Colom-Pellicer M, Blanco J,
Calvo E, Aragonès G and Mulero M: Grape-Seed procyanidin extract
(GSPE) Seasonal-Dependent modulation of glucose and lipid
metabolism in the liver of healthy F344 rats. Biomolecules.
12(839)2022.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Sharma SD, Meeran SM and Katiyar SK:
Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative
stress and activation of mitogen-activated protein kinases and
nuclear factor-kappaB signaling in in vivo SKH-1 hairless mice. Mol
Cancer Ther. 6:995–1005. 2007.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Divya SP, Wang X, Pratheeshkumar P, Son
YO, Roy RV, Kim D, Dai J, Hitron JA, Wang L, Asha P, et al:
Blackberry extract inhibits UVB-induced oxidative damage and
inflammation through MAP kinases and NF-κB signaling pathways in
SKH-1 mice skin. Toxicol Appl Pharmacol. 284:92–99. 2015.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Ma X, Wang R, Yu S, Lu G, Yu Y and Jiang
C: Anti-Inflammatory activity of oligomeric proanthocyanidins via
inhibition of NF-κB and MAPK in LPS-Stimulated MAC-T Cells. J
Microbiol Biotechnol. 30:1458–1466. 2020.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Rajput SA, Sun L, Zhang NY, Khalil MM,
Ling Z, Chong L, Wang S, Rajput IR, Bloch DM, Khan FA, et al: Grape
seed proanthocyanidin extract alleviates aflatoxin B1-induced
immunotoxicity and oxidative stress via modulation of NF-κB and
Nrf2 signaling pathways in broilers. Toxins (Basel).
11(23)2019.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Jia Z, Song Z, Zhao Y, Wang X and Liu P:
Grape seed proanthocyanidin extract protects human lens epithelial
cells from oxidative stress via reducing NF-кB and MAPK protein
expression. Mol Vis. 17:210–217. 2011.PubMed/NCBI
|
|
113
|
Schmuch J, Beckert S, Brandt S, Löhr G,
Hermann F, Schmidt TJ, Beikler T and Hensel A: Extract from
Rumex acetosa L. for prophylaxis of periodontitis:
Inhibition of bacterial in vitro adhesion and of gingipains of
Porphyromonas gingivalis by
epicatechin-3-O-(4β→8)-epicatechin-3-O-gallate
(procyanidin-B2-Di-gallate). PLoS One. 10(e0120130)2015.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Steffen Y, Gruber C, Schewe T and Sies H:
Mono-O-methylated flavanols and other flavonoids as inhibitors of
endothelial NADPH oxidase. Arch Biochem Biophys. 469:209–219.
2008.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Álvarez E, Rodiño-Janeiro BK, Jerez M,
Ucieda-Somoza R, Núñez MJ and González-Juanatey JR: Procyanidins
from grape pomace are suitable inhibitors of human endothelial
NADPH oxidase. J Cell Biochem. 113:1386–1396. 2012.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Pinna C, Morazzoni P and Sala A:
Proanthocyanidins from Vitis vinifera inhibit oxidative
stress-induced vascular impairment in pulmonary arteries from
diabetic rats. Phytomedicine. 25:39–44. 2017.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Weseler AR and Bast A: Masquelier's grape
seed extract: From basic flavonoid research to a well-characterized
food supplement with health benefits. Nutr J. 16(5)2017.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Yang LJ, Zhu DN, Dang YL and Zhao X:
Treatment of condyloma acuminata in pregnant women with cryotherapy
combined with proanthocyanidins: Outcome and safety. Exp Ther Med.
11:2391–2394. 2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Sano A: Safety assessment of 4-week oral
intake of proanthocyanidin-rich grape seed extract in healthy
subjects. Food Chem Toxicol. 108:519–523. 2017.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Occhipinti A, Germano A and Maffei ME:
Prevention of urinary tract infection with oximacro, a cranberry
extract with a high content of A-type proanthocyanidins: A
pre-clinical double-blind controlled study. Urol J. 13:2640–2649.
2016.PubMed/NCBI
|
|
121
|
Serrano J, Casanova-Martí À, Gil-Cardoso
K, Blay MT, Terra X, Pinent M and Ardévol A: Acutely administered
grape-seed proanthocyanidin extract acts as a satiating agent. Food
Funct. 7:483–490. 2016.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Zhao Y, Xie Y, Li X, Song J, Guo M, Xian D
and Zhong J: The protective effect of proanthocyanidins on the
psoriasis-like cell models via PI3K/AKT and HO-1. Redox Rep.
27:200–211. 2022.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Omma A, Colak S, Can Sandikci S, Yucel C,
Erden A, Sertoglu E and Ozgurtas T: Serum neopterin and ischemia
modified albumin levels are associated with the disease activity of
adult immunoglobulin A vasculitis (Henoch-Schönlein purpura). Int J
Rheum Dis. 22:1920–1925. 2019.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Wang B, Shao-Kuan FR and Dong C:
Expression and significance of follicular helper T cells and
galactose-deficient IgA1 in children with Henoch-Schönlein purpura.
Zhongguo Dang Dai Er Ke Za Zhi. 22:473–477. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
125
|
Long M, Zhang Y, Li P, Yang SH, Zhang WK,
Han JX, Wang Y and He JB: Intervention of Grape seed
proanthocyanidin extract on the subchronic immune injury in mice
induced by Aflatoxin B1. Int J Mol Sci. 17(516)2016.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Liu X, Qiu J, Zhao S, You B, Ji X, Wang Y,
Cui X, Wang Q and Gao H: Grape seed proanthocyanidin extract
alleviates ouabain-induced vascular remodeling through regulation
of endothelial function. Mol Med Rep. 6:949–954. 2012.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Di Pietro GM, Castellazzi ML, Mastrangelo
A, Montini G, Marchisio P and Tagliabue C: Henoch-Schönlein Purpura
in children: Not only kidney but also lung. Pediatr Rheumatol
Online J. 17(75)2019.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Padeh S and Passwell JH: Successful
treatment of chronic Henoch-Schonlein purpura with colchicine and
aspirin. Isr Med Assoc J. 2:482–483. 2000.PubMed/NCBI
|
|
129
|
Pan S and Zhong J: Construction and
identification of simplified rat model of Henoch-Schönlein Purpura.
Sichuan Med J. 42:669–672. 2021.(In Chinese).
|
|
130
|
Li Y, Feng X, Huang L, Zhu H, Xu Y, Sui X,
Xu Y, Han Y and Qin C: Hematologic and immunological
characteristics of Henoch-Schönlein purpura in rat and rabbit
models induced with ovalbumin based on type III hypersensitivity.
Sci Rep. 5(8862)2015.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Kaegi C, Wuest B, Schreiner J, Steiner UC,
Vultaggio A, Matucci A, Crowley C and Boyman O: Systematic review
of safety and efficacy of rituximab in treating immune-mediated
disorders. Front Immunol. 10(1990)2019.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Gohari A, Matsell DG, Mammen C and Goldman
RD: Henoch-Schönlein purpura in children: Use of corticosteroids
for prevention and treatment of renal disease. Can Fam Physician.
66:895–897. 2020.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Du Y, Hou L, Zhao C, Han M and Wu Y:
Treatment of children with Henoch-Schönlein purpura nephritis with
mycophenolate mofetil. Pediatr Nephrol. 27:765–771. 2012.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Nikibakhsh AA, Mahmoodzadeh H, Karamyyar
M, Hejazi S, Noroozi M, Macooie AA, Gholizadeh A and Gholizadeh L:
Treatment of complicated henoch-schönlein purpura with
mycophenolate mofetil: A retrospective case series report. Int J
Rheumatol. 2010(254316)2010.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Fujimoto M, Katayama K, Nishikawa K,
Mizoguchi S, Oda K, Hirabayashi Y, Suzuki Y, Haruki A, Ito T,
Murata T, et al: A Kidney transplant recipient with recurrent
Henoch-Schönlein Purpura nephritis successfully treated with
steroid pulse therapy and epipharyngeal abrasive therapy. Nephron.
144 (Suppl 1):S54–S48. 2020.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Kurnia B: Henoch-Schonlein purpura in
children: The role of corticosteroids. Open Access Maced J Med Sci.
7:1812–1814. 2019.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Ozen S, Marks SD, Brogan P, Groot N, de
Graeff N, Avcin T, Bader-Meunier B, Dolezalova P, Feldman BM,
Kone-Paut I, et al: European consensus-based recommendations for
diagnosis and treatment of immunoglobulin A vasculitis-the SHARE
initiative. Rheumatology (Oxford). 58:1607–1616. 2019.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Han F, Chen LL, Ren PP, Le JY, Choong PJ,
Wang HJ, Xu Y and Chen JH: Mycophenolate mofetil plus prednisone
for inducing remission of Henoch-Schönlein purpura nephritis: A
retrospective study. J Zhejiang Univ Sci B. 16:772–779.
2015.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Lu Z, Song J, Mao J, Xia Y and Wang C:
Evaluation of mycophenolate mofetil and Low-dose steroid combined
therapy in moderately severe Henoch-Schönlein Purpura nephritis.
Med Sci Monit. 23:2333–2339. 2017.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Hamilton P, Ogundare O, Raza A, Ponnusamy
A, Gorton J, Alachkar H, Choudhury J, Barratt J and Kalra PA:
Long-term therapeutic plasma exchange to prevent End-stage kidney
disease in adult severe resistant Henoch-Schonlein purpura
nephritis. Case Rep Nephrol. 2015(269895)2015.PubMed/NCBI View Article : Google Scholar
|