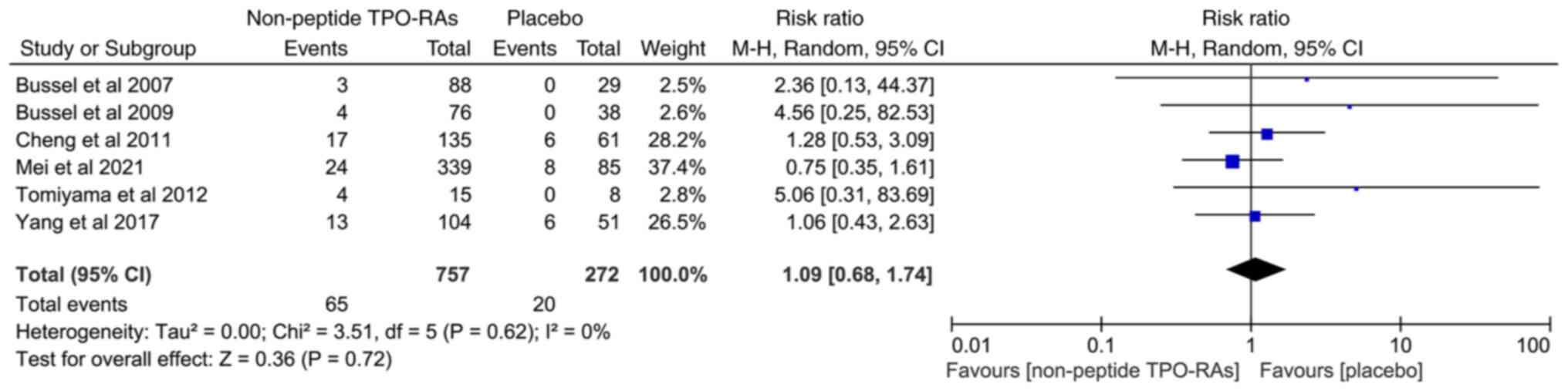
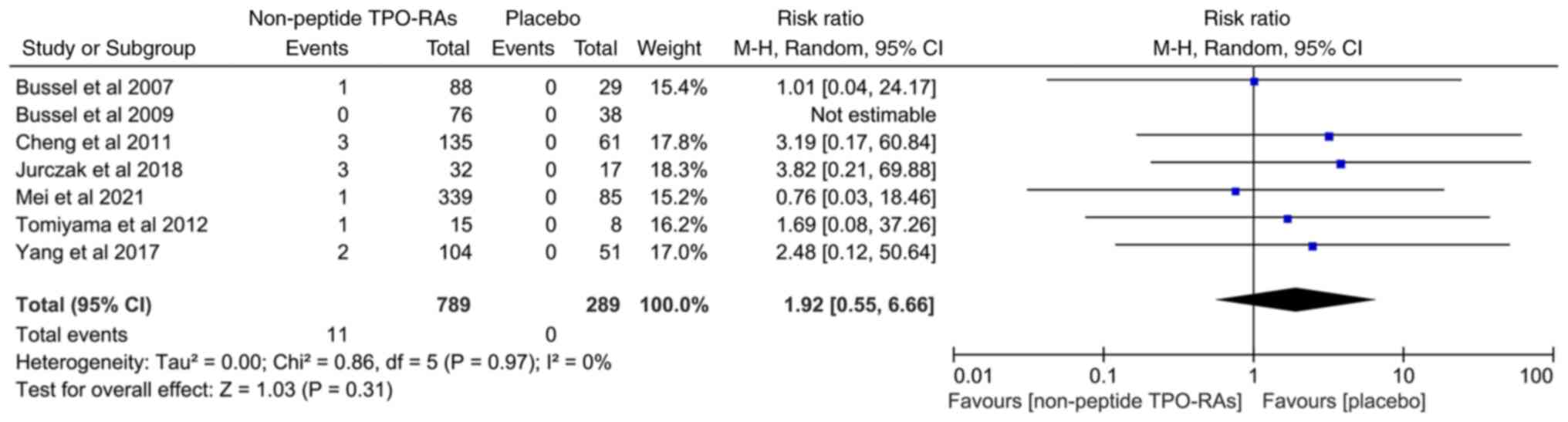
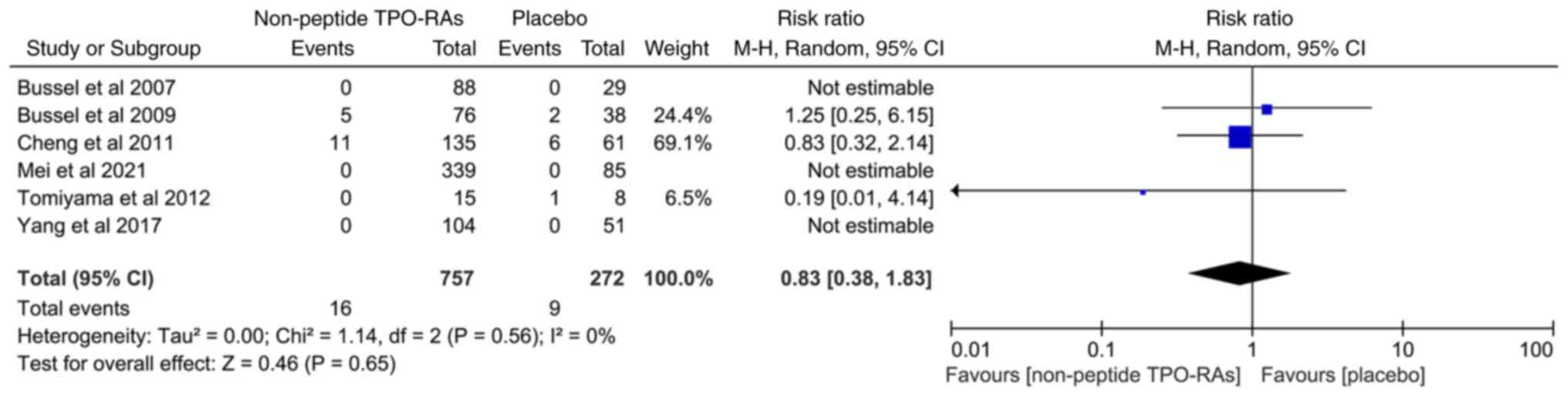
|
1
|
Neunert C, Terrell DR, Arnold DM, Buchanan
G, Cines DB, Cooper N, Cuker A, Despotovic JM, George JN, Grace RF,
et al: American society of hematology 2019 guidelines for immune
thrombocytopenia. Blood Adv. 3:3829–3866. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Palaniappan G and Jennings W: Idiopathic
thrombocytopenic purpura. Mo Med. 106:69–73. 2009.PubMed/NCBI
|
|
3
|
Ahn YS and Horstman LL: Idiopathic
thrombocytopenic purpura: Pathophysiology and management. Int J
Hematol. 76:123–131. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nugent D, McMillan R, Nichol JL and
Slichter SJ: Pathogenesis of chronic immune thrombocytopenia:
Increased platelet destruction and/or decreased platelet
production. Br J Haematol. 146:585–596. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cooper N, Kruse A, Kruse C, Watson S,
Morgan M, Provan D, Ghanima W, Arnold DM, Tomiyama Y, Santoro C, et
al: Immune thrombocytopenia (ITP) World Impact Survey (I-WISh):
Impact of ITP on health-related quality of life. Am J Hematol.
96:199–207. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sestøl HG, Trangbæk SM, Bussel JB and
Frederiksen H: Health-related quality of life in adult primary
immune thrombocytopenia. Expert Rev Hematol. 11:975–985.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mokhtar GM, Farid SM, Shaker NM and Farrag
KE: Health-related quality of life of Egyptian children with immune
thrombocytopenia and their parents. J Pediatr Hematol Oncol.
36:194–199. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun Y, Long S and Liu W: Risk factors and
psychological analysis of chronic immune thrombocytopenia in
children. Int J Gen Med. 13:1675–1683. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Towner S, Berger ZE, Titman P, New HV,
Theodore K, Brown G and Sibson KR: Fatigue, executive function and
psychological effects in children with immune thrombocytopenia: A
cross-sectional study. Br J Haematol. 189:534–542. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mareddy C, Kalra M and Sachdeva A: Generic
romiplostim for children with persistent or chronic immune
thrombocytopenia: Experience from a tertiary care centre in North
India. Br J Haematol. 197:618–626. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Maitland HS: Avatrombopag effectively
maintained platelet counts in a patient with immune
thrombocytopenia who was intolerant to tyrosine kinase inhibitor
therapy. Am J Case Rep. 22(e933788)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bohn JP and Steurer M: Current and
evolving treatment strategies in adult immune thrombocytopenia.
Memo. 11:241–246. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sandal R, Mishra K, Jandial A, Sahu KK and
Siddiqui AD: Update on diagnosis and treatment of immune
thrombocytopenia. Expert Rev Clin Pharmacol. 5:553–568.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Beltrami-Moreira M and Bussel JB: Low-dose
rituximab in immune thrombocytopenia: One and done. Am J Hematol.
97:388–389. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mishra K, Pramanik S, Sandal R, Jandial A,
Sahu KK, Singh K, Khera S, Meshram A, Khurana H, Somasundaram V, et
al: Safety and efficacy of azathioprine in immune thrombocytopenia.
Am J Blood Res. 11:217–226. 2021.PubMed/NCBI
|
|
16
|
Fresneau B, Petit A, Courcoux MF, Tabone
MD, Auvrignon A, Landman-Parker J and Leverger G: Vinblastine in
the treatment of children and adolescents with refractory immune
thrombocytopenia. Am J Hematol. 86:785–787. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Feng FE, Feng R, Wang M, Zhang JM, Jiang
H, Jiang Q, Lu J, Liu H, Peng J, Hou M, et al: Oral all-trans
retinoic acid plus danazol versus danazol as second-line treatment
in adults with primary immune thrombocytopenia: A multicentre,
randomised, open-label, phase 2 trial. Lancet Haematol.
4:e487–e496. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bussel J, Kulasekararaj A, Cooper N, Verma
A, Steidl U, Semple JW and Will B: Mechanisms and therapeutic
prospects of thrombopoietin receptor agonists. Semin Hematol.
56:262–278. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Basciano PA and Bussel JB:
Thrombopoietin-receptor agonists. Curr Opin Hematol. 19:392–398.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Neunert CE: Thrombopoietin receptor
agonist use for immune thrombocytopaenia. Hamostaseologie.
39:272–278. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cohn CS and Bussel JB: Romiplostim: A
second-generation thrombopoietin agonist. Drugs Today (Barc).
45:175–188. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Stasi R: Eltrombopag: The discovery of a
second generation thrombopoietin-receptor agonist. Expert Opin Drug
Discov. 4:85–93. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Philippart M, Schmidt J and Bittner B:
Oral delivery of therapeutic proteins and peptides: An overview of
current technologies and recommendations for bridging from approved
intravenous or subcutaneous administration to novel oral regimens.
Drug Res (Stuttg). 3:113–120. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dova Pharmaceuticals: DOPTELET prescribing
information, 2020. https://dova.com/wp-content/uploads/2019/06/doptelet-prescribing-information.pdf.
Accessed January, 2021.
|
|
25
|
Syed YY: Hetrombopag: First Approval.
Drugs. 81:1581–1585. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nguyen TT, Palmaro A, Montastruc F,
Lapeyre-Mestre M and Moulis G: Signal for thrombosis with
eltrombopag and romiplostim: A disproportionality analysis of
spontaneous reports within VigiBase®. Drug Saf.
38:1179–1186. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Teekaput C, Nadsasarn A, Tanprawate S,
Soontornpun A, Thiankhaw K, Wantaneeyawong C, Teekaput K and
Chai-Adisaksopha C: Cerebral venous sinus thrombosis in immune
thrombocytopenia patients treated with thrombopoietin receptor
agonist: Case reports and literature review. Ann Med Surg (Lond).
79(104116)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cuker A, Chiang EY and Cines DB: Safety of
the thrombopoiesis-stimulating agents for the treatment of immune
thrombocytopenia. Curr Drug Saf. 5:171–181. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim TO, Despotovic J and Lambert MP:
Eltrombopag for use in children with immune thrombocytopenia. Blood
Adv. 2:454–461. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang Y and Kolesar JM: Eltrombopag: An
oral thrombopoietin receptor agonist for the treatment of
idiopathic thrombocytopenic purpura. Clin Ther. 33:1560–1576.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med.
6(e1000097)2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Aertgeerts B and Cools F: The Cochrane
Collaboration and systematic literature reviews about the
efficiency of a treatment. Verh K Acad Geneeskd Belg. 69:335–350.
2007.PubMed/NCBI(In Dutch).
|
|
33
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
3:176–181. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sterne JAC, Savović J, Page MJ, Elbers RG,
Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge
SM, et al: RoB 2: A revised tool for assessing risk of bias in
randomised trials. BMJ. 366(l4898)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bussel JB, Cheng G, Saleh MN, Psaila B,
Kovaleva L, Meddeb B, Kloczko J, Hassani H, Mayer B, Stone NL, et
al: Eltrombopag for the treatment of chronic idiopathic
thrombocytopenic purpura. N Engl J Med. 357:2237–2247.
2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bussel JB, Provan D, Shamsi T, Cheng G,
Psaila B, Kovaleva L, Salama A, Jenkins JM, Roychowdhury D, Mayer
B, et al: Effect of eltrombopag on platelet counts and bleeding
during treatment of chronic idiopathic thrombocytopenic purpura: A
randomised, double-blind, placebo-controlled trial. Lancet.
373:641–648. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cheng G, Saleh MN, Marcher C, Vasey S,
Mayer B, Aivado M, Arning M, Stone NL and Bussel JB: Eltrombopag
for management of chronic immune thrombocytopenia (RAISE): A
6-month, randomised, phase 3 study. Lancet. 377:393–402.
2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tomiyama Y, Miyakawa Y, Okamoto S,
Katsutani S, Kimura A, Okoshi Y, Ninomiya H, Kosugi H, Nomura S,
Ozaki K, et al: A lower starting dose of eltrombopag is efficacious
in Japanese patients with previously treated chronic immune
thrombocytopenia. J Thromb Haemost. 10:799–806. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang R, Li J, Jin J, Huang M, Yu Z, Xu X,
Zhang X and Hou M: Multicentre, randomised phase III study of the
efficacy and safety of eltrombopag in Chinese patients with chronic
immune thrombocytopenia. Br J Haematol. 176:101–110.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jurczak W, Chojnowski K, Mayer J, Krawczyk
K, Jamieson BD, Tian W and Allen LF: Phase 3 randomised study of
avatrombopag, a novel thrombopoietin receptor agonist for the
treatment of chronic immune thrombocytopenia. Br J Haematol.
183:479–490. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mei H, Liu X, Li Y, Zhou H, Feng Y, Gao G,
Cheng P, Huang R, Yang L, Hu J, et al: A multicenter, randomized
phase III trial of hetrombopag: A novel thrombopoietin receptor
agonist for the treatment of immune thrombocytopenia. J Hematol
Oncol. 14(37)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Rodeghiero F: Is ITP a thrombophilic
disorder? Am J Hematol. 91:39–45. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wong RSM, Saleh MN, Khelif A, Salama A,
Portella MSO, Burgess P and Bussel JB: Safety and efficacy of
long-term treatment of chronic/persistent ITP with eltrombopag:
Final results of the EXTEND study. Blood. 130:2527–2536.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cuker A: Toxicities of the thrombopoietic
growth factors. Semin Hematol. 3:289–298. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Promacta prescribing information.
http://www.promactacares.com/prescribing_information.pdf.
Accessed January 14, 2009.
|
|
46
|
Cuker A, Chiang EY and Cines DB: Safety of
the thrombopoiesis-stimulating agents for the treatment of immune
thrombocytopenia. Curr Drug Saf. 2:171–181. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
James ER: The etiology of steroid
cataract. J Ocul Pharmacol Ther. 23:403–420. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Edwards IR: Adverse drug effects and their
clinical management: A personal view. Drug Saf. 6:383–390.
2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wang L, Gao Z, Chen XP, Zhang HY, Yang N,
Wang FY, Guan LX, Gu ZY, Zhao SS, Luo L, et al: Efficacy and safety
of thrombopoietin receptor agonists in patients with primary immune
thrombocytopenia: A systematic review and meta-analysis. Sci Rep.
6(39003)2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Deng J, Hu H, Huang F, Huang C, Huang Q,
Wang L, Wu A, Yang J, Qin D, Zou W and Wu J: Comparative efficacy
and safety of thrombopoietin receptor agonists in adults with
thrombocytopenia: A systematic review and network meta-analysis of
randomized controlled trial. Front Pharmacol.
12(704093)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Li T, Liu Q, Pu T, Liu J and Zhang A:
Efficacy and safety of thrombopoietin receptor agonists in children
and adults with persistent and chronic immune thrombocytopenia: A
meta-analysis. Expert Opin Pharmacother. 6:763–774. 2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Audia S, Mahévas M, Samson M, Godeau B and
Bonnotte B: . Pathogenesis of immune thrombocytopenia. Autoimmun
Rev. 6:620–632. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Fan J and de Lannoy IA: Pharmacokinetics.
Biochem Pharmacol. 1:93–120. 2014.PubMed/NCBI View Article : Google Scholar
|