Introduction
Immune thrombocytopenia (ITP) is a common
hemorrhagic disease (1-3).
The main pathogenesis of this disease stems from autoantibodies
mediated by T cells and B cells that specifically adhere to the
platelet and megakaryocyte membranes in the bone marrow, leading to
increased platelet destruction and megakaryocyte maturation
disorder (4). A platelet count
<100x109/l in peripheral blood and skin purpura are
the most characteristic manifestations of the disease and are the
basis for an ITP diagnosis (1,3). The
bleeding symptoms of ITP are typically mild and not fatal. However,
potential bleeding events seriously affect the quality of life and
psychological status of patients (5-9).
Generally, the purpose of ITP treatment is to
maintain a relatively safe platelet count
(>50x109/l), which can reduce the risk of severe
bleeding (10,11). Glucocorticoids and intravenous
immunoglobulins are the first-line drugs for ITP treatment since
their initial treatment effectiveness is 60-80% (12). However, only ~30% of patients
experience a sustained response (13). Due to the side effects of the
long-term use of glucocorticoids, such as osteoporosis, infections
and emotional disorders, second-line drugs have become a necessary
choice for certain patients (13-17).
In recent years, second-line drugs, thrombopoietin receptor
agonists (TPO-RAs), have been used in ITP treatment (1,10,11).
TPO-RAs can simulate the binding of natural
thrombopoietin to receptors on the surface of megakaryocytes and
bone marrow hematopoietic stem cells, specifically promoting the
differentiation and proliferation of megakaryocytes, thereby
increasing platelet production (18-20).
TPO-RAs can be mainly divided into two categories: Peptide TPO-RAs
(subcutaneous injection) and non-peptide TPO-RAs (oral
administration), typically administered as romiplostim and
eltrombopag, respectively (1,21,22).
Compared with subcutaneous injection, the oral dosage form
significantly improves the continuity of treatment for patients
with ITP (23).
Avatrombopag and hetrombopag are other non-peptide
TPO-RAs used to treat ITP, which were approved by the USA in 2020
and China in 2021, respectively (24,25).
With the gradual increased use of non-peptide TPO-RAs in clinical
treatment, controversial adverse events (AEs) such as thrombosis,
cataracts and aminotransferase abnormalities have become the focus
for clinicians (26-30).
Therefore, the present study aimed to update and summarize the AEs
of non-peptide TPO-RAs (including eltrombopag, avatrombopag and
hetrombopag), compared with placebo, from previous studies to
provide a theoretical basis for monitoring clinical AEs.
Materials and methods
Literature search
This analysis was completed according to the PRISMA
guidelines (31) and the Cochrane
Handbook (32). Information from
PubMed (www.pubmed.gov), Web of Science
(www.webofscience.com) and Embase
(www.embase.com) was retrieved using a computer by
combining mesh terms and near-synonyms. For example, the key word
searches for the PubMed database were: (Idiopathic thrombocytopenic
purpura*) OR (purpura*, idiopathic thrombocytopenic) OR
(thrombocytopenic purpura*, idiopathic) OR (immune thrombocytopenic
purpura*) OR (purpuras, immune thrombocytopenic) OR
(thrombocytopenic purpura*, immune) OR (immune thrombocytopenia*)
OR (thrombocytopenia*, immune) OR (thrombocytopenic purpura,
autoimmune) OR (autoimmune thrombocytopenia*) OR
(thrombocytopenia*, autoimmune) OR (autoimmune thrombocytopenic
purpura*) OR (purpura*, autoimmune thrombocytopenic) OR (purpura,
thrombocytopenic, autoimmune) AND (thrombopoietin receptor
agonist*) OR (eltrombopag) OR (avatrombopag) OR (hetrombopag) OR
(TPO-RA*). The key words used to search Web of Science and Embase
were similar. The references of all the articles included in each
study were also searched. If the original data in trials were
incomplete or missing, the author was contacted via email to
supplement the missing data. Filters were not used for any database
retrieval. The last day of literature search was November 5th,
2022.
Eligibility criteria
The inclusion criteria to exclude confounders were:
i) The study was a randomized double-blind clinical trial with a
placebo as the control; ii) all patients who received non-peptide
TPO-RAs were adults (aged >18 years old) with ITP; iii) the
duration of the double-blind study was at least 6 weeks, and the
AEs data during this period could be extracted; and iv) the study
was a multicenter trial, regardless of ethnicity or region.
Exclusion criteria: i) Literature with duplicate publications of
the same data; ii) articles were not published or it was not
possible to obtain the full text.
Data extraction
Two researchers (YJ and ML) assessed all titles
and/or abstracts of the retrieved literature to exclude articles
that did not meet the inclusion criteria. The selected literature
was then imported into EndNote software to delete duplicates. The
full text of the selected literature was then reviewed.
Data were extracted using standardized data
collection tables. The information extracted from each study
included the first author, publication year, clinical trial design,
duration of the double-blind study, study population, clinical
classification of ITP, name and dosage of the drug and number and
type of AEs. AEs were graded according to the National Cancer
Institute Common Terminology Criteria for AEs (version 3.0)
(33). If the clinical trials
included a double-blind and open-label extension phase, only data
from the double-blind period were collected. All research data
included in the present study were obtained from the previous
literature. Therefore, approval from an ethics committee and
informed consent of the participants were not required.
Quality assessment
Bias risk assessment was conducted according to Risk
of Bias (RoB) 2.0, a revised tool for assessing risk of bias in
randomised trials developed by the Cochrane Collaboration (34). The evaluation content includes
random sequence generation, allocation concealment, blinding of
participants and personnel, blinding of outcome assessment,
incomplete outcome data, selective reporting and other bias. All
seven items evaluated as low RoB led to an overall rating of ‘low
risk’, >1 item evaluated as high RoB led to an overall rating of
‘high risk’ and the remaining studies were rated as having ‘unclear
risk’. In case of any discrepancy in the quality evaluation, the
team discussed it collectively or negotiated with a third
investigator (HY).
Statistical analysis
RevMan 5.4.1 software (The Cochrane Collaboration)
was selected to analyze the data of all included studies. The
heterogeneity of the collected studies was tested using the
chi-square test and I2 test. Regardless of the P-value
and I²-value, the random-effects model was chosen. The relative
risk (RR) index was used to evaluate the strength of the
association between non-peptide TPO-RAs and AEs. P<0.05 was
considered to indicate a statistically significant difference.
Results
Study selection
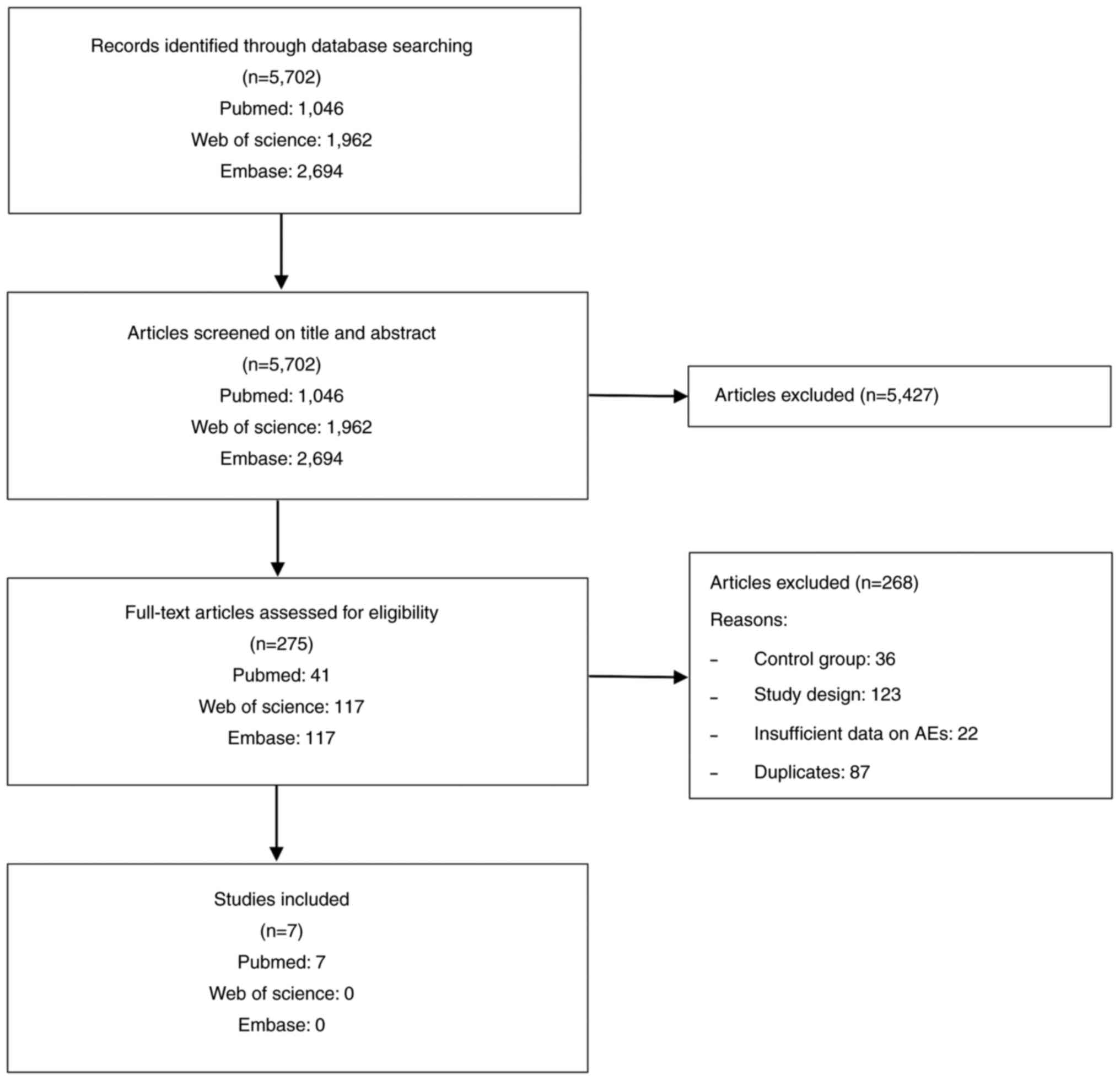
From the screening process, 5,702 records were
initially obtained from the databases. A total of 5,427 records
were removed after assessing the titles and/or abstracts. After
reading the entire text, 268 articles that were repetitive or did
not conform to the inclusion criteria were removed. Finally, seven
articles (35-41)
were used for the present meta-analysis, with a publication period
of 2007 to 2021. The screening process is illustrated in Fig. 1.
Study characteristics
Of the seven included studies, five were Phase III
clinical trials that were all multicenter studies (36,37,39-41).
However, two of these trials were conducted in a single country
(39,41). A total of four studies added an
open-label stage after the double-blind period (38-41).
However, data from the added period were not collected. In total,
three non-peptide TPO-RAs were selected as interventions:
Eltrombopag was used in five studies, avatrombopag in one study and
hetrombopag in one study. A total of 1,078 adult patients with ITP
were enrolled, including 789 and 289 patients in the intervention
and placebo groups, respectively. All enrolled patients had
persistent or chronic ITP. The number of participants in the
selected studies ranged from 23 to 424. All studies used a placebo
as a control. The duration of double-blinding was between 6 weeks
and 6 months. Thrombosis was the only AE not observed in the
placebo group. Table I provides
further details on study characteristics.
 | Table IStudy characteristics. |
Table I
Study characteristics.
| | Intervention | Control | Intervention (n) /
control (n) | |
|---|
| First author,
year | Type of ITP
(n) | Country | Study duration | Drug | Dose per day,
mg | Number of
patients | Number of
patients | Any AEs | Grade 3/4 AEs | Elevated
transaminase levels | Thrombosis | Cataracts | (Refs.) |
|---|
| Bussel et
al, 2007 | Persistent
(117) | Multiple | 6 weeks | Eltrombopag | 30, 50 or 75 | 29 | 88 | 45/17 | 9/4 | 3/0 | 1/0 | 0/0 | (35) |
| Bussel et
al, 2009 | Persistent
(114) | Multiple | 6 weeks | Eltrombopag | 50 | 38 | 76 | 45/14 | 2/1 | 4/0 | 0/0 | 5/2 | (36) |
| Cheng et al,
2011 | Persistent
(196) | Multiple | 6 months | Eltrombopag | 50 | 61 | 135 | N | 20/7 | 17/6 | 3/0 | 11/6 | (37) |
| Tomiyama et
al, 2012 | Persistent
(23) | Japan | 6 weeks | Eltrombopag | 12.5-50 | 8 | 15 | 11/2 | 1/0 | 4/0 | 1/0 | 0/1 | (38) |
| Yang et al,
2017 | Chronic (155) | China | 8 weeks | Eltrombopag | 25-75 | 51 | 104 | 66/34 | 8/5 | 13/6 | 2/0 | 0/0 | (39) |
| Jurczak et
al, 2018 | Chronic (49) | Multiple | 6 months | Avatrombopag | 20 | 17 | 32 | 31/10 | 6/0 | N | 3/0 | N | (40) |
| Mei et al,
2021 | Persistent
(424) | China | 10 weeks | Hetrombopag | 2.5 or 5 | 85 | 339 | 316/81 | N | 24/8 | 1/0 | 0/0 | (41) |
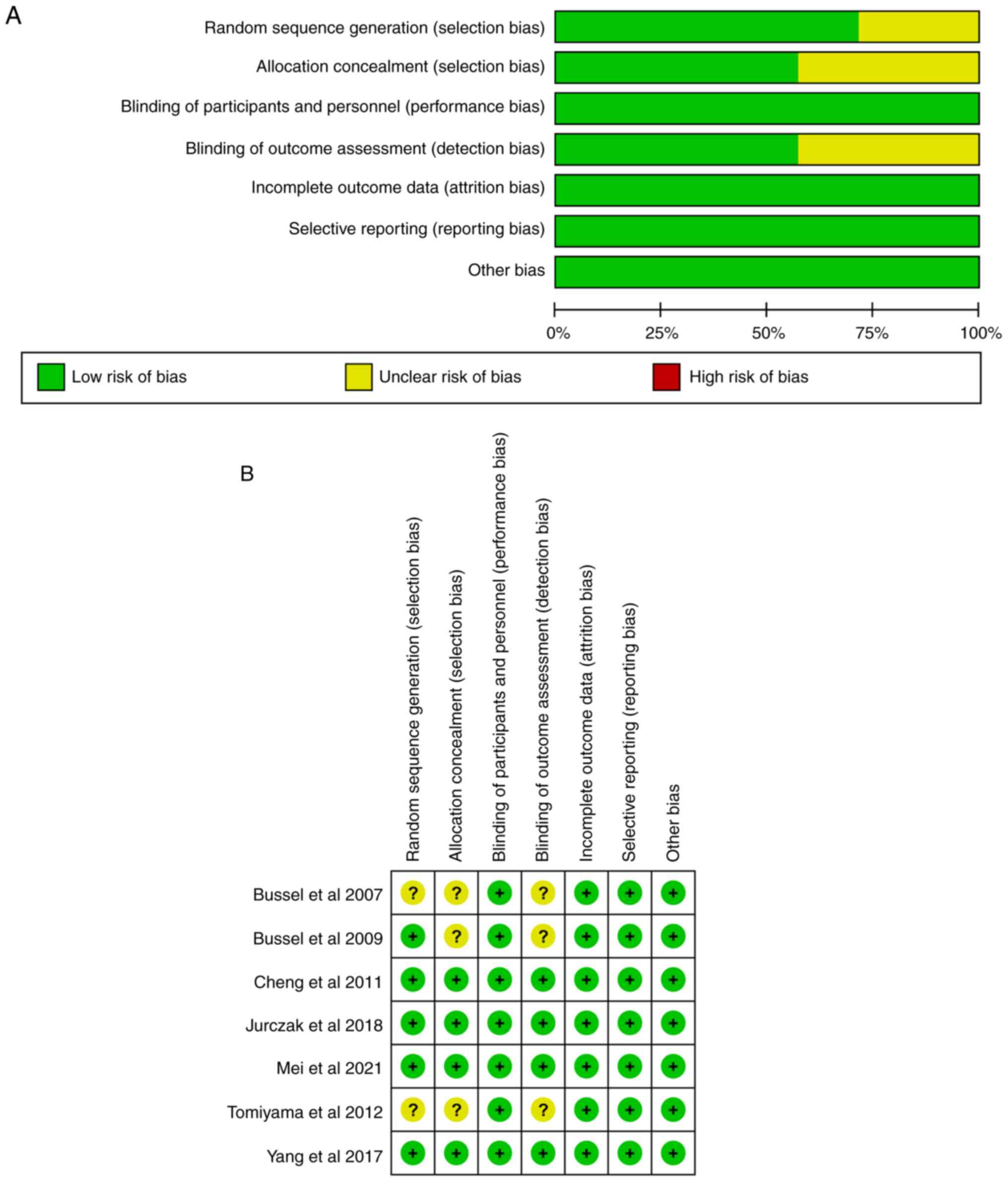
Quality assessment
Cochrane Collaboration RoB 2.0 was used to assess
the RoB in all selected studies. The evaluation revealed that none
of the seven randomized controlled trials had a high RoB. ‘Unclear’
RoB occurred only in selection and detection biases. The RoB graph
and summary are illustrated in Fig.
2A and B, respectively.
Incidence of any AEs and grade 3/4
AEs
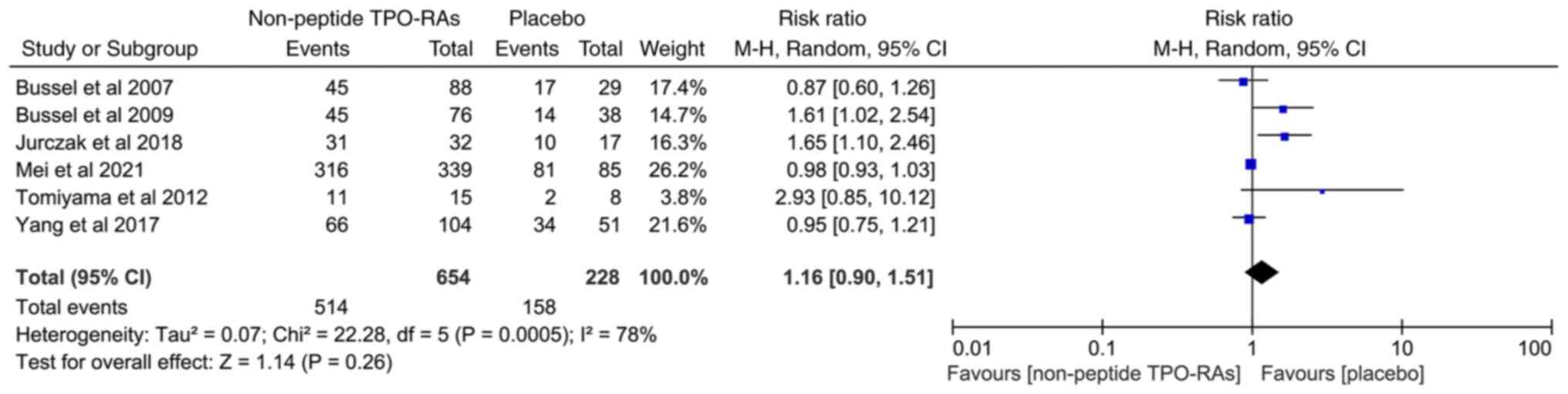
A total of six studies involving 882 patients with
ITP were included to compare the incidence of any AE between both
the non-peptide TPO-RA treated and placebo groups. The results
revealed no significant difference in the incidence of any AEs
between the two groups (RR=1.16; 95% CI, 0.90-1.51;
I2=78%; P=0.26; Fig.
3). Due to high heterogeneity, this result must be interpreted
carefully. There was also no significant difference in the
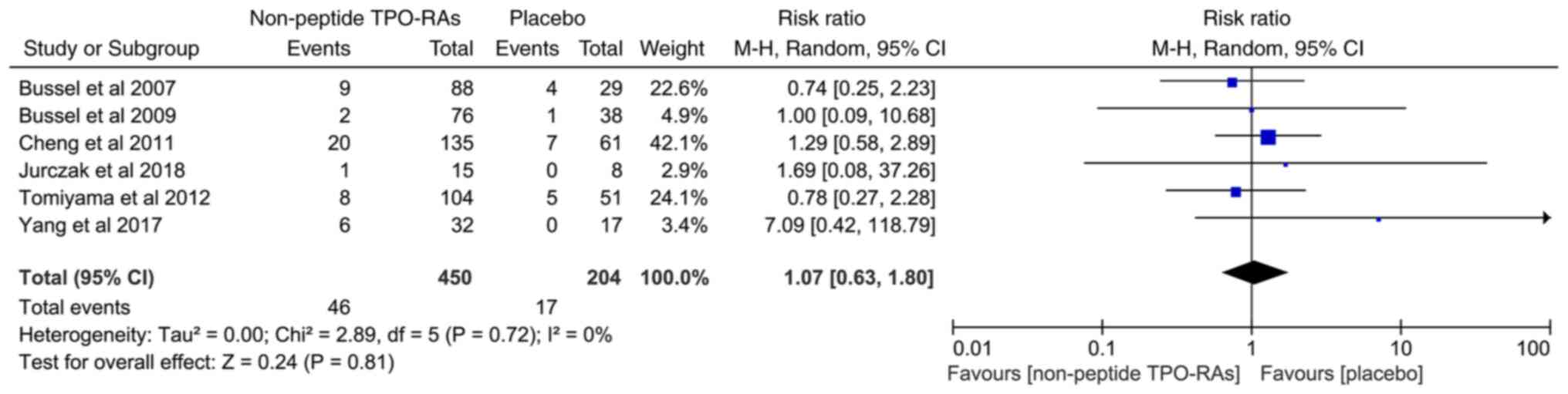
incidence of grade 3/4 AEs between both groups (RR=1.07; 95% CI,
0.63-1.80; I2=0%; P=0.81; Fig. 4).
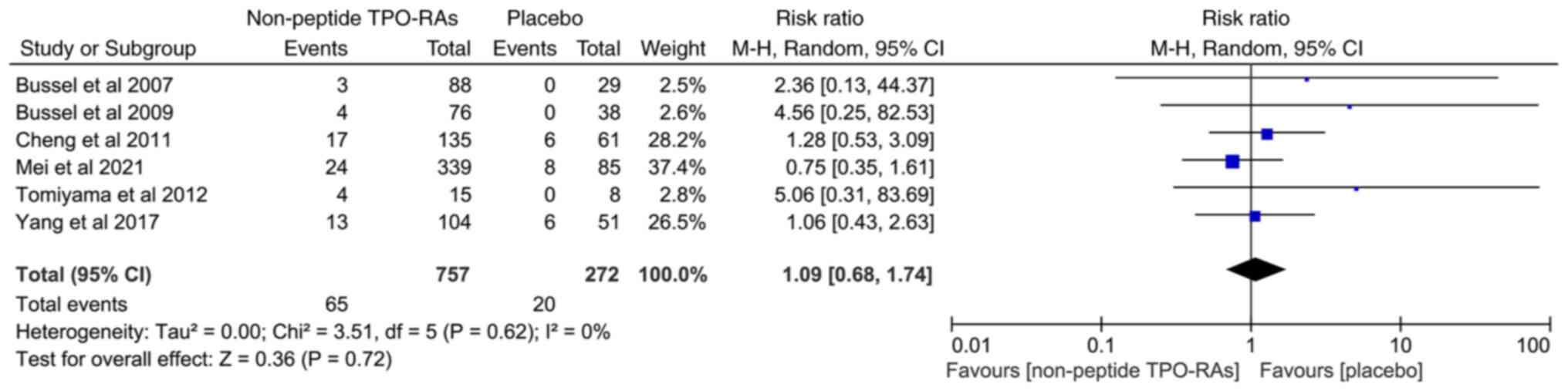
Incidence of elevated transaminase
levels/hepatotoxicity
Transaminases include alanine aminotransferase and
aspartate aminotransferase. The summary results based on six
studies revealed no significant difference in the incidence of
elevated transaminase levels between the non-peptide TPO-RA treated
and placebo groups (RR=1.09; 95% CI, 0.68-1.74; I2=0%;
P=0.72; Fig. 5).
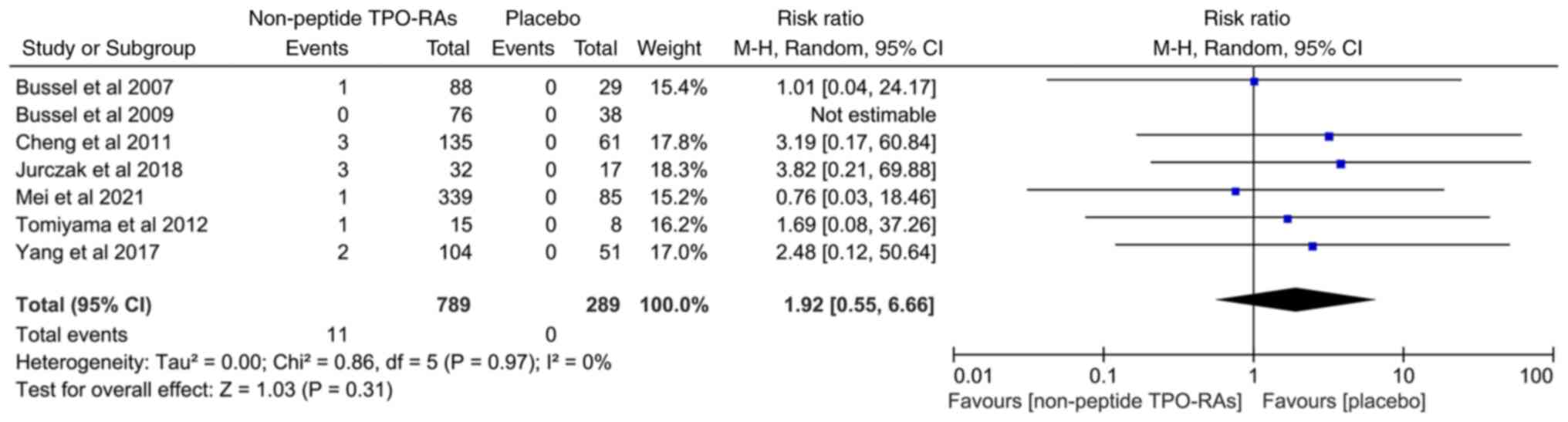
Incidence of thrombosis and
cataracts
Thrombotic events were fully described in all the
included studies. The incidence of thrombotic events was 1.39 and
0% in the non-peptide TPO-RA treated and placebo groups,
respectively. From the RR value, the incidence of thrombosis in the
non-peptide TPO-RA treated group was 1.92 times higher than that in
the placebo group (RR=1.92; 95% CI, 0.55-6.66; I2=0%;
P=0.31; Fig. 6). However, the
P-value revealed no statistically significant difference.
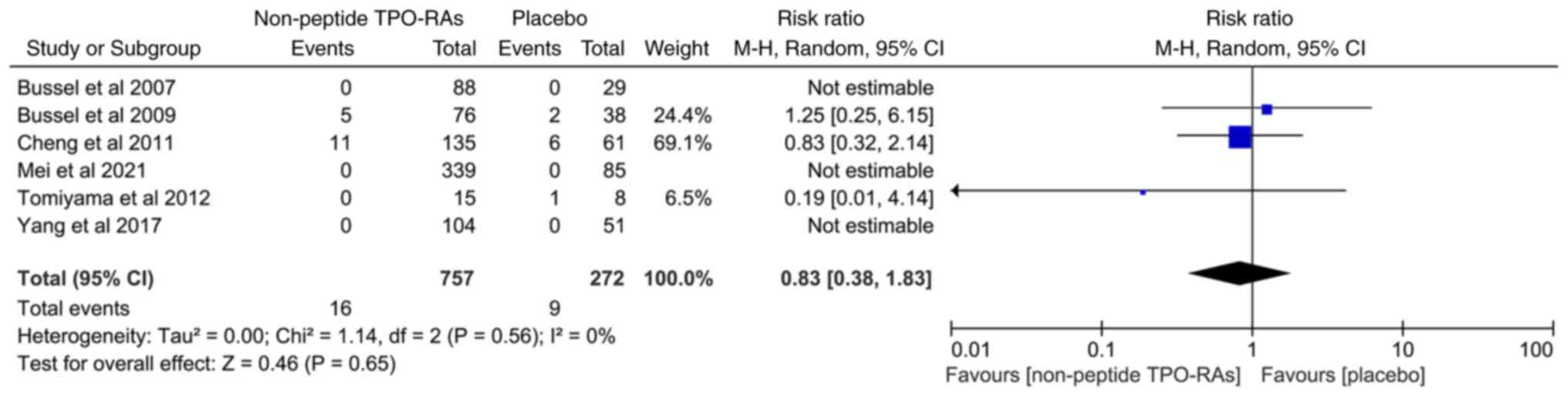
The data on cataracts (new or aggravated) were
available in six articles. Its incidence rate was 2.11% in the
non-peptide TPO-RAs group and 3.30% in the placebo group. However,
there was no significant difference between the two groups
(RR=0.83; 95% CI, 0.38-1.83; I2=0%; P=0.65; Fig. 7).
Discussion
In the present study, the safety of non-peptide
TPO-RAs and placebo in patients with ITP was compared. The results
revealed no significant differences in the incidence of any AEs,
grade 3/4 AEs, elevated transaminase levels, thrombosis or
cataracts between the two study groups. Thus, it is concluded that
it is relatively safe to use non-peptide TPO-RAs to treat ITP
within at least 6 months of treatment.
Thrombosis is one of the most damaging effects of
ITP treatment with TPO-RAs (27).
TPO-RAs increase the risk of thrombosis by increasing platelet
count and stimulating the production of young and more hemostatic
platelets (42). Thrombosis was
the only event in the present meta-analysis that occurred in the
intervention group but not in the placebo group, with an incidence
of 1.39%. As this study only collected thrombosis events during the
double-blind clinical trial period, the incidence rate may have
been underestimated. A large phase III RCT on the safety of
eltrombopag reported a thrombosis incidence of 2% (37). However, in an expanded study, the
median treatment time for eltrombopag was 2.37 years and the
incidence of thrombosis reached 6% (43). This indicates that treatment
duration may be one of the main factors affecting the incidence of
thrombosis.
It was discovered in preclinical animal model
experiments that TPO-RAs may cause cataracts in rodents (44-46).
Thus, eye examinations have become screening criteria for patients
using TRO-RAs (35). In the
present study, the incidence rates of cataracts in the non-peptide
TPO-RA and placebo groups were 2.11 and 3.30%, respectively.
TPO-RAs are second-line drugs for ITP (1). They are recommended only when
glucocorticoid drugs are ineffective, as observed in all the
included studies. Therefore, all patients with cataracts had used
glucocorticoids in the past, which is an important risk factor for
cataract formation (47). Thus,
whether non-peptide TPO-RAs can cause cataracts in ITP patients
requires further clarification.
Any AEs, grade 3/4 AEs and elevated transaminase
levels are side effects of most drugs (48). The results revealed that, compared
with placebo, non-peptide TPO-RAs did not increase the total number
of AEs, serious AEs or elevated transaminase levels, which is
consistent with previous meta-analyses on TPO-RAs (49-51).
However, unlike in the past, this study is the meta-analysis on the
safety of non-peptide TPO-RAs, and we have demonstrated that it is
relatively safe as a second-line drug for the treatment of ITP.
The mechanism of ITP varies between children and
adults, and the self-reported symptoms of adverse reactions in
children may be inacurrate (52).
Therefore, age is an important factor affecting drug-related AEs.
In addition, the route of administration generally affects the
absorption rate and metabolism of drugs, so it may be also related
to AEs (53). The present study
only included adult patients treated with oral TPO-RAs for ITP,
effectively avoiding the impact of age and medication route in the
results. Moreover, clinical data were from randomized double-blind
placebo-controlled clinical trials, which further enhanced the
reliability of the results.
The present study does however have some
limitations. Firstly, the treatment period of ITP with non-peptide
TPO-RAs in all clinical trials included was ≤6 months. Thus, it was
impossible to analyze the occurrence of long-term AEs. Moreover,
time has a significant effect on the incidence of AEs. Secondly,
the present analysis only included adults as study participants
thus, the results may not be applicable to children. Finally, a
strict inclusion standard was set to improve the accuracy of the
results, which reduced the study sample size.
In conclusion, the safety of non-peptide TPO-RAs for
ITP treatment was evaluated and it was discovered that the
incidence of any AEs, grade 3/4 AEs, elevated transaminase levels,
thrombosis and cataracts were not statistically different from
those in the placebo group. These results indicate that non-peptide
TPO-RAs are relatively safe for patients with ITP, within at least
6 months of treatment.
Acknowledgements
The authors would like to thank Dr Youqing Shen and
Dr Jing Zhang (Department of Pediatrics, Suqian Hospital Affiliated
to Xuzhou Medical University) for their assistance during the
writing period.
Funding
Funding: This study was supported by the Jiangsu Province
Maternal and Child Health Research Project (grant nos. F201941 and
F202153) and Suqian Science and Technology Plan Project (grant nos.
Z2019154 and K202002).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SZ and JL designed the study. YJ, ML, HY and JQ
collected the data. NS, JQ and SZ performed the data analysis and
wrote the manuscript. All authors have read and approved the final
manuscript. NS and SZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neunert C, Terrell DR, Arnold DM, Buchanan
G, Cines DB, Cooper N, Cuker A, Despotovic JM, George JN, Grace RF,
et al: American society of hematology 2019 guidelines for immune
thrombocytopenia. Blood Adv. 3:3829–3866. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Palaniappan G and Jennings W: Idiopathic
thrombocytopenic purpura. Mo Med. 106:69–73. 2009.PubMed/NCBI
|
|
3
|
Ahn YS and Horstman LL: Idiopathic
thrombocytopenic purpura: Pathophysiology and management. Int J
Hematol. 76:123–131. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nugent D, McMillan R, Nichol JL and
Slichter SJ: Pathogenesis of chronic immune thrombocytopenia:
Increased platelet destruction and/or decreased platelet
production. Br J Haematol. 146:585–596. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cooper N, Kruse A, Kruse C, Watson S,
Morgan M, Provan D, Ghanima W, Arnold DM, Tomiyama Y, Santoro C, et
al: Immune thrombocytopenia (ITP) World Impact Survey (I-WISh):
Impact of ITP on health-related quality of life. Am J Hematol.
96:199–207. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sestøl HG, Trangbæk SM, Bussel JB and
Frederiksen H: Health-related quality of life in adult primary
immune thrombocytopenia. Expert Rev Hematol. 11:975–985.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mokhtar GM, Farid SM, Shaker NM and Farrag
KE: Health-related quality of life of Egyptian children with immune
thrombocytopenia and their parents. J Pediatr Hematol Oncol.
36:194–199. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun Y, Long S and Liu W: Risk factors and
psychological analysis of chronic immune thrombocytopenia in
children. Int J Gen Med. 13:1675–1683. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Towner S, Berger ZE, Titman P, New HV,
Theodore K, Brown G and Sibson KR: Fatigue, executive function and
psychological effects in children with immune thrombocytopenia: A
cross-sectional study. Br J Haematol. 189:534–542. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mareddy C, Kalra M and Sachdeva A: Generic
romiplostim for children with persistent or chronic immune
thrombocytopenia: Experience from a tertiary care centre in North
India. Br J Haematol. 197:618–626. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Maitland HS: Avatrombopag effectively
maintained platelet counts in a patient with immune
thrombocytopenia who was intolerant to tyrosine kinase inhibitor
therapy. Am J Case Rep. 22(e933788)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bohn JP and Steurer M: Current and
evolving treatment strategies in adult immune thrombocytopenia.
Memo. 11:241–246. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sandal R, Mishra K, Jandial A, Sahu KK and
Siddiqui AD: Update on diagnosis and treatment of immune
thrombocytopenia. Expert Rev Clin Pharmacol. 5:553–568.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Beltrami-Moreira M and Bussel JB: Low-dose
rituximab in immune thrombocytopenia: One and done. Am J Hematol.
97:388–389. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mishra K, Pramanik S, Sandal R, Jandial A,
Sahu KK, Singh K, Khera S, Meshram A, Khurana H, Somasundaram V, et
al: Safety and efficacy of azathioprine in immune thrombocytopenia.
Am J Blood Res. 11:217–226. 2021.PubMed/NCBI
|
|
16
|
Fresneau B, Petit A, Courcoux MF, Tabone
MD, Auvrignon A, Landman-Parker J and Leverger G: Vinblastine in
the treatment of children and adolescents with refractory immune
thrombocytopenia. Am J Hematol. 86:785–787. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Feng FE, Feng R, Wang M, Zhang JM, Jiang
H, Jiang Q, Lu J, Liu H, Peng J, Hou M, et al: Oral all-trans
retinoic acid plus danazol versus danazol as second-line treatment
in adults with primary immune thrombocytopenia: A multicentre,
randomised, open-label, phase 2 trial. Lancet Haematol.
4:e487–e496. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bussel J, Kulasekararaj A, Cooper N, Verma
A, Steidl U, Semple JW and Will B: Mechanisms and therapeutic
prospects of thrombopoietin receptor agonists. Semin Hematol.
56:262–278. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Basciano PA and Bussel JB:
Thrombopoietin-receptor agonists. Curr Opin Hematol. 19:392–398.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Neunert CE: Thrombopoietin receptor
agonist use for immune thrombocytopaenia. Hamostaseologie.
39:272–278. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cohn CS and Bussel JB: Romiplostim: A
second-generation thrombopoietin agonist. Drugs Today (Barc).
45:175–188. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Stasi R: Eltrombopag: The discovery of a
second generation thrombopoietin-receptor agonist. Expert Opin Drug
Discov. 4:85–93. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Philippart M, Schmidt J and Bittner B:
Oral delivery of therapeutic proteins and peptides: An overview of
current technologies and recommendations for bridging from approved
intravenous or subcutaneous administration to novel oral regimens.
Drug Res (Stuttg). 3:113–120. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dova Pharmaceuticals: DOPTELET prescribing
information, 2020. https://dova.com/wp-content/uploads/2019/06/doptelet-prescribing-information.pdf.
Accessed January, 2021.
|
|
25
|
Syed YY: Hetrombopag: First Approval.
Drugs. 81:1581–1585. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nguyen TT, Palmaro A, Montastruc F,
Lapeyre-Mestre M and Moulis G: Signal for thrombosis with
eltrombopag and romiplostim: A disproportionality analysis of
spontaneous reports within VigiBase®. Drug Saf.
38:1179–1186. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Teekaput C, Nadsasarn A, Tanprawate S,
Soontornpun A, Thiankhaw K, Wantaneeyawong C, Teekaput K and
Chai-Adisaksopha C: Cerebral venous sinus thrombosis in immune
thrombocytopenia patients treated with thrombopoietin receptor
agonist: Case reports and literature review. Ann Med Surg (Lond).
79(104116)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cuker A, Chiang EY and Cines DB: Safety of
the thrombopoiesis-stimulating agents for the treatment of immune
thrombocytopenia. Curr Drug Saf. 5:171–181. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim TO, Despotovic J and Lambert MP:
Eltrombopag for use in children with immune thrombocytopenia. Blood
Adv. 2:454–461. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang Y and Kolesar JM: Eltrombopag: An
oral thrombopoietin receptor agonist for the treatment of
idiopathic thrombocytopenic purpura. Clin Ther. 33:1560–1576.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med.
6(e1000097)2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Aertgeerts B and Cools F: The Cochrane
Collaboration and systematic literature reviews about the
efficiency of a treatment. Verh K Acad Geneeskd Belg. 69:335–350.
2007.PubMed/NCBI(In Dutch).
|
|
33
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
3:176–181. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sterne JAC, Savović J, Page MJ, Elbers RG,
Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge
SM, et al: RoB 2: A revised tool for assessing risk of bias in
randomised trials. BMJ. 366(l4898)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bussel JB, Cheng G, Saleh MN, Psaila B,
Kovaleva L, Meddeb B, Kloczko J, Hassani H, Mayer B, Stone NL, et
al: Eltrombopag for the treatment of chronic idiopathic
thrombocytopenic purpura. N Engl J Med. 357:2237–2247.
2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bussel JB, Provan D, Shamsi T, Cheng G,
Psaila B, Kovaleva L, Salama A, Jenkins JM, Roychowdhury D, Mayer
B, et al: Effect of eltrombopag on platelet counts and bleeding
during treatment of chronic idiopathic thrombocytopenic purpura: A
randomised, double-blind, placebo-controlled trial. Lancet.
373:641–648. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cheng G, Saleh MN, Marcher C, Vasey S,
Mayer B, Aivado M, Arning M, Stone NL and Bussel JB: Eltrombopag
for management of chronic immune thrombocytopenia (RAISE): A
6-month, randomised, phase 3 study. Lancet. 377:393–402.
2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tomiyama Y, Miyakawa Y, Okamoto S,
Katsutani S, Kimura A, Okoshi Y, Ninomiya H, Kosugi H, Nomura S,
Ozaki K, et al: A lower starting dose of eltrombopag is efficacious
in Japanese patients with previously treated chronic immune
thrombocytopenia. J Thromb Haemost. 10:799–806. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang R, Li J, Jin J, Huang M, Yu Z, Xu X,
Zhang X and Hou M: Multicentre, randomised phase III study of the
efficacy and safety of eltrombopag in Chinese patients with chronic
immune thrombocytopenia. Br J Haematol. 176:101–110.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jurczak W, Chojnowski K, Mayer J, Krawczyk
K, Jamieson BD, Tian W and Allen LF: Phase 3 randomised study of
avatrombopag, a novel thrombopoietin receptor agonist for the
treatment of chronic immune thrombocytopenia. Br J Haematol.
183:479–490. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mei H, Liu X, Li Y, Zhou H, Feng Y, Gao G,
Cheng P, Huang R, Yang L, Hu J, et al: A multicenter, randomized
phase III trial of hetrombopag: A novel thrombopoietin receptor
agonist for the treatment of immune thrombocytopenia. J Hematol
Oncol. 14(37)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Rodeghiero F: Is ITP a thrombophilic
disorder? Am J Hematol. 91:39–45. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wong RSM, Saleh MN, Khelif A, Salama A,
Portella MSO, Burgess P and Bussel JB: Safety and efficacy of
long-term treatment of chronic/persistent ITP with eltrombopag:
Final results of the EXTEND study. Blood. 130:2527–2536.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cuker A: Toxicities of the thrombopoietic
growth factors. Semin Hematol. 3:289–298. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Promacta prescribing information.
http://www.promactacares.com/prescribing_information.pdf.
Accessed January 14, 2009.
|
|
46
|
Cuker A, Chiang EY and Cines DB: Safety of
the thrombopoiesis-stimulating agents for the treatment of immune
thrombocytopenia. Curr Drug Saf. 2:171–181. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
James ER: The etiology of steroid
cataract. J Ocul Pharmacol Ther. 23:403–420. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Edwards IR: Adverse drug effects and their
clinical management: A personal view. Drug Saf. 6:383–390.
2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wang L, Gao Z, Chen XP, Zhang HY, Yang N,
Wang FY, Guan LX, Gu ZY, Zhao SS, Luo L, et al: Efficacy and safety
of thrombopoietin receptor agonists in patients with primary immune
thrombocytopenia: A systematic review and meta-analysis. Sci Rep.
6(39003)2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Deng J, Hu H, Huang F, Huang C, Huang Q,
Wang L, Wu A, Yang J, Qin D, Zou W and Wu J: Comparative efficacy
and safety of thrombopoietin receptor agonists in adults with
thrombocytopenia: A systematic review and network meta-analysis of
randomized controlled trial. Front Pharmacol.
12(704093)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Li T, Liu Q, Pu T, Liu J and Zhang A:
Efficacy and safety of thrombopoietin receptor agonists in children
and adults with persistent and chronic immune thrombocytopenia: A
meta-analysis. Expert Opin Pharmacother. 6:763–774. 2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Audia S, Mahévas M, Samson M, Godeau B and
Bonnotte B: . Pathogenesis of immune thrombocytopenia. Autoimmun
Rev. 6:620–632. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Fan J and de Lannoy IA: Pharmacokinetics.
Biochem Pharmacol. 1:93–120. 2014.PubMed/NCBI View Article : Google Scholar
|