Introduction
Gouty arthritis (GA) is a common condition that is
induced by the precipitation and deposition of uric acid in
articular structures (1). The most
critical risk factor for the development of GA is a high serum
level of urate (2). The disorder
involves severe pain, swelling, warmth and redness at the gouty
joint (3). To prevent GA flares,
urate deposits must be eliminated by maintaining a low serum level
below the saturation point of 6.0 mg/dl or less (4).
Numerous studies have concentrated on the
development of novel medications to treat GA. Currently, the major
medications used in the clinical therapy of gout are non-steroidal
anti-inflammatory drugs, uricosuria agents and xanthine oxidase
inhibitors. However, none of the commercial drugs available can
both decrease serum urate levels and treat inflammation (5). Furthermore, there is compelling
evidence for associations between GA and an increased risk of
serious complications, including gouty nephropathy, hypertension,
cardiovascular disease and diabetes (6). Thus, new, safe, and effective
medicines for GA are needed.
The mTOR signaling pathway modulates the NALP3
inflammasome, which influences the production of interleukin
(IL)-1β and leads to GA attacks (7,8).
mTOR is an evolutionarily conservative serine-threonine protein
kinase that can recognize and integrate a series of intracellular
and environmental signals, including growth factors and insulin, to
regulate cellular and organic reactions. The mTOR signaling pathway
is highly conservative in eukaryotes and is related to the
proliferation of lymphocytes, the activation of immune cells,
autophagy and the metabolism of lipids and glucose, among other
functions. Due to its central role in signaling pathways, recent
studies have shown that mTOR correlates with GA, and it is the
common pathogenic signal pathway in numerous diseases, such as
vascular diseases, inflammation, obesity, progressive nephropathy
and diabetes (9-13).
During the early stage of GA, monosodium urate (MSU)
stimulates macrophages and produces numerous pro-inflammatory
factors, such as IL-1β, which are activated by the NALP3
inflammasome. When high concentrations of IL-1β are released, the
inflammatory reaction is amplified. At the same time, MSU
stimulates neutrophils (NG) and accumulates them at sites of joint
inflammation. MSU activates NG by the release of inflammatory
factors and the formation of neutrophil extracellular traps (NETs).
NETs are extracellular reticular fiber structures composed of
proteinase 3 (PR3), neutrophil elastase (NE), MMP9 (matrix
metalloproteinase 9), cathepsin G (CTSG), lactoferrin (LTF),
myeloperoxidase (MPO), peptidoglycan recognition protein (PRPG) and
high-mobility group protein B1 (HMGB1), among others (14-16).
When NETs are formed, the release of serine protein kinase may
activate pro-IL-1β and the NALP3 inflammasome, promote the
production of inflammatory mediators, and amplify the inflammatory
response.
Dioscorea nipponica Makino (TSDN) is used as
a natural medicine in China and is effective in treating GA. The
dried bark from its rhizome is considered to have effects of
relaxing the sinews, speeding up the ‘collaterals’, stimulating
blood flow, relieving pain, dispersing ‘wind’, and removing
moisture. The primary active components are total saponins from
TSDN, which include dioscin, protodioscin, and pseudo-protodioscin.
TSDN contains both anti-inflammatory and uric acid-lowering
properties.
TSDN also decreases the activities of xanthine
oxidase and adenosine deaminase, thus decreasing uric acid
production. It also reduces the expression levels of urate anion
transporter 1 and glucose transporter 9 while increasing the
expression of organic anion transporter (OAT)1 and OAT3, which
promotes uric acid excretion (17). TSDN showed potent anti-inflammatory
activity in previous studies and affected both the toll-like
receptor (TLR)2/4-IL-1R and the NALP3 inflammasome, thus regulating
the production and release of IL-1β (18,19).
Since the mTOR signaling pathway is upstream of TLR2/4-IL-1R, it
was aimed to determine whether TSDN has an effect on this pathway
and the formation of NETs in GA rats.
Materials and methods
Preparation of TSDN
TSDN was supplied by the Tongrentang Drug Company.
The plant material (voucher specimen: hlj-202028) was verified by
Professor Zhenyue Wang of Heilongjiang University of Chinese
Medicine. Using D-101 macroporous adsorption resin, 1 g of crude
drug was separated three times for 1.5 h with 6 ml of 50% ethanol
as previously described (18). A
4.97% (w/w) extraction ratio was recorded. Chemical standardization
of the herbal extract was accomplished using liquid
chromatography-tandem mass spectrometry (LC-MS) (Agilent
Technologies, Inc.; 6470B). The three main compounds found in the
herb were dioscin, protodioscin and pseudo-protodioscin (18). According to ultraviolet
spectrophotometry, the extraction rate of TSDN in the extract was
~55.9%.
Animals
A total of 40 male SPF Wistar rats (8 weeks old;
250±15 g) were provided by Changsheng Biotechnology Co., Ltd. and
maintained at the Heilongjiang University of Traditional Chinese
Medicine. Before the experiments, the rats were housed under
specific pathogen-free conditions at 22±24˚C and a relative
humidity of 55±5% on a 12-h/12-h light/dark cycle with water and
regular food ad libitum for 7 days and allowed to adjust to
their surroundings. Animal studies were approved (approval no.
2021052801) by The Animal Ethics Committee of Heilongjiang
University of Traditional Chinese Medicine (Harbin, China).
Preparation of the GA rat model
To induce the GA model, the ankle joints of rats
were injected bilaterally with a suspension of MSU (cat. no. U2625;
Sigma-Aldrich; Merck KGaA) on the third day of treatment (18). After modeling, the behavior of rats
was monitored each day until the experiment was completed.
Animal grouping and drug
administration
The rats were separated into 4 groups: i) normal,
ii) model, iii) TSDN (160 mg/kg) and iv) rapamycin groups (2.5
mg/kg; rapamycin purity >99%; Nanjiang Dulai Biotechnology Co.,
Ltd.) (18,20). TSDN and rapamycin treatments were
given every 24 h for one week. An equivalent dose of sterile saline
was administered to the normal and model groups. On the seventh
day, the rats were anesthetized with 3% sodium pentobarbital (45
mg/kg) by intraperitoneal injection. All rats were sacrificed by
cervical dislocation after collecting 4 ml of blood from the
abdominal artery in the dissection room of Heilongjiang University
of Traditional Chinese Medicine, and then synovial tissue samples
were collected. The blood was allowed to coagulate for 1 h at 37˚C
before being centrifuged at 3,500 g for 15 min at 4˚C. It was then
kept at -20˚C until testing. The tissues were stored at -80˚C. At
the end of the trials, the rats were sacrificed by cervical
dislocation.
Reverse-transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.). RNAiso Plus (cat. no. 9109), a
reverse transcription kit (cat. no. RR047Q), and a SYBR Green kit
(cat. no. RR420Q) were purchased from Takara Biomedical Technology
(Beijing) Co., Ltd. The reverse transcription and SYBR Green kits
were used for reverse transcription and gene amplification
according to the manufacturer's instructions. The qPCR
thermocycling conditions were as follows: Predenaturation at 95˚C
for 30 sec, followed by 40 cycles of 95˚C for 5 sec (denaturation),
60˚C for 30 sec (annealing) and 72˚C for 30 sec (extension). The
primer sequences are listed in Table
I. All primers and kits were obtained from Dalian Bao Biology
Co., Ltd., and GAPDH was employed as a normalization standard. The
mRNA levels were quantitatively analyzed by the 2-ΔΔCq
method (21).
 | Table IThe primer sequences used for reverse
transcription-quantitative PCR. |
Table I
The primer sequences used for reverse
transcription-quantitative PCR.
| Gene name | Primer sequence
(5'-3') |
|---|
| PI3K | F:
TCCCCGAGCTCACATCAGTCAA |
| |
CCCTGAGTGCCTCATCAAACTTC |
| AKT | F:
ATGGACTTCCGGTCAGGTTCA |
| | R:
GCCCTTGCCCAGTAGCTTCA |
| AMPK | F:
TGAGCTTACAGCTTTACCTGGTTGA |
| | R:
CACTTGACCGAGGTCTGTGGA |
| PTEN | F:
CCAATGGCTAAGTGAAGACGACAA |
| | R:
CATAGCGCCTCTGACTGGGAATA |
| mTOR | F:
GCTTATCAAGCAAGCGACATCTCA |
| | R:
TCCACTGGAAGCACAGACCAAG |
| Raptor | F:
ATGCTGGCCTCACCAGGTTC |
| | R:
GCATCATGCAACCTCAGTCACA |
| GAPDH | F:
TGCACCACCAACTGCTTAG |
| | R:
GATGCAGGGATGATGTTC |
Western blot analysis
Synovial tissues were extracted and homogenized, and
the samples were lysed with RIPA lysate containing a protease
inhibitor before being centrifuged for 30 min at 10,000 x g at 4˚C.
The bicinchoninic acid method was used to calculate the protein
concentration of the supernatant. SDS-PAGE with 10% gels was
performed with 20 µg proteins/lane, which were transferred to
nitrocellulose filter membranes (Pierce; Thermo Fisher Scientific,
Inc.). The proteins were then blocked with 5% non-fat milk for 1 h
at room temperature and subsequently incubated overnight at 4˚C
with the appropriate primary antibodies. Following the primary
incubation, membranes were incubated for 2 h at 37˚C with goat
anti-mouse IgG/HRP secondary antibodies (1:4,000; cat. no.
abs20001; Absin Bioscience, Inc.), and finally visualized using the
Hypersensitive ECL Chemiluminescence Kit (cat. no. BL520B; Beijing
Labgic Technology Co., Ltd.). The loading control was β-actin, and
the densities of bands were calculated using Image Pro-Plus 6.0
software (Media Cybernetics, Inc). The specific antibodies that
were employed are listed in Table
II.
 | Table IIList of antibodies used in western
blot analysis. |
Table II
List of antibodies used in western
blot analysis.
| Primary antibody
name | Molecular weight
(kDa) | Dilution | Cat. no. | Supplier |
|---|
| PI3K | ≈85 | 1:1,000 | ab86714 | Abcam |
| p-PI3K (Y607) | ≈84 | 1:500 | ab182651 | Abcam |
| Akt | ≈60 | 1:500 | 9272 | Cell Signaling
Technology, Inc. |
| p-AKT (Ser473) | ≈60 | 1:1,000 | ab81283 | Abcam |
| AMPK | ≈62 | 1:500 | 40585 | Signalway Antibody
LLC |
| p-AMPKd | ≈63 | 1:500 | 11174 | Signalway Antibody
LLC |
| PTEN | ≈55 | 1:1,000 | 60300-1-Ig | Proteintech Group,
Inc. |
| mTOR | ≈289 | 1:1,000 | 2972 | Cell Signaling
Technology, Inc. |
| Raptor | ≈140 | 1:500 | 20984-1-AP | Proteintech Group,
Inc. |
| β-actin | 42 | 1:2,000 | 66009-1-Ig | Proteintech Group,
Inc. |
Immunohistochemical analysis
Immunohistochemical staining was performed for NE,
PR3, CTSG, LTF and MPO according to the manufacturer's instructions
of each corresponding kit. Synovial tissue samples were fixed in 4%
paraformaldehyde at 4˚C overnight. Sections (5-µm thick) were
deparaffinized with xylene and rehydrated in a declining alcohol
series. Following the addition of a citric acid repair solution for
antigen recovery, 3% hydrogen peroxide was added to inactivate
endogenous peroxidase. The sections were blocked for 1 h at room
temperature with 1% goat serum (cat. no. BL210A; Biosharp Life
Sciences), then incubated with the primary antibody for 1 h at room
temperature. After that, the sections were treated for 10 min at
room temperature with goat anti-mouse IgG/HRP secondary antibodies
(1:4,000; cat. no. abs20001; Absin Bioscience, Inc.). Finally,
diaminobenzidine solution was used to stain the slices, which were
then counterstained with hematoxylin and examined under an optical
microscope. Image Pro-Plus 6.0 software was used to quantify the
expression levels by examining the mean values of integrated
optical density. The primary antibodies used are provided in
Table III.
 | Table IIIList of antibodies used in
immunohistochemical analysis. |
Table III
List of antibodies used in
immunohistochemical analysis.
| Antibody name | Cat. no. | Supplier | Dilution |
|---|
| Neutrophil
elastase | ab183342 | Abcam | 1:100 |
| Proteinase 3 | ab270441 | Abcam | 1:100 |
| Cathepsin G | 63665 | Cell Signaling
Technology, Inc. | 1:200 |
| Lactoferrin | sc-53498 | Santa Cruz
Biotechnology, Inc. | 1:100 |
|
Myeloperoxidase | ab208670 | Abcam | 1:1,000 |
Immunofluorescent analysis
Ankle-joint specimens were sectioned to obtain
3-µm-thick slices for observation. They were then stained with
citrullinated histone 3 (CitH3; 1:100; cat. no. ab5103; Abcam) and
Alexa Fluor® 488 goat anti-rabbit IgG (H+L) (1:100; cat.
no. A23220; Abbkine Scientific Co., Ltd.). A Leica TCS-SP5 confocal
microscope was used for imaging of the samples. Image Pro-Plus 6.0
software was used to evaluate the percentage of positive
expression.
ELISA analysis
Manufacturer-recommended kits (Shanghai Bolilai
Science and Technology Co., Ltd.) were used to quantify the levels
of IL-1β (cat. no. BLL101857E) and TNF-α (cat. no. BLL100946E) in
serum.
Statistical analysis
All results are presented as the mean ± SEM.
Statistical comparisons were performed using SPSS 25.0 software
(IBM Corp.). One-way analysis of variance (ANOVA) followed by
Dunnett's post hoc test was used to examine the differences between
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
TSDN regulates the PI3K/AKT/mTOR
axis
To determine the impact of TSDN on the PI3K/AKT/mTOR
axis in GA rats, the mRNA and protein expression levels of the
aforementioned pathway were examined using RT-qPCR (Fig. 1) and western blot analysis
(Fig. 2), respectively. It was
revealed that PI3K mRNA expression was significantly lower in the
model group than the normal group (Fig. 1A). However, no statistical
difference was identified between the model and TSDN groups. The
protein expression of p-PI3K was significantly lower in the model
group than in the normal group (Fig.
2C), but TSDN increased it significantly.
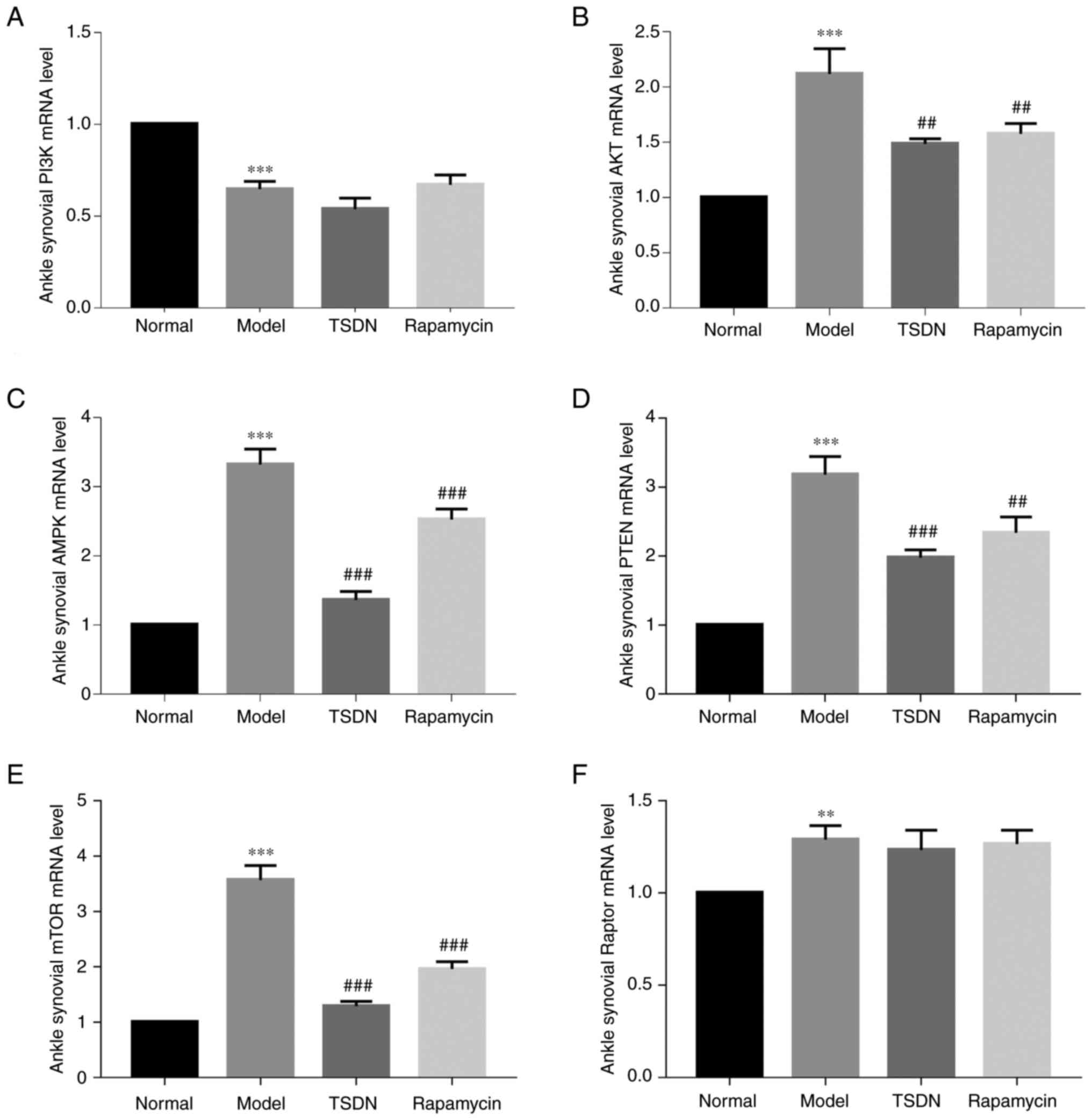 | Figure 1TSDN regulates the mRNA levels of
PI3K, AKT, AMPK, PTEN, mTOR and Raptor in GA rats. (A) PI3K, (B)
AKT, (C) AMPK, (D) PTEN, (E) mTOR and (F) Raptor. Data are shown as
the mean ± SEM (n=6). **P<0.01 and
***P<0.001 vs. normal. ##P<0.01 and
###P<0.001 vs. model. TSDN, Dioscorea
nipponica Makino; AMPK, activated protein kinase; Raptor,
regulatory-associated protein of mTOR. |
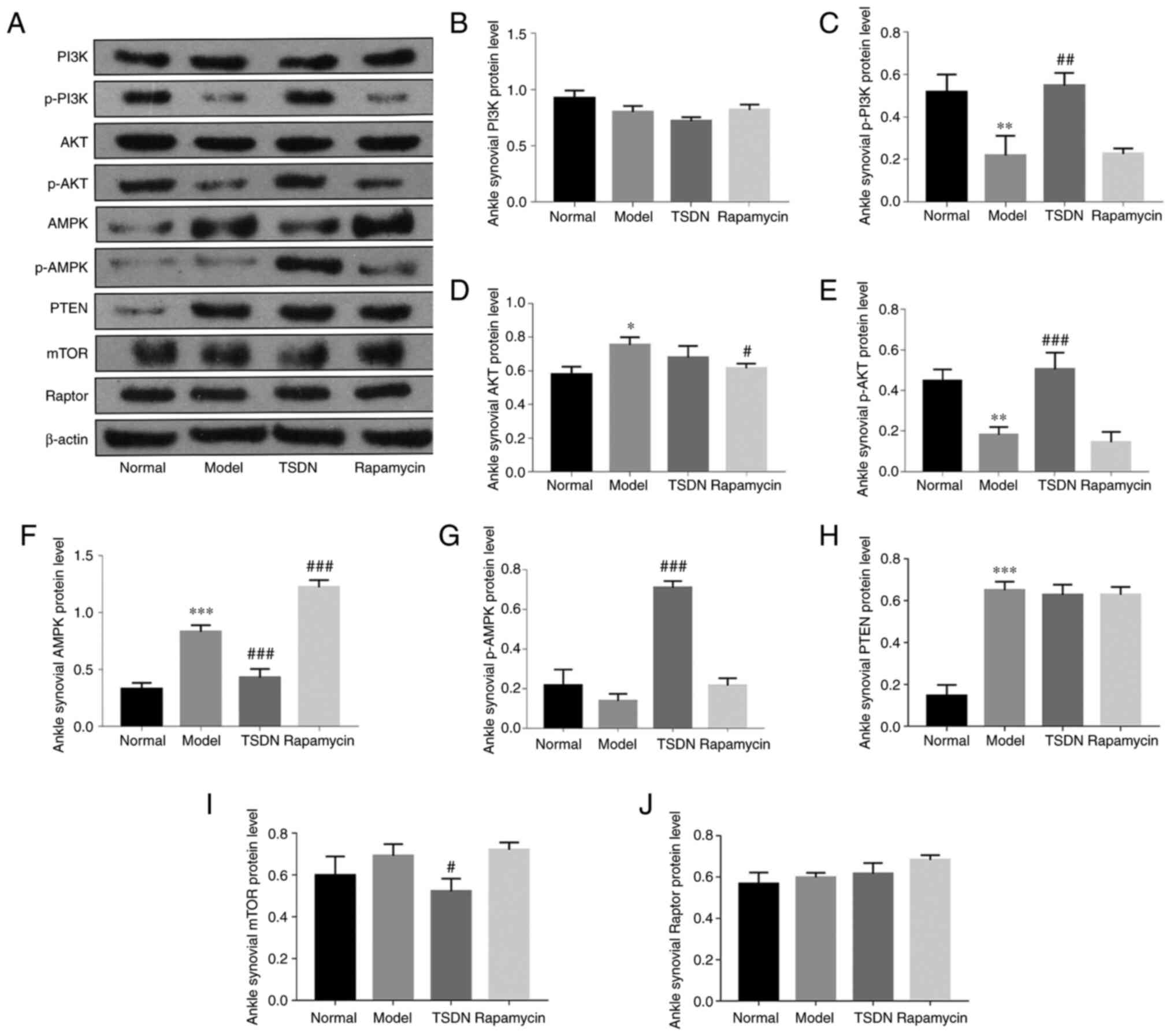 | Figure 2TSDN modulates the protein expression
of PI3K, p-PI3K, AKT, p-AKT, AMPK, p-AMPK, PTEN, mTOR and Raptor.
(A) PI3K, p-PI3K, AKT, p-AKT, AMPK, p-AMPK, PTEN, mTOR and Raptor
were detected using western blot analysis. Densitometric analysis
of (B) PI3K, (C) p-PI3K, (D) AKT, (E) p-AKT, (F) AMPK, (G) p-AMPK,
(H) PTEN, (I) mTOR and (J) Raptor. Data are presented as the mean ±
SEM (n=3). *P<0.05, **P<0.01 and
***P<0.001 vs. normal. #P<0.05,
##P<0.01 and ###P<0.001 vs. model.
TSDN, Dioscorea nipponica Makino; p-, phosphorylated; AMPK,
activated protein kinase; Raptor, regulatory-associated protein of
mTOR. |
Both the mRNA and protein expression of AKT were
significantly enhanced in the model group compared with the normal
group (Figs. 1B and 2D). TSDN and rapamycin significantly
reduced its mRNA expression (Fig.
1B), whereas rapamycin decreased its protein expression
(Fig. 2D). The protein expression
of p-AKT was significantly lower in the model group than in the
control group (Fig. 2E), and TSDN
significantly increased it.
In the model group, both mRNA and protein expression
of AMPK were significantly higher than in the normal group
(Figs. 1C and 2F). Rapamycin and TSDN both significantly
reduced its mRNA expression. TSDN also significantly reduced AMPK
protein expression (Fig. 2F), but
rapamycin significantly increased it. There was no change in the
protein expression of p-AMPK in the model group compared with the
normal group, while TSDN significantly improved its protein
expression (Fig. 2G).
Both the mRNA and protein expression of PTEN in the
model group were elevated compared with the normal group (Fig. 1D and Fig. 2H), but TSDN and rapamycin
significantly reduced the mRNA expression. In the model group, the
mRNA expression of mTOR was higher than in the control group
(Fig. 1E), while TSDN and
rapamycin significantly decreased its expression. TSDN also
decreased mTOR protein expression (Fig. 2I). The mRNA expression of Raptor in
the model group was significantly higher than that of the normal
group (Fig. 1F), but neither TSDN
nor rapamycin had any impact on it. Additionally, the protein
expression of Raptor did not differ statistically between groups
(Fig. 2J). These findings
suggested that in GA rats, TSDN modulated the PI3K/AKT/mTOR
signaling pathway.
TSDN decreases the expression levels
of NE, PR3, CTSG, LTF and MPO
NE, PR3, CTSG, LTF and MPO are critical components
of NETs. As demonstrated in Fig.
3A-F, the protein expression levels of NE, PR3, CTSG, LTF and
MPO in the model group were significantly higher than in the
control group. TSND significantly reduced the levels of NE, PR3,
CTSG, LTF and MPO. Rapamycin significantly decreased the levels of
NE, PR3, CTSG and LTF. These results demonstrated that TSDN
suppresses the formation of NETs.
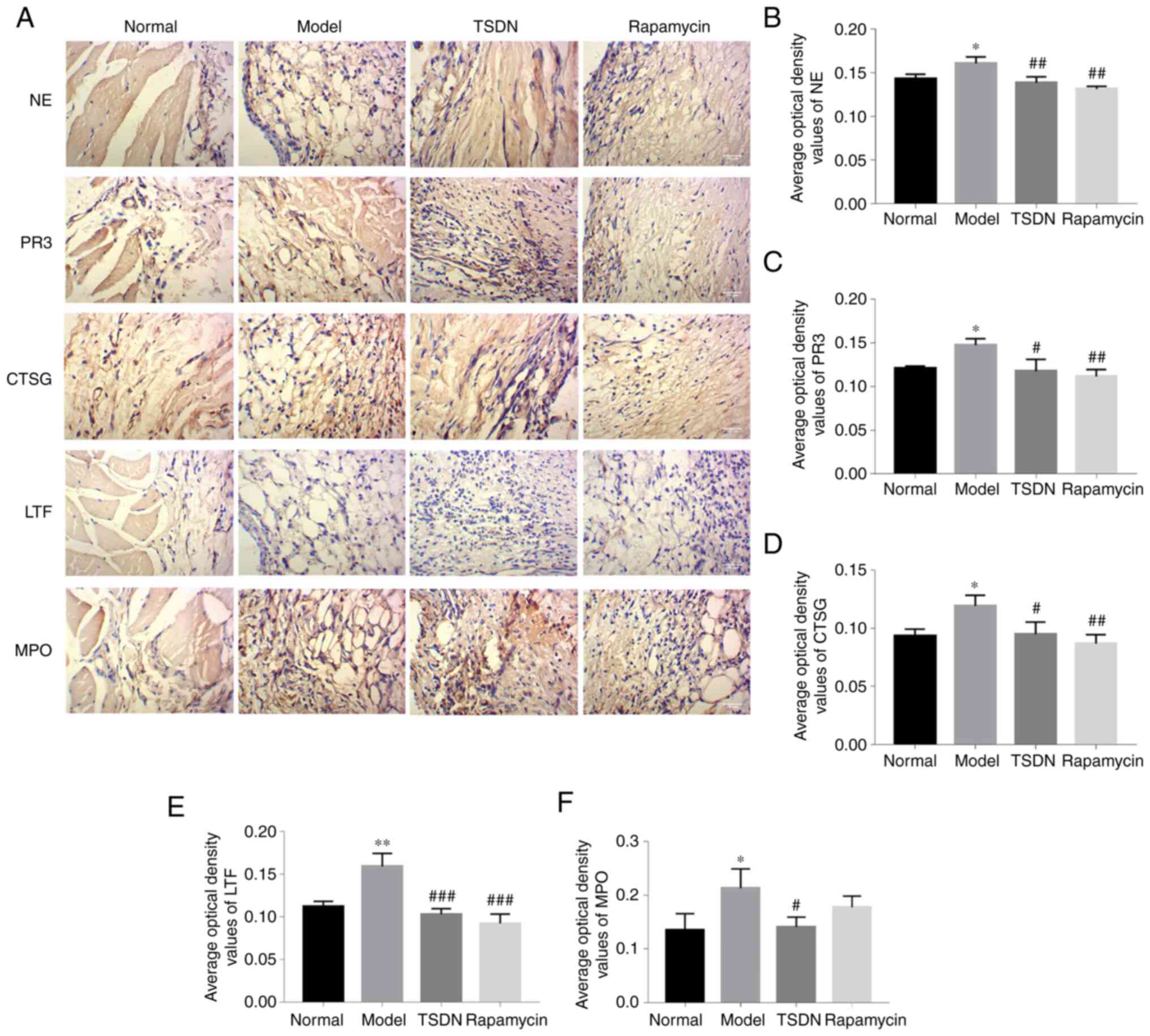 | Figure 3TSDN decreases the levels of NE, PR3,
CTSG, LTF and MPO. (A) Immunohistochemical staining of NE, PR3,
CTSG, LTF and MPO (magnification, x400; scale bar, 100 µm).
Quantified expression levels of (B) NE, (C) PR3, (D) CTSG, (E) LTF
and (F) MPO. Data are shown as the mean ± SEM (n=3).
*P<0.05 and **P<0.01 vs. normal.
#P<0.05, ##P<0.01 and
###P<0.001 vs. model. TSDN, Dioscorea
nipponica Makino; NE, neutrophil elastase; PR3, Proteinase 3;
CTSG, cathepsin G; LTF, lactoferrin; MPO, myeloperoxidase. |
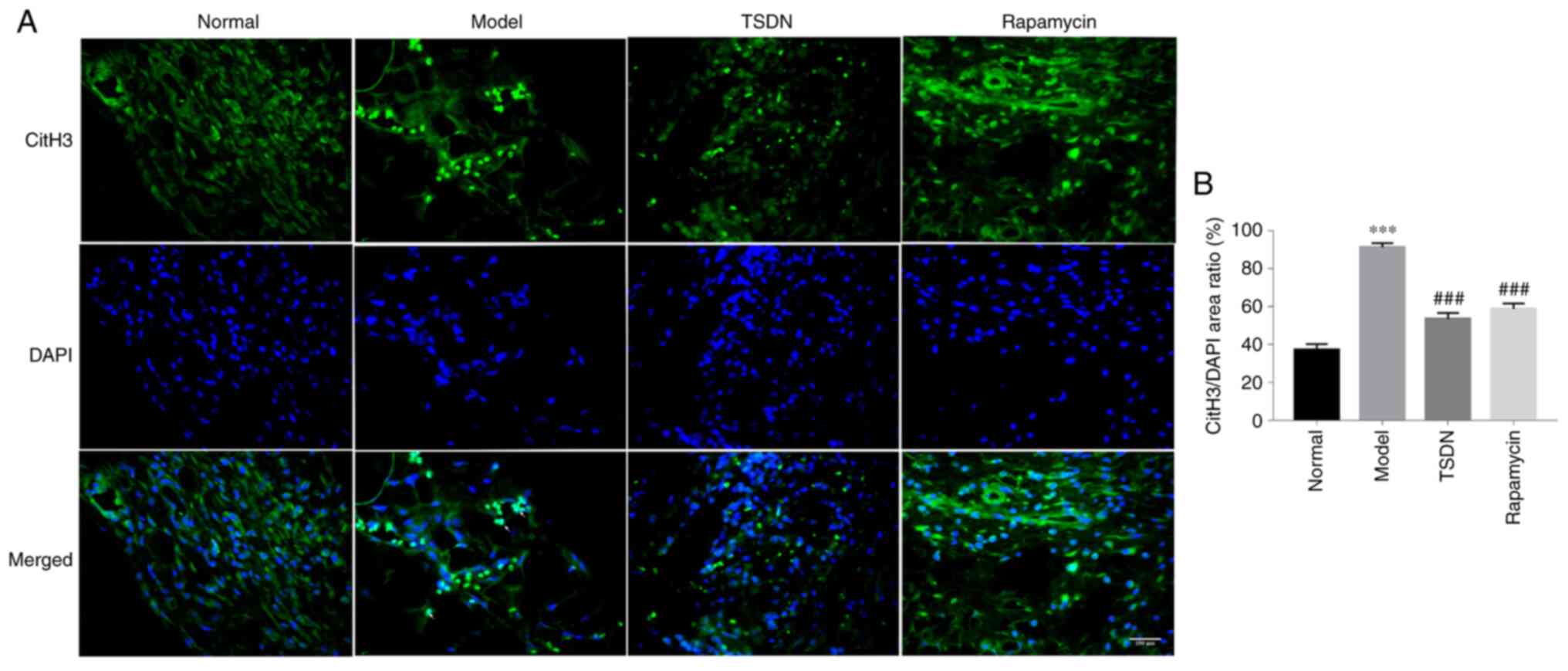
TSDN lowers CitH3+
cells
NETs are cloud-like structures that are co-localized
with DNA and CitH3 and are involved in cell membrane degradation.
As revealed in Fig. 4A and
B, the number of CitH3+
cells increased significantly in the model group compared with the
control group, but TSDN and rapamycin significantly decreased the
number. Immunofluorescent data demonstrated that TSDN inhibits the
formation of NETs.
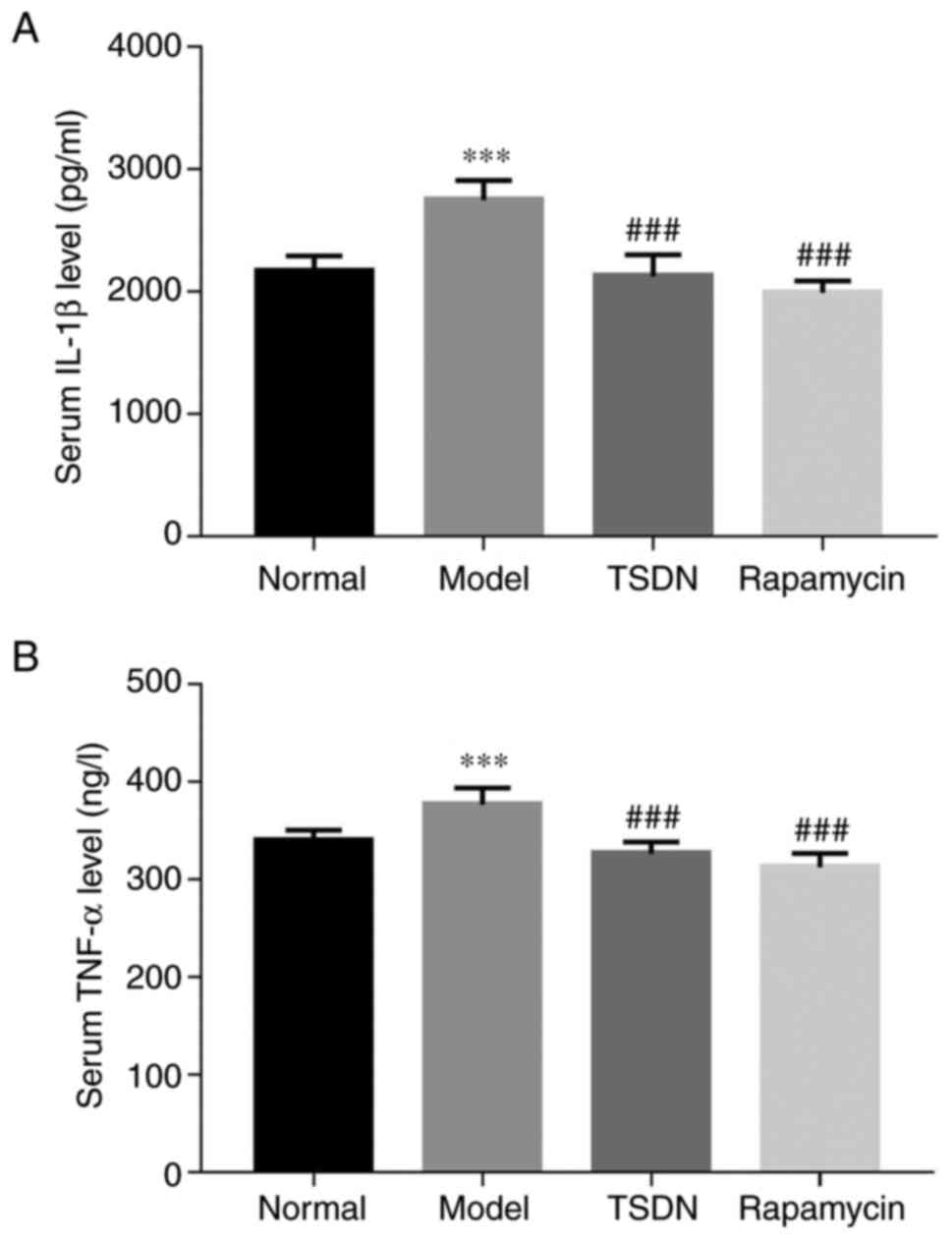
TSDN inhibits the levels of IL-1β and
TNF-α
As indicated in Fig.
5A and B, IL-1β and TNF-α were
significantly enhanced in the model group compared with the control
group. Both TSDN and rapamycin significantly decreased the release
of IL-1β and TNF-α. These results suggested that TSDN has an
anti-inflammatory effect in GA rats.
Discussion
GA is the most frequent type of inflammatory
arthritis in elderly men and is 3-4 times more common than
rheumatoid arthritis (22). GA can
potentially lead to severe consequences and considerable challenges
for patients and society. TSDN is used as a natural medicine that
has been revealed to be effective in treating GA in clinical
trials, and its major active component is TSDN. However, the
pharmacological mechanism of TSDN's effect on GA needs
clarification. Our previous studies showed that TSDN has uric
acid-lowering and anti-inflammation effects and that it influences
the NALP3 inflammasome, thus regulating the activation of IL-1β
(17-19).
Since mTOR is the upstream signaling pathway of the NALP3
inflammasome, it also correlates with numerous diseases. The goal
of the present study was to determine whether TSDN regulates the
mTOR signaling pathway as a potential treatment for GA.
The present results showed that TSDN reduced the
mRNA and protein expression levels of AMPK and mTOR, as well as the
mRNA expression levels of AKT and PTEN, while it increased the
protein expression levels of p-PI3K, p-AKT and P-AMPK. TSDN also
reduced the levels of NE, PR3, CTSG, LTF and MPO, the number of
CitH3+ cells, and the levels of IL-1β and TNF-α. TSDN
downregulated the mRNA expression of AKT and PI3K, but it did not
influence their protein expression. It also downregulated both the
mRNA and protein expression of AMPK. This may have occurred because
TSDN regulates transcription and translation in a distinct way.
The present study demonstrated that TSDN enhanced
the protein expression levels of p-PI3K and p-AKT. When p-PI3K is
activated, it inhibits tuberous sclerosis complex 2 (TSC2). p-AKT
also inhibits TSC2. When TSC2 is inhibited, the Ras homolog
enriched in the brain is activated, thus mTORC1 is activated. When
mTORC1 is activated, autophagy is activated since mTORC1 is the
main gateway to autophagy (23).
According to previous studies, autophagy (a cellular
waste-clearance and renewal mechanism) plays a significant role as
a macrophage-intrinsic negative regulator of the NALP3 inflammasome
(24). Another study found that
Sirt1 suppresses macrophage polarization towards the M1 phenotype
by stimulating the PI3K/AKT/STAT6 pathway, resulting in an
anti-inflammatory effect in GA (25). TSDN increased the protein
expression of p-AMPK. When p-AMPK is activated, it inhibits Raptor.
Activated AMPK decreases mononuclear phagocyte responses to urate
crystals in vitro, as well as the activation of the NLRP3
inflammasome and IL-1β. AMPK also stimulates autophagy and
anti-inflammatory M2 macrophage polarization (26).
TSDN also decreased the protein expression of mTOR,
which is in accordance with a previous study by the authors, since
mTOR is the upstream regulator of the NALP3 inflammasome. However,
between the normal and model groups, there was no discernible
difference in the protein expression of mTOR. This may be due to it
being a total protein, but not in its active phosphorylated state
(27).
PTEN has been inversely connected to the PI3K/AKT
signaling pathway. The findings of the present study revealed that
the mRNA and protein expression of PTEN were increased in the model
group. TSDN decreased its mRNA expression, but it did not influence
the protein expression. The mRNA expression of Raptor was enhanced
in the model group, but there was no effect of TSDN on its
expression.
During the formation of NETs, a kind of specially
programmed cell death occurs, which is called NETosis, which is
different from apoptosis and necrosis. When cell membranes are
damaged, numerous molecules enter the cytoplasm, including
histones, NE, MPO, PR3, LTF, CTSG, MMP, PRPG, HMGB1 and pentraxin.
Li et al (14) proposed
that NETosis-products may be used as targets for crystal-induced
diseases. NET-like structures consist of DNA decorated with CitH3
and elastase (28). TSDN reduced
the levels of NE, PR3, CTSG, LTF and MPO, as well as the number of
CitH3+ cells and the production of IL-1β and TNF-α.
There are certain limitations to the present study.
Only GA rats were studied, and no clinical samples were acquired.
Therefore, the findings do not accurately represent the clinical
application of TSDN. The aim of the study was to shed light on the
crucial role that NETs play in GA in the treatment of TSDN, yet the
detection index was somewhat limited. In future studies, more
in-depth research on the mechanism of NET generation will be
conducted.
Additionally, the present study demonstrated the
regulatory function of the PI3K/AKT/mTOR pathway in the TSDN
intervention of production of NETs, but the primary regulatory
targets were not identified. Pathway inhibitors, gene silencing and
overexpression will be used in future studies to elucidate the
mechanism. Finally, there was lack of neutrophil labeling to
determine neutrophil infiltration, which is another limitation to
the study.
In conclusion, TSDN regulated the PI3K/AKT/mTOR
axis, NET formation and inflammatory factors. This helped to
explain the anti-inflammatory effect of TSDN on GA. The results
suggested that TSDN may be used in conditions of comorbidity and
has favorable potential to treat GA. The findings could provide a
solid foundation for extending the therapeutic application of TSDN
and provide new insight into the development of novel medications
for GA.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Joint Guidance
Project of Heilongjiang Natural Science Foundation (grant no.
LH2021H099), the Start Fund of Postdoctoral Research in
Heilongjiang (grant no. LRB146914), the Outstanding Youth
Development Fund of Heilongjiang University of Chinese Medicine
(grant no. 2019JC06), the Project of Heilongjiang Administration of
Traditional Chinese Medicine (grant no. ZHY202094), the Graduate
Innovative Scientific Research Project of Heilongjiang University
of Chinese Medicine (grant no. 2020yjscx057) and the Natural
Science Foundation of Heilongjiang (key project; grant no.
ZD2020H006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ designed and carried out the experiment and wrote
the manuscript. LL and HS carried out part of the experiment. SL
assisted in designing the experiment and writing the manuscript.
QZ, LL and SL confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by (approval
no. 2021052801) and conducted in accordance with The Animal Ethics
Committee of Heilongjiang University of Traditional Chinese
Medicine (Harbin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wen X, Lou Y, Song S, He Z, Chen J, Xie Z,
Shi X, Wen C and Shao TX: Qu-Zhuo-Tong-Bi decoction alleviates
gouty arthritis by regulating butyrate-producing bacteria. Front
Pharmacol. 11(610556)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dalbeth N, Gosling AL, Gaffo A and
Abhishek A: Gout. Lancet. 388:2039–2052. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cheng JJ, Ma XD, Ai GX, Yu QX, Chen XY,
Yan F, Li YC, Xie JH, Su ZR and Xie QF: Palmatine protects against
msu-induced gouty arthritis via regulating the NF-κB/NLRP3 and Nrf2
pathways. Drug Des Devel Ther. 16:2119–2132. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Keller SF and Mandell BF: Management and
cure of gouty arthritis. Med Clin North Am. 105:297–310.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bernardes ACFPF, Matosinhos RC, de Paula
Michel Araújo MC, Barros CH, de Oliveira Aguiar Soares RD, Costa
DC, Sachs D and Saúde-Guimarães DA: Sesquiterpene lactones from
lychnophora species: Antinociceptive, anti-inflammatory, and
antioxidant pathways to treat acute gout. J Ethnopharmacol.
269(113738)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sun Z, Li Z, Tan Y, Wang X, Wang C, Dong
M, Liu H, Chen H, Li Y, Li L and Wang D: Anti-gouty arthritis and
anti-hyperuricemia properties of sanghuangporus vaninii and
inonotus hispidus in rodent models. Nutrients.
14(4421)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cabău G, Crișan TO, Klück V, Popp RA and
Joosten LAB: Urate-induced immune programming: Consequences for
gouty arthritis and hyperuricemia. Immunol Rev. 294:92–105.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vazirpanah N, Ottria A, van der Linden M,
Wichers CGK, Schuiveling M, van Lochem E, Phipps-Green A, Merriman
T, Zimmermann M, Jansen M, et al: mTOR inhibition by metformin
impacts monosodium urate crystal-induced inflammation and cell
death in gout: A prelude to a new add-on therapy. Ann Rheum Dis.
78:663–671. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu GY and Sabatini DM: mTOR at the nexus
of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol.
21:183–203. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Al-Bari MAA and Xu P: Molecular regulation
of autophagy machinery by mTOR-dependent and -independent pathways.
Ann N Y Acad. 1467:3–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
De Vita V and Melnik BC: Activation of
mechanistic target of rapamycin complex 1: The common link between
rheumatoid arthritis and diabetes mellitus. Rheumatology.
58:377–379. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mirza-Aghazadeh-Attari M, Ekrami EM,
Aghdas SAM, Mihanfar A, Hallaj S, Yousefi B, Safa A and Majidinia
M: Targeting PI3K/Akt/mTOR signaling pathway by polyphenols:
implication for cancer therapy. Life Sci.
255(117481)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang N, Feng T, Liu X and Liu Q: Curcumin
inhibits migration and invasion of non-small cell lung cancer cells
through up-regulation of miR-206 and suppression of PI3K/AKT/mTOR
signaling pathway. Acta Pharm. 70:399–409. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li Y, Cao X, Liu Y, Zhao Y and Herrmann M:
Neutrophil extracellular traps formation and aggregation
orchestrate induction and resolution of sterile crystal-mediated
inflammation. Front Immunol. 9(1559)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hidalgo A, Libby P, Soehnlein O, Aramburu
IV, Papayannopoulos V and Silvestre-Roig C: Neutrophil
extracellular traps: from physiology to pathology. Cardiovasc Res.
118:2737–2753. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Caution K, Young N, Robledo-Avila F,
Krause K, Abu Khweek A, Hamilton K, Badr A, Vaidya A, Daily K, et
al: Caspase-11 mediates neutrophil chemotaxis and extracellular
trap formation during acute arthritis through alteration of cofilin
phosphorylation. Front Immunol. 10(2519)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou Q, Yu DH, Liu Y and Liu SM: Total
saponins from Discorea nipponica Makino ameliorate urate excretion
inhyperuricemic rats. Pharmacogn Mag. 11:567–573. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou Q, Yu DH, Zhang N and Liu SM:
Anti-inflammatory effect of total saponins from Dioscorea
nipponica Makino on gouty arthritis and its influence on NALP3
inflammasome. Chin J Integr Med. 25:663–670. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhou Q, Lin FF, Liu SM and Sui XF:
Influence of the total saponin fraction from Dioscorea
nipponica Makino on TLR2/4-IL-1R receptor signal pathway in
rats of gouty arthritis. J Ethnopharmacol. 206:274–282.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dai Q, Zhou D, Xu L and Song X: Curcumin
alleviates rheumatoid arthritis-induced inflammation and synovial
hyperplasia by targeting mTOR pathway in rats. Drug Des Devel Ther.
12:4095–4105. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Singh JA and Gaffo A: Gout epidemiology
and comorbidities. Semin Arthritis Rheum. 50:S11–S16.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rabanal-Ruiz Y, Otten EG and Korolchuk VI:
mTORC1 as the main gateway to autophagy. Essays Biochem.
61:565–584. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhong Z, Sanchez-Lopez E and Karin M:
Autophagy, NLRP3 inflammasome and autoinflammatory/immune diseases.
Clin Exp Rheumatol. 34 (4 Suppl 98):S12–S16. 2016.PubMed/NCBI
|
|
25
|
Liu L, Zhu X, Zhao T, Yu Y, Xue Y and Zou
H: Sirt1 ameliorates monosodium urate crystal-induced inflammation
by altering macrophage polarization via the PI3K/Akt/STAT6 pathway.
Rheumatology (Oxford). 58:1674–1683. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Terkeltaub R: What makes gouty
inflammation so variable. BMC Med. 15(158)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Presneau N, Shalaby A, Idowu B, Gikas P,
Cannon SR, Gout I, Diss T, Tirabosco R and Flanagan AM: Potential
therapeutic targets for chordoma: PI3K/AKT/TSC1/TSC2/mTOR pathway.
Br J Cancer. 100:1406–1414. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jiang A, Zhang Y, Wu D, Li S, Liu Z, Yang
Z and Wei Z: Sodium molybdate induces heterophil extracellular
traps formation in chicken. Ecotoxicol Environ Saf.
210(111886)2021.PubMed/NCBI View Article : Google Scholar
|