1. Introduction
Osteoarthritis (OA), also known as chronic
degenerative arthritis, is characterized by low-grade inflammation
in the joints. In total, >250 million patients suffer from OA;
thus, posing a serious threat to human health (1). Notably, OA may induce the disability
of joints, and cause a loss of labor production and economic
burden. At present, there are no effective strategies for the
treatment of OA, and the majority of drugs available for the
treatment of OA, including non-steroidal anti-inflammatory drugs
and glucosamine, only relieve the symptoms. Notably, surgery is
often considered last for effectively managing OA of the knee
(2). To the best of our knowledge,
although numerous previous studies aimed to improve the available
treatment options for OA, the results of clinical trials are not
satisfactory at present (3,4).
This may be explained by a lack of understanding of the potential
pathological mechanisms underlying OA.
Pathologically, abnormal metabolic changes may lead
to the pathogenesis of OA and these modifications include
inflammatory stress, increased chondrocyte apoptosis and
extracellular matrix (ECM) degradation (5). Chondrocytes are a unique cell type
found in the cartilage that maintains the balance of ECM metabolism
(6). However, avascular cartilage
with limited capacity for repair is impacted by detrimental
stimuli, negatively influencing the biological functions of
chondrocytes and subsequently inducing pathological changes
(7). Typically, the pathological
development of OA is orchestrated by a network of signaling
pathways, including the Wnt/β-catenin (8), PI3K/AKT (9), mitogen-activated protein kinases
(MAPK)/NF-κB (10) and Notch
pathways (11). These key
signaling pathways are considered potential targets for the
development of novel drugs. In recent years, research has focused
on the use of Traditional Chinese Medicine (TCM) in the prevention
of OA development (12).
Ginseng, belonging to the genus Panax in the
Araliaceae family, is a common TCM used in East Asian
countries for the treatment of numerous diseases. Ginseng exhibits
dietary, nutraceutical and medicinal uses. The bioactive compounds
of ginseng, namely ginsenosides, are classified as steroidal
saponins with a triterpene dammarane chemical structure and a
steroid-like configuration. To date, ~200 ginsenosides and >40
different subtypes have been discovered (13). Among these ginsenosides, Rb1, Rb2,
Rg1, Rc, Rd, Re and Rg1 are the most abundant (14) (Fig.
1). Moreover, ginsenosides are divided into protopanaxadiol,
protopanaxatriol and other subtypes, according to the structure of
the backbone. The different modified groups and sugars attached to
the backbone produce various distinctive structures of ginsenosides
with distinct biological activities (15). Results of recent studies
demonstrated that ginsenosides exhibit numerous beneficial
properties, including cardiovascular protection (16), neuroprotection (17), liver protection (18), antitumor (19), anti-diabetes (20) and bone protection (21). Moreover, ginsenosides exhibit
numerous pharmacological activities, including anti-inflammatory
(22), which is the main
therapeutic strategy for the clinical management of OA. The present
study aimed to review and discuss the protective activities of
ginseng and ginsenosides by inhibiting inflammation, oxidative
stress and ECM degradation during OA development (Fig. 1).
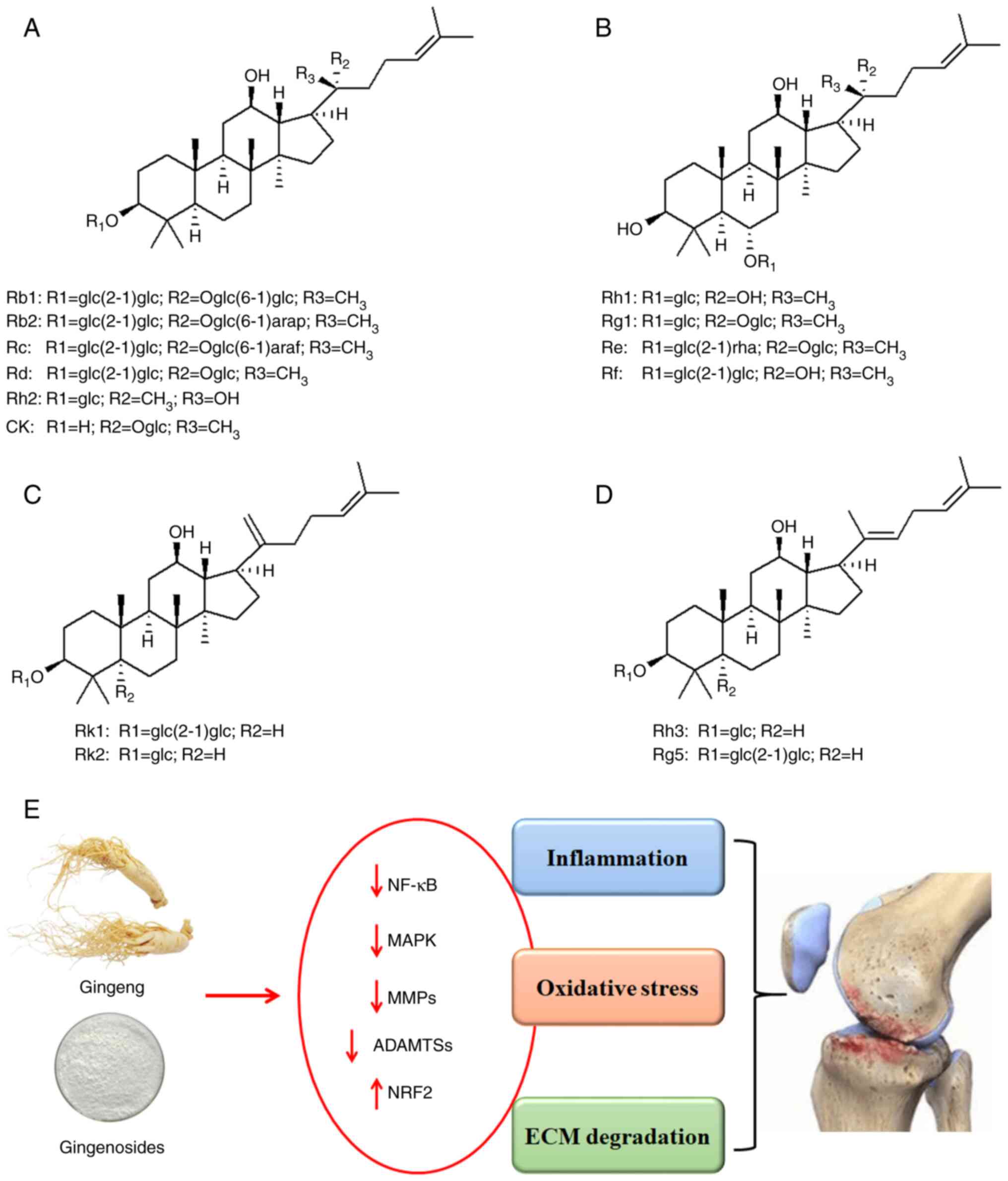 | Figure 1Chemical structures of ginsenosides
and the protective effects of ginseng and ginsenosides against
osteoarthritis. (A) Different ginsenosides of the protopanaxadiol
type. Ginsenoside Rb1 has a glc(2-1)glc group at the R1 position, a
Oglc(6-1)glc group at the R2 position, and a methyl group at the R3
position. Ginsenoside Rb2 and Rc have the same groups as Rb1 at the
R1 and R3 position, while the R2 position is replaced by
Oglc(6-1)arap and Oglc(6-1)araf groups, respectively. The R1, R2
and R3 positions of Ginsenosides Rd, Rh2 and CK are glc(2-1)glc,
glc, and H, Oglc, CH3 and Oglc, and CH3, OH
and CH3, respectively. (B) Different ginsenosides of the
protopanaxatriol type. (C) Derivatives of protopanaxadiol:
Ginsenosides Rk1 and Rk2. (D) Derivatives of protopanaxadiol:
Ginsenosides Rh3 and Rg5. (E) The protective activity of ginseng
and ginsenosides against osteoarthritis development by inhibiting
inflammation, oxidative stress and ECM degradation. ECM,
extracellular matrix. |
2. Protective effects of ginseng and
ginsenosides against OA development
The pathological development of
OA
The pathological development of OA is multifactorial
and affected by the activation of signaling cascades. Inflammatory
responses and oxidative stress are involved in the progression of
OA (23). Inflammation is an
innate immune response triggered by pathogens or danger-related
signals. Results of a previous study described the association
between immune cells and OA development (24). Notably, chondrocytes and
synoviocytes are the two cell types responsible for producing
inflammatory cytokines and chemokines, and these are involved in
the pathogenesis of OA (25). More
specifically, the increased production of inflammatory cytokines
may induce the aberrant expression of cell signaling pathways,
transcriptional expression and joint cartilage destruction. The
altered expression of cell signaling pathways may further enhance
the release of inflammatory cytokines, forming a positive loop
(26). For example, IL-1β and TNFα
are pro-inflammatory cytokines secreted by chondrocytes,
synoviocytes and mononuclear cells in early OA. Both IL-1β and TNFα
stimulate the signaling cascade of inflammation, producing IL-6,
IL-1β, TNFα and prostaglandin E2 (PGE2) in chondrocytes (27). In addition, IL-1β, IL-6 and TNFα
are important regulators in the promotion of articular cartilage
destruction and synovium inflammatory responses (28). Activation of NF-κB signaling is
associated with increased expression of pro-inflammatory cytokines
in OA, including cyclooxygenase-2 (COX-2), PGE2 and inducible
nitric oxide synthase (iNOS), the increased expression of MMPs and
A disintegrin and metalloproteinase with thrombospondin motifs
(ADAMTSs), and the decreased expression of collagen II and
aggrecans (29).
Dysregulated expression in both pro- and
anti-oxidant systems may induce oxidative stress, which is
associated with excessive reactive oxygen species (ROS) production
(30). Notably, oxidative stress
exerts detrimental effects on macromolecules and stimulates various
disorders in the human body. Moreover, oxidative stress is
considered a complex pathological process that impacts numerous
target organelles, including mitochondria and the endoplasmic
reticulum (ER). Initiation of oxidative stress may stimulate
organelles to adaptively modify their metabolism, to protect from
injury and maintain cellular homeostasis (31). In addition, increased oxidative
stress may impair DNA, protein and lipid production, leading to
cellular injury (32). Notably,
mitochondria are both producers and targets of ROS. Specifically,
the mitochondrial respiratory chain produces ROS, which may induce
mitochondrial dysfunction and subsequently enhance the production
of ROS with a positive loop (33).
Under oxidative stress, increased mitochondrial fission and
decreased mitochondrial fusion induce the imbalance of
mitochondrial metabolism, leading to the increased expression of
Bax and cytochrome c, and the initiation of mitochondrial apoptosis
(34). Results of a previous study
demonstrated the close association between OA progression and
oxidative stress; notably, oxidative stress induces the increased
production of ROS in OA chondrocytes (35).
Protective activities of ginseng and
ginsenosides against OA
The participation of inflammatory responses and
oxidative stress in OA indicates that the cellular processes of OA
chondrocytes may be modulated by appropriate anti-inflammatory
agents and anti-oxidants. For example, in lipopolysaccharide
(LPS)-treated RAW 264.7 macrophage cells, a ginsenoside Rh2 mixture
[consisting of 20(S)-Rh2, 20(R)-Rh2, Rk2 and Rh3] exerted
anti-inflammatory effects by inhibiting the expression of NF-κB
signaling (36). In IL-1β-treated
SW1353 cells, Korean red ginseng suppressed the expression of
MMP-13 and the release of glycosaminoglycan, by inhibiting the
activation of p38 MAPK, JNK and STAT1/2 signaling pathways
(37). In addition, the results of
a previous study demonstrated that extracts of Notoginseng
Radix and Rehmanniae Radix Preparata alleviate joint
pain and inhibited cartilage degeneration in rat OA models
(38). Similarly, Panax
quinquefolium saponin, isolated from Radix panacis
quinquefolia (American ginseng) inhibited IL-1β-induced ER
stress, NF-κB-mediated inflammatory responses and cell apoptosis in
rat chondrocytes (39,40). Red ginseng also exhibits
anti-oxidative activity, which may be an advantage in protecting
against the destruction of joint cartilages (41). In a double-blind randomized trial,
patients treated with red ginseng demonstrated improved joint pain,
higher disability of the arm, shoulder and hand scores, increased
production of antioxidant enzymes, and decreased expression of
oxidative stress markers (42).
Maltol, a compound in red ginseng, reduces the levels of
pro-inflammatory cytokines and the production of catabolic factors,
such as MMP-13 and ADAMTS-5, by suppressing NF-κB activity and
increasing the nuclear factor (erythroid-derived 2)-like 2 (NRF2)
pathway (43,44). These results demonstrated the
protective activity of ginseng and the corresponding bioactive
ginsenosides. Moreover, these results highlighted the molecular
mechanisms that may exhibit potential in the suppression of
inflammatory responses and oxidative stress in OA chondrocytes.
3. Anti-inflammatory properties of
ginsenosides
Results of a previous study suggested that
inflammasomes exhibit potential as biomarkers in inflammatory
diseases. Activation of inflammasomes may trigger caspase-1, which
activates IL-1β, IL-18 and IL-33(45). Results of a previous study
demonstrated the biological activity of ginsenosides in the
activation of inflammasomes (46).
Notably, ginsenoside Rg1 and Rh3 inhibit the activation of
inflammasomes by inhibiting NOD-like receptor thermal protein
domain associated protein 3 (NLRP3) and absent in melanoma 2
activity in mouse and human macrophages (47). Moreover, the inhibitory activity of
Rg1 against inflammasomes has been demonstrated in numerous
diseases (48). In IL-1β-treated
human OA chondrocytes, Rg1 significantly decreased the production
of COX-2 and PGE2(49) (Table I). In addition, ginsenoside Rb1 and
Rb2 may decrease the levels of TNFα in RAW 264.7 cells with
IC50 values of 56.5 and 27.5 µM, respectively, and in
U937 cells with IC50 values of 51.3 and 26.8 µM,
respectively (50). Moreover, Rb1
inhibited the IL-1β-induced expression of COX-2/PGE2 and iNOS/NO,
and caspase-3 and PARP mRNA expression in primary human OA
chondrocytes (51) (Table I). Results of a previous study
demonstrated that ginsenoside Rf decreases the serum levels of
IL-6, IL-1β and TNFα (52).
 | Table IProtective activity of ginsenosides
against OA. |
Table I
Protective activity of ginsenosides
against OA.
| Compound | First author/s,
year | Model | Concentrations | Biological
functions | (Refs.) |
|---|
| Rb1 | Cheng et al,
2013 | Human OA
chondrocytes | 1, 10 and 100
µg/l | COX-2↓, PGE2↓,
iNOS↓, NO↓, MMP-13↓, caspase-3↓, poly (ADP-ribose) polymerase↓,
collagen II↑, aggrecan↑ | (51) |
| | Aravinthan et
al, 2021 | MIA-induced rat
OA | 3-10 µg/kg bw | Bone morphogenetic
protein 2↑, collagen II↑, MMP-13↓, IFNγ↓, monocyte chemoattractant
protein-1/C-C motif chemokine 2 ↓, IL-1β↓, IL-6↓ | (53) |
| | Luan et al,
2022 | MIA-induced rat
OA | 5 and 10 mg/kg
bw | Histological
improvement, IL-1β↓, IL-6↓, TNFα↓, miR-21-5p↓, fibroblast growth
factor 18↑ | (56) |
| | Luan et al,
2022 | Rat
chondrocytes | 10 µM | IL-1β↓, IL-6↓,
TNFα↓, miR-21-5p↓, fibroblast growth factor 18↑ | (56) |
| | Hossain et
al, 2022 | Rabbit knee OA | 30 and 100
µg/kg | MMPs↓, TNFα↓,
caspase-3↓, Bax↓, ROS↓, NF-κB↓, p38 MAPK↓, PI3K/AKT↓ | (67) |
| | Na et al,
2012 | Rat
chondrocytes | 50 and 100 µM | Caspase-3↓, Bax↓,
Bcl-xL↑, apoptosis↓ | (70) |
| | Kim et al,
2012 | Rat
chondrocytes | 100 µM | ROS↓, NO↓, iNOS↓,
collagen II↑, SOX9↑, MMP-1↓, MMP-13↓ | (71) |
| | Chen et al,
2016 | ACLT+MMx rat
models; C5.18 cells | 300 µM; 100
µg/ml | IL-1β↓,
histological improvement, MMP-13↓, collagen X↓ | (86) |
| Rg1 | Cheng et al,
2017 | Human OA
chondrocytes | 0.1, 1 and l0
µg/ml | MMP-13↓, COX-2↓,
PGE2↓, collagen II↑, aggrecan↑ | (49) |
| | Cheng et al,
2017 | ACLT-induced rat
OA | 30 and 60
mg/kg | MMP-13↓, collagen
II↑ | (49) |
| | Huang et al,
2014 | Rat
chondrocytes | 10 µg/ml | p-AKT↑, Bcl-2,
Bax↓, Cyto c↓, caspase-3↓, MMP-13↓, TIMP-1↑, PI3K/AKT↑ | (72) |
| | Xu et al,
2022 | Human
chondrocytes | 10, 50 and 100
µg/ml | Bax↓, Bcl-2↑,
caspase-3↓, caspase-8↓, caspase-9↓, Fas ligand↓, apoptosis inducing
factor ↓, cytochrome c↓, ROS↓, malondialdehyde↓ | (73) |
| Rg3 | Ma et al,
2021 | TC28a2 cells | 3 µM | Sirt1↑, peroxisome
proliferator-activated receptor gamma coactivator 1-α↑, Sirt3↑,
acetylated CypD↓, mitochondrial functions↑, apoptosis↓, NF-κB↓,
MAPK↓ | (74) |
| | So et al,
2013 | Human OA
chondrocytes | 1 and 2.5 µM | MMP-1↓, MMP-3↓,
MMP-13↓, collagen II↑, ACAN↑, β-galactosidase↓ | (85) |
| Rg5 | Zhang, 2017 | Rat OA models | 1, 2, 5, 10, 15
mg/kg | MMP-13↓, TIMP-1↓,
collagen II↑, IL-1β↓, TNFα↓, NO↓, iNOS↓, BMP-2↑, TGFβ1↑ | (90) |
| Ro | Zhang et al,
2015 | Rat
chondrocytes | 50, 100 and 200
µM | Bax↓, Bad↓, p-p53↓,
Bcl-xL↑, proliferating cell nuclear antigen ↑, caspase-3↓, COX-2↓,
MMP-3↓, MMP-9↓, p-p65↓ | (62) |
In monoiodoacetate (MIA)-induced OA in
ovariectomized (OVX) rats, Rb1 exhibited inhibitory activity
against inflammatory responses, as indicated by the decreased
expression of IL-1β, IL-6, monocyte chemoattractant protein-1/C-C
motif chemokine 2 and PGE2/COX-2 (Table I) (53). Fibroblast growth factor 18 (FGF18)
plays a critical role in cartilage formation, osteogenesis and bone
development (54). Increased
expression of FGF18 is associated with anabolic activity in
cartilaginous tissues and this may act as a potential target for
the therapeutical management of OA (55). Results of a previous study
demonstrated that Rb1 enhances the expression of FGF18 by sponging
miR-21-5p in MIA-induced OA rats, protecting against OA
development. Overexpression of miR-21-5p abolished the
chondroprotective effects of Rb1 by stimulating inflammatory
responses, decreasing cell viability and attenuating FGF18-mediated
chondroprotection (56) (Table I).
NF-κB signaling plays a crucial role in inflammatory
responses. Activation of the NF-κB signaling pathway includes
phosphorylation of IκB kinases (IKKs), IκB and p65, nuclear
translocation of p65, and transcriptional regulation of target
genes (57) (Fig. 2). Moreover, ginsenoside Rk1 may
ameliorate inflammation by inhibiting LPS-induced phosphorylation
of NF-κB, JAK2 and STAT3-Ser727/-Tyr705 in RAW 264.7 cells
(58). In murine models of sepsis
in vivo and in vitro, ginsenosides exerted inhibitory
activity against inflammation by suppressing NF-κB and MAPK
signaling pathways (59).
Moreover, the expression of NF-κB signaling is activated in primary
human OA chondrocytes (60).
Ginsenoside Ro also exhibits potential as an inhibitor of
inflammation by suppressing NF-κB signaling pathways. Results of a
previous study demonstrated that Ro inactivates the TNFα-induced
NF-κB signaling pathway (61)
(Table I). More specifically, Ro
exhibited inhibitory activity against the IL-1β-induced
upregulation of COX-2, Bax, Bad and caspase-3 expression, the
downregulation of Bcl-xL and proliferating cell nuclear antigen
expression, and phosphorylation of p65 and p53, inhibiting
NF-κB-associated inflammation and chondrocyte apoptosis (62).
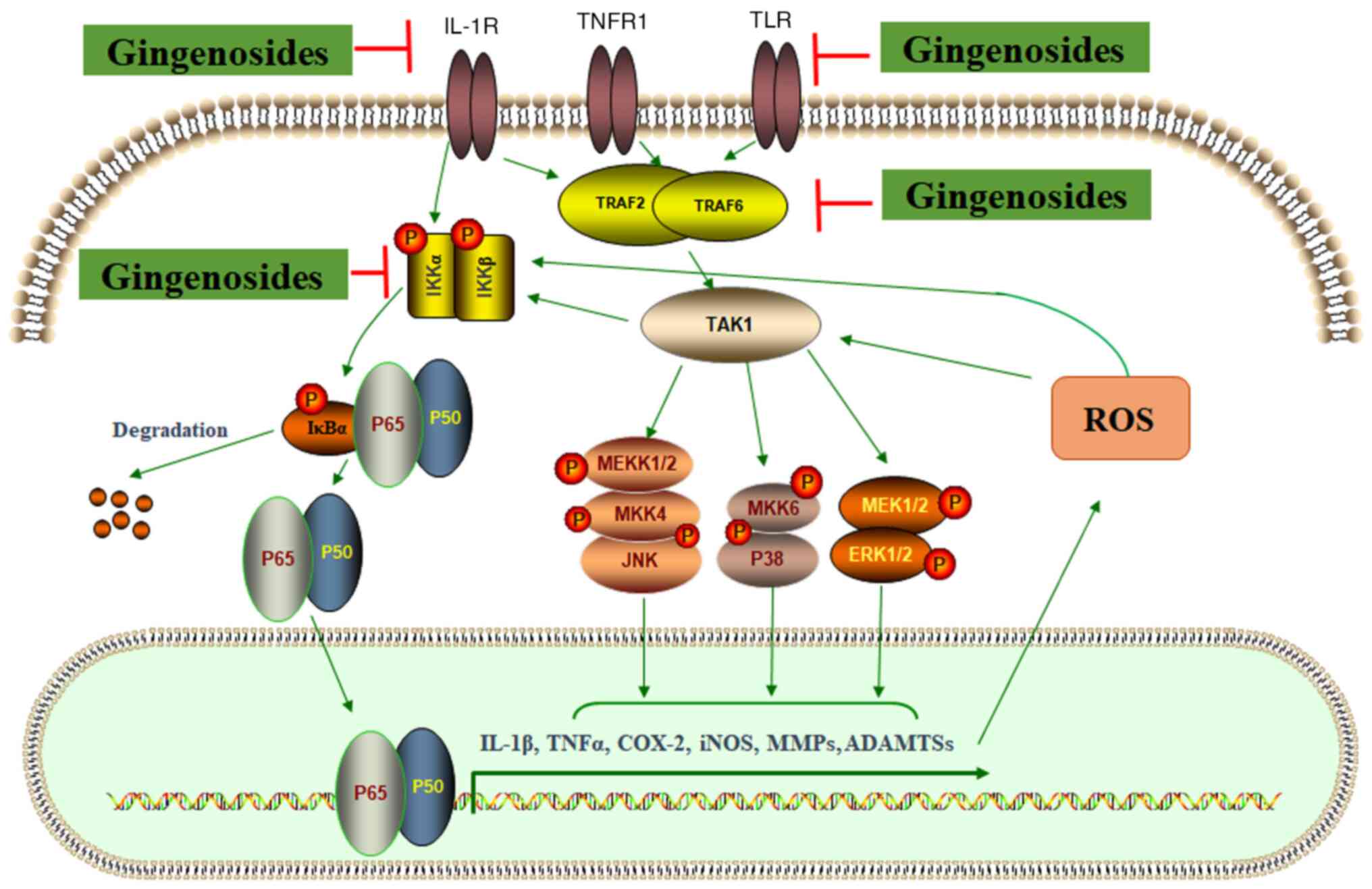 | Figure 2Activation of NF-κB and MAPK
signaling pathways regulates the expression of catabolic factors.
External signals activate TRAF2/TRAF6, and induce the
phosphorylation of MAPK (JNK, p38 MAPK and ERK1/2) by mediating
TAK1. The phosphorylation of IKKα/IKKβ is induced by TAK1 and
external signals. The IKK-induced phosphorylation of IκBα may lead
to its degradation and the release of p65/p50, which enter the
nucleus for the transcriptional regulation of target genes,
including IL-1β, TNFα, COX-2, iNOS, MMPs and ADAMTSs. Ginsenosides
may inhibit NF-κB and MAPK signaling pathways, and suppress
inflammatory responses. TRAF, TNF receptor-associated factor; TAK,
transforming growth factor-β activated kinase; IKK, inhibitor of
IκB kinase; COX, cyclooxygenase; iNOS, inducible isoform of nitric
oxide synthase; ADAMTSs, A disintegrin and metalloproteinase with
thrombospondin motifs; MEKK, mitogen-activated protein kinase
kinase kinase 1. |
MAPKs exhibit an essential role in cell responses to
stimuli, such as inflammatory cytokines. GTPase-induced activation
of MAPK kinase kinases induces phosphorylation of MAPK kinases
which activate p38 MAPK (63)
(Fig. 2). Moreover, P38 MAPK is
activated by IL-1β and TNFα, and suppression of p38 MAPK may lead
to the decreased production of inflammation cytokines (64). P38 MAPK is involved in activation
of the NF-κB signaling pathway. Results of a previous study
demonstrated that ginsenosides exert their therapeutic effects by
targeting p38 MAPK (65). Notably,
Rb1 inhibited 2,4,6-trinitrobenzene sulfuric acid-stimulated COX-2
and iNOS expression, the NF-κB signaling pathway, and LPS-induced
NF-κB and MAPK (p38, ERK1/2 and JNK) pathways (66). In a rabbit OA model, Rb1 inhibited
the activity of NF-κB, p38 MAPK and PI3K/AKT signaling pathways to
inhibit inflammatory responses, ameliorate histopathological
changes and protect rabbit knee articular cartilages (67) (Table
I).
4. Anti-oxidative activity of
ginsenosides
Hydrogen peroxide (H2O2)
exhibits various biological effects by generating ROS, which is
associated with oxidative stress and increased chondrocyte
apoptosis (68). Mechanistically,
H2O2 enhances the permeability of the
mitochondrial membrane and promotes the translocation of cytochrome
c from the mitochondria to the cytoplasm, leading to the initiation
of apoptotic pathways (69).
Results of a previous study demonstrated that Rb1 exhibits
inhibitory activity against H2O2-induced
mitochondrial permeability transition and caspase-3 expression, and
exerted effects on Bcl-xL expression, leading to suppression of
cell apoptosis in rat chondrocytes (70) (Table
I). Similarly, Rb1 treatment ameliorated the decreased
viability caused by H2O2, increased the
production of ROS and NO, and decreased the expression of
chondrogenic genes, including Sox9 and collagen II in rat
chondrocytes (71) (Table I). In IL-1β-treated rat
chondrocytes, Rg1 maintained mitochondrial functions and
ameliorated mitochondrial-mediated apoptosis, demonstrated by the
increased expression of Bcl-2 and the decreased expression of Bax,
cytochrome c and caspase-3. Interestingly, treatment with the PI3K
inhibitor, LY294002, may reverse the protective effects of
Rg1(72).
In IL-1β-treated human OA chondrocytes, Rg1 reduced
the levels of ROS, decreased the production of malondialdehyde
(MDA), improved the mitochondrial membrane potential, upregulated
the expression of Bcl-2, downregulated the expression of Bax,
caspase-3, caspase-9, factor-related apoptosis ligand,
apoptosis-inducing factor and cytochrome c, and inhibited
IL-1β-induced chondrocyte apoptosis by decreasing the
PI3K/AKT-mediated mitochondrial signaling pathway (73). TNFα stimulation may induce the loss
of mitochondrial mass, DNA copy number and the generation of ROS,
decrease the mitochondrial membrane potential and upregulate IL-8
and MMP-9, eliciting chondrocyte apoptosis and ECM degradation
(74). Results of a recent study
demonstrated that Rg3 activates Sirt3/PGC-1α expression and
reversed the effects of TNFα on the acetylation of cyclophilin D
and mitochondrial dysfunction through downregulation of NF-κB and
p38 MAPK signaling pathways (74)
(Table I).
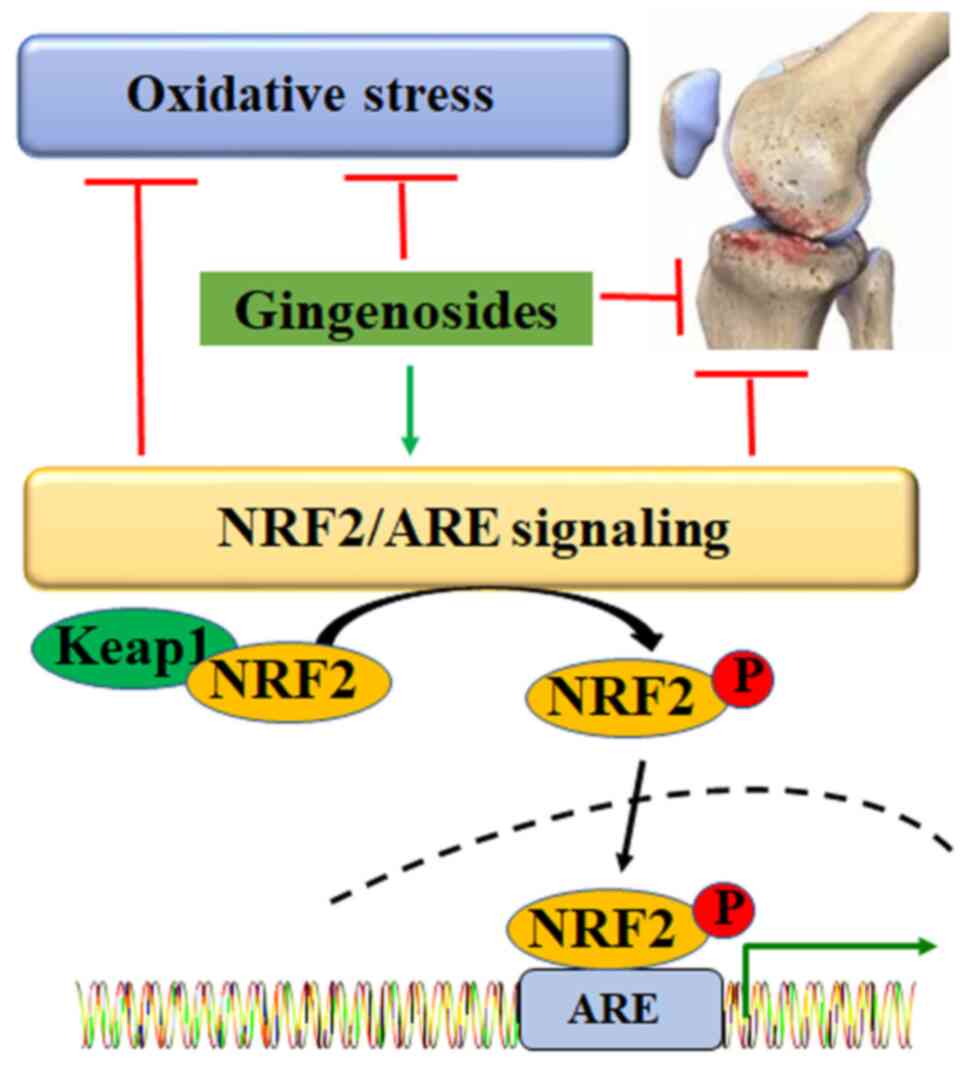
NRF2, a key transcriptional factor in the redox
system, regulates the anti-oxidative defence at multiple levels
(75). Under physiological
conditions, NRF2 is inactivated via interaction with Kelch-like
ECH-associated protein 1. Under oxidative stress, NRF2 is released,
phosphorylated, activated and translocated into the nucleus to bind
with anti-oxidant response elements, mediating the expression of
target genes, such as heme oxygenase 1 (HO-1) (76) (Fig.
3). Results of a previous study demonstrated that Rb1 decreases
the levels of MDA, increases the production of glutathione and
activates the NRF2 signaling pathway (77). Ginsenoside compound K (CK)
exhibited neuroprotective activity by stimulating the NRF2/HO-1
signaling pathway and suppressing oxidative stress (78). In SH-SY5Y cells, Rb1 inhibited the
6-hydroxydopamine-induced expression of caspase-3 by upregulating
the activity of the PI3K/AKT/NRF2 signaling pathway (79). However, further investigations into
the specific effects of ginsenosides on the NRF2 signaling pathway
in chondrocytes are required.
5. Inhibitory activity of ginsenosides
against ECM degradation
ECM in articular cartilage mainly consists of
collagen II and aggrecan. Collagen II is a fibrillar collagen with
triple-helical homotrimers of collagen type II α1 chain (Col2a1),
which form heterotypic fibrils with collagen IX and XI in the
cartilage. Aggrecan is formed by a core protein with the attachment
of ~200 glycosaminoglycan chains (80). MMPs play a critical role in ECM
degradation. Notably, MMP-13 is the key degrading enzyme in
collagen II cleavage (81).
ADAMTSs, namely ADAMTS-4 and ADAMTS-5, degrade aggrecan (82). Several signaling pathways, such as
NF-κB, MAPK and PI3K/AKT, are involved in mediating the
corresponding catabolic activity (27). Inhibition of catabolic enzymes is
considered an effective therapeutic strategy for the clinical
management of OA.
A previous study screened 11 ginseng saponins and
the results demonstrated that these ginseng saponins inhibit the
activity of MMP-13, which degrades the major collagens in rabbit OA
cartilage (83). Ginsenoside Rc,
Rd, Rf, Rg3 and F4 may decrease the expression of MMP-13 in
IL-1β-treated SW1353 cells. Moreover, 10, 30 and 50 µM F4 decreased
the expression of MMP-13 by 33.5, 57.9 and 90.0%, respectively, by
suppressing the MAPK pathway (83). Panax ginseng and the
associated bioactive compounds, ginsenosides Rd and Rb3, decreased
MMP-3 expression and increased collagen II expression in
IL-1β-treated S12 murine cartilage cells by suppressing the
phosphorylation of p38 MAPK but not ERK (84). Results of a previous study
suggested that Rg3 may decrease MMP-1, MMP-13 and β-galactosidase
expression, and increase collagen II and aggrecan production in
IL-1β-treated human OA chondrocytes (Table I) (85).
Results of a previous study demonstrated that Rg1
downregulates the expression of MMP-13 and upregulates the
production of aggrecan and collagen II in IL-1β-treated human OA
chondrocytes (49). In
IL-1β-treated rat chondrocytes, Rg1 also suppressed the expression
of MMP-13 and enhanced the expression of tissue inhibitor of matrix
metalloproteinase-1 (TIMP-1) by mediating the PI3K/AKT signaling
pathway (72). In anterior
cruciate ligament transection-induced rat OA models, Rg1 exhibited
protective activity against cartilage erosion by decreasing MMP-13
expression and increasing collagen II production (49). Moreover, Rb1 reduced MMP-13 mRNA
expression and enhanced aggrecan and Col2a1 mRNA expression in
IL-1β-treated human OA chondrocytes (Table I) (51). In addition, Rb1 increased the
expression of BMP2 and collagen II, decreased the expression of
MMP-13 and ameliorated the histopathological changes in MIA-induced
OA in OVX rats (53). Results of a
previous study demonstrated that Rb1 may attenuate IL-1β-induced
MMP-13 and collagen type X (ColX) expression in C5.18 cells
(86). In anterior cruciate
ligament transection and medial meniscus resection-induced OA rat
models, Rb1 ameliorated cartilage degeneration and histological
damage scores, and decreased the percentage of chondrocytes with
positive MMP-13 and ColX staining; thus, inhibiting the progression
of arthritis (86) (Table I).
In IL-1β-treated rat chondrocytes, Ro may decrease
the expression of MMP-3 and MMP-9 by inhibiting the activity of the
NF-κB signaling pathway (62).
Moreover, Rb1 downregulated the expression of MMPs, inhibited
chondrocyte apoptosis and protected knee articular cartilage by
inhibiting NF-κB, p38 MAPK and PI3K/AKT signaling pathways in
rabbit OA models (67). Results of
a recent study demonstrated the inhibition of MMPs and
anti-inflammatory activity of Rg1 in various tissues (87). Rg1 synergistically increased MMP
inhibition in combination with other drugs, such as Timosaponin
AIII in MG63 and U2OS cells (88).
TIMP-1 is an inhibitor of MMPs and is associated with the
inhibition of ECM degradation and chondrocyte apoptosis in OA
cartilage (89). Results of a
previous study demonstrated that ginsenoside Rg5 exhibits
protective activity against OA development by decreasing the
expression of MMP-13 by 45% and increasing the expression of TIMP-1
by 67% in OA rat knee cartilages. In addition, following treatment
with Rg5, the production of collagen II and proteoglycan were
enhanced, the expression levels of IL-1β, TNFα and NO/iNOS were
decreased, and the apoptotic ratio of OA chondrocytes was decreased
(90) (Table I).
The Notch signaling pathway mediates cell-to-cell
interactions and determines cell fate. It consists of Notch 1-4
receptors and five ligands [jagged1, jagged2, delta-like 1 (DLL1),
DLL3 and DLL4] (Fig. 4) (91). Of note, Notch signaling is involved
in the pathophysiological alterations of OA. Expression levels of
ligand jagged1 and Notch1 are upregulated in OA chondrocytes, and
inflammatory cytokines, such as IL-1β and TNFα may increase Notch
signaling (92). Similar to the
effects of Notch inhibitor γ-secretase inhibitor
N-[N-(3,5difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl
ester, results of a previous study demonstrated that Rb1 inhibits
the expression of MMP-13, collagen II, Notch1 and jagged1 in
experimental OA rats and IL-1β-treated SW1353 cells, protecting
against cartilage lesions (93).
In addition, H2O2 is associated with the
inhibition of proteoglycan synthesis and degradation of aggrecan in
articular cartilage, leading to ECM degradation and cartilage
erosion (94). In
H2O2-treated rat chondrocytes, Rb1 reversed
the increased expression of MMP-1 and MMP-13, and the decreased
expression of collagen II (71)
(Table I).
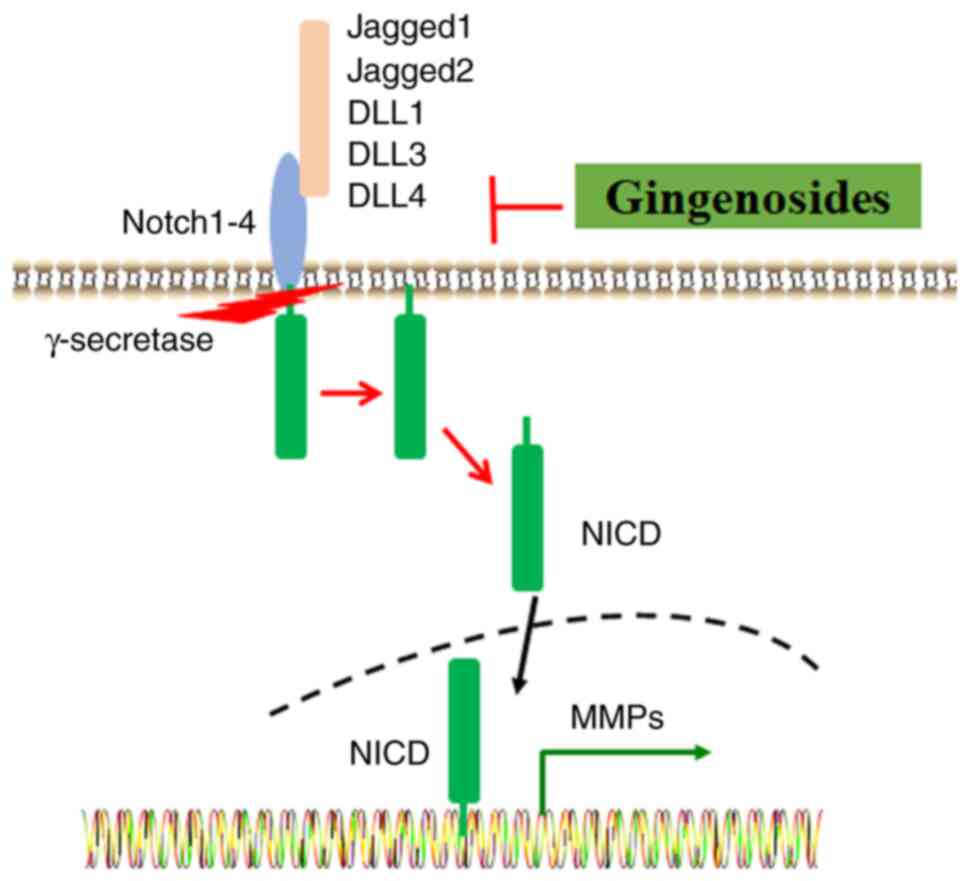 | Figure 4Ginsenosides protect against ECM
degradation through the inhibition of Notch signaling. Ligands,
such as Jagged1, Jagged2, DLL1, DLL3 and DLL4, bind to the Notch1-4
receptors. A series of proteolytic events are activated by
γ-secretase, and these may induce the release of NCID, which enters
the nucleus and regulates the expression of target genes, such as
MMPs. ECM, extracellular matrix; DLL, delta-like 1; NCID, Notch
intracellular domain. |
6. Pharmacokinetic properties of
ginsenosides
Rb1 is a hydrophile and the most abundant
ginsenoside in ginseng. In addition, Rb1 is the parent compound of
less hydrophilic ginsenosides, such as Rd, Rg3, Rg5, Rk1, F2 and CK
(95). Notably, gut microbiota
carries out hydrolysis of hydrophilic ginsenosides via
deglycosylation, to convert them into hydrophobic ginsenosides
(96). This conversion may be
associated with increased bioavailability. A recent study aimed to
determine the safety of red ginseng extract via oral administration
in 13 healthy Korean male participants, and the results
demonstrated that there are no associated adverse events. In
addition, the bioconverted red ginseng extract possesses a higher
maximum plasma concentration, area under curve
(AUC)(0-t) and AUC(0-∞), and a shorter time
to maximum plasma concentration following oral administration,
compared with those of the red ginseng extract (97). Rd is distributed to various organs
and oxidation and glycosylation are the main metabolic pathways of
Rd in rats. Results of a previous study demonstrated that the
absolute bioavailability of Rd is 0.26% in dogs (98). The recommended intravenous
administration dose range of Rd is 10-75 mg and this range was
generally well tolerated in clinical trials (99). The main pharmacokinetic parameters
of Re have been comprehensively discussed (100) and Re exhibits a poor
bioavailability of ~0.24% (101).
Similarly, following oral administration of 50 mg/kg Rb1, Rb2 and
Rb3 in rats, the AUC values were 66.8, 9.7 and 55.1 mg h/l,
respectively, and the Cmax values were 6.1, 0.4
and 3.3 mg/l, respectively. These results may be associated with
poor bioavailability, respectively ~0.78, 0.08 and 0.52% for Rb1,
Rb2 and Rb3 (102,103). 20(S)-Protopanaxadiol [20(S)-PPD]
is a ginsenoside metabolite with full deglycosylation. Notably,
20(S)-PPD exhibits a half-life of 3 h in rats and 10.6-16.7 h in
humans (104). The
bioavailability of 20(S)-PPD following oral administration was 31.0
and 9.6% in rats and dogs, respectively (105). This indicates that
deglycosylation significantly enhances bioavailability. Results of
a previous study demonstrated that the absolute bioavailability of
PDD in rats is 36.8%, which is ~10 times higher than that of Rg3
and Rh2(106).
The majority of ginsenosides are characterized by a
lipophilic steroid skeleton, a low oral absorption rate, rapid
clearance and a short half-life, with low levels of bioavailability
at <5% (107). Interestingly,
novel drug delivery systems may provide improved solubility, oral
absorption rates and bioavailability. A series of nano delivery
systems, such as liposomes, polymeric nanoparticles (NPs),
micelles, microemulsions, metal and inorganic NPs, biomimetic NPs,
and protein NPs, have been developed to improve efficiency and
reduce associated adverse effects, such as hypertension, insomnia,
anxiety, diarrhea and vomiting (107,108). Notably, ginsenoside CK prepared
with polymer micelles exhibits good biodegradability and
biocompatibility, with antitumor effects stronger than those of
free CK (109). Folic acid (FA)
is considered an optimal targeting moiety that is used for
antitumor drug delivery. Results of a previous study demonstrated
that Rg5 released from FA-modified bovine serum albumin NPs may
accumulate at the tumor site within 8 h, improving the therapeutic
efficacy and tumor targetability (110). To improve levels of
bioavailability, polymeric nano-capsules have been employed to
encapsulate Rb1 to become nano-Rb1, which significantly inhibited
the activity of NF-κB and NLRP3 inflammasomes (111). The conjugation of
superparamagnetic iron oxide nanoparticles (SPIONs) with Rg3 has
been developed. Results of a previous study demonstrated that
SPION-Rg3 exhibits increased anti-oxidative and anti-inflammatory
activities in RAW 264.7 cells (112). A variety of ginsenoside
nanodrugs, such as Doxil, Onivyde and Vyxeos, were developed with
different physiochemical properties, leading to differences in
pharmacokinetics, biodistribution, efficacy and safety (113).
7. Clinical perspectives
Results of a recent study demonstrated the effective
use of TCM in the management of OA (114). Huoxuezhitong capsule (HXZT), a
compound derived from Angelica sinensis (Oliv.)
Diels, Panax notoginseng (Burkill) F. H. Chen ex C. H.,
Boswellia sacra, Borneol, Eupolyphaga sinensis
Walker and Pyritum, demonstrated efficacy against OA (115). Ginsenosides Rg1 and Rb1, and
Noto-ginsenoside R1, are the main effective compounds of HXZT,
which inhibited inflammatory responses by inhibiting NF-κB and
PI3K/AKT signaling pathways in LPS-treated RAW264.7 and ATDC5
cells, and in MIA-induced rat OA models (115). However, the limited water
solubility of ginsenosides may impact the potential clinical
applications.
Ginsenosides are not directly absorbed with intact
structures in vivo and deglycosylation by intestinal
bacteria or gastric acid is required before absorption in the
intestinal tract. Ginsenoside CK, the main metabolite of
ginsenosides, exhibits a pharmacological activity higher than that
of ginsenosides (116). However,
to the best of our knowledge, there are no CK preparations that are
available for use in patients, despite advances in TCM development.
At present, CK capsules are undergoing clinical trials for the
treatment of rheumatoid arthritis (117). In LPS-treated RAW 264.7 cells, CK
decreased the levels of NO/iNOS and PGE2/COX-2, and exhibited
inhibitory activity against inflammation by suppressing the NF-κB
and MAPK signaling pathways (118). Consistently, a previous study
demonstrated that CK can suppress the NF-κB pathway by inhibiting
IKK in H2O2-treated MC3T3-E1 cells (119).
Glucocorticoids (GCs) have been extensively used for
the treatment of inflammation and immune disorders (120). However, the use of GCs in
clinical practice is complex due to reduced sensitivity and the
potential resistance to GCs. Moreover, the acquired resistance to
GCs may lead to the abnormal upregulation of inflammatory
transcriptional factors, such as activator protein 1, to interrupt
the competitive binding of GC receptor (GR) to DNA (121). In clinic practice, GCs are
frequently used in the treatment and improvement of OA; however,
the recommendations for treatment with GCs have not been updated
since 2013(122). This may be due
to associated negative outcomes, such as accelerated progression of
OA and increased joint destruction (123). Notably, the results of a previous
study demonstrated that Rh1 inhibited GC-induced downregulation of
GR expression and DNA binding in RAW 264.7 cells (124). Mechanistically, a combination of
Rh1 with dexamethasone (DEX) may inhibit the phosphorylation of
IκBα and p65, and the nuclear translocation of p65; thus,
inhibiting the NF-κB signaling pathway. Combined with DEX, Rh1
enhanced the expression of Dual specificity phosphatase 1, which
specifically blocks the phosphorylation and activation of the MAPK
family, including p38, MAPK, ERK1/2 and JNK (124,125).
Poor levels of bioavailability and the metabolites
of ginsenosides may impact the corresponding clinical applications.
Therefore, the development of strategies to increase structural
stability and enhance absorption in the gastrointestinal tract is
required. Moreover, research should focus on effective delivery
systems within the human body. Results of previous studies
demonstrated the therapeutic effects of ginsenosides both in
vivo and in vitro; however, further studies into the
absorption, distribution, metabolism, excretion and toxicity of
ginsenosides in humans are required. Notably, animal models have
been used for evaluating the safety of potential drugs for the
treatment of OA. However, there are numerous differences between
animals and humans, and toxicological responses in animals may not
be applicable in humans. Moreover, additional clinical trials are
required to assess the safety and associated adverse events of
ginsenosides, to determine levels of toxicity and facilitate their
use in the clinic.
8. Conclusions
OA is characterized by low-grade chronic
inflammation and its pathological development is orchestrated by a
complex network of signaling pathways. Further understanding of the
molecular mechanisms underlying OA is essential for the development
of novel drugs. TCM and associated bioactive compounds may exhibit
potential in drug screening. Results of previous studies have
demonstrated the anti-inflammatory activity of ginseng and
ginsenosides in OA development (67). Mechanistically, ginsenosides may
inhibit inflammation and oxidative stress, and suppress ECM
degradation by targeting NF-κB and MAPK signaling pathways.
Numerous strategies, including nanotechnologies, have been
developed to improve bioavailability. However, further clinical
trials are required to determine the potential pharmacological
effects of ginsenosides. The pathological development of OA is
multifactorial and the present literature review focused on
inflammatory responses, oxidative stress and ECM degradation. The
present review exhibits numerous limitations; for example, aging,
metabolic diseases and drug-drug interactions were not discussed.
Moreover, a combination of ginsenosides with other drugs may
exhibit potential in the management of OA and ginsenosides may also
be used as carriers to deliver other drugs. Thus, further
investigations into the synergic pharmacology between ginsenosides
and other drugs are required.
Acknowledgements
Not applicable.
Funding
Funding: The present study was financially supported by the
Science and Technology Research Project of the Education Department
of Jiangxi Province (grant no. GJJ2201446) and Ganzhou United
Science and Technology Program (grant no. 2022-YB1495).
Availability of data and materials
Not applicable.
Authors' contributions
XL was responsible for conceptualization and
methodology. JC, LH and XL were responsible for data curation,
writing the final article, draft preparation, data curation, data
authentication, validation, reviewing and editing. Data
authentication is not applicable. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu SY, Lin CH, Chang NJ, Hu WL, Hung YC,
Tsao Y and Kuo CA: Combined effect of laser acupuncture and
electroacupuncture in knee osteoarthritis patients: A protocol for
a randomized controlled trial. Medicine (Baltimore).
99(e19541)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang W, Ouyang H, Dass CR and Xu J:
Current research on pharmacologic and regenerative therapies for
osteoarthritis. Bone Res. 4(15040)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jones IA, Togashi R, Wilson ML, Heckmann N
and Vangsness CT Jr: Intra-articular treatment options for knee
osteoarthritis. Nat Rev Rheumatol. 15:77–90. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Charlesworth J, Fitzpatrick J, Perera NKP
and Orchard J: Osteoarthritis-a systematic review of long-term
safety implications for osteoarthritis of the knee. BMC
Musculoskelet Disord. 20(151)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang J, Li J, Song D, Ni J, Ding M, Huang
J and Yan M: AMPK: Implications in osteoarthritis and therapeutic
targets. Am J Transl Res. 12:7670–7681. 2020.PubMed/NCBI
|
|
6
|
Dilley JE, Bello MA, Roman N, McKinley T
and Sankar U: Post-traumatic osteoarthritis: A review of pathogenic
mechanisms and novel targets for mitigation. Bone Rep.
18(101658)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Goldring MB and Marcu KB: Cartilage
homeostasis in health and rheumatic diseases. Arthritis Res Ther.
11(224)2009.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Jiang J, Feng S, Li Z, Luo Y, Wang Z, Li M
and Wu G: The expression of MDM2 gene promoted chondrocyte
proliferation in rats with osteoarthritis via the Wnt/β-catenin
pathway. Cell Mol Biol (Noisy-le-grand). 67:236–241.
2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang Y, Zhao H, Jia S, Wang Q, Yao W, Yang
Y and Bai L: Senomorphic agent pterostilbene ameliorates
osteoarthritis through the PI3K/AKT/NF-κB axis: An in vitro and in
vivo study. Am J Transl Res. 14:5243–5262. 2022.PubMed/NCBI
|
|
10
|
Qiu J, Jiang T, Yang G, Gong Y, Zhang W,
Zheng X, Hong Z and Chen H: Neratinib exerts dual effects on
cartilage degradation and osteoclast production in Osteoarthritis
by inhibiting the activation of the MAPK/NF-κB signaling pathways.
Biochem Pharmacol. 205(115155)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Minguzzi M, Panichi V, D'Adamo S, Cetrullo
S, Cattini L, Flamigni F, Mariani E and Borzì RM: Pleiotropic roles
of NOTCH1 signaling in the loss of maturational arrest of human
osteoarthritic chondrocytes. Int J Mol Sci.
22(12012)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang M, Jiang L, Wang Q, Chen H and Xu G:
Traditional Chinese medicine for knee osteoarthritis: An overview
of systematic review. PLoS One. 12(e0189884)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim JH: Pharmacological and medical
applications of Panax ginseng and ginsenosides: A review for
use in cardiovascular diseases. J Ginseng Res. 42:264–269.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kang OJ and Kim JS: Comparison of
ginsenoside contents in different parts of Korean ginseng (Panax
ginseng C.A. Meyer). Prev Nutr Food Sci. 21:389–392.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fan M, Lan X, Wang Q, Shan M, Fang X,
Zhang Y, Wu D, Luo H, Gao W and Zhu D: Renal function protection
and the mechanism of ginsenosides: Current progress and future
perspectives. Front Pharmacol. 14(1070738)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhao T, Wang X, Liu Q, Yang T, Qu H and
Zhou H: Ginsenoside Rd promotes cardiac repair after myocardial
infarction by modulating monocytes/macrophages subsets conversion.
Drug Des Devel Ther. 16:2767–2782. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou AF, Zhu K, Pu PM, Li ZY, Zhang YY,
Shu B, Cui XJ, Yao M and Wang YJ: Neuroprotective effect and
possible mechanisms of ginsenoside-Rd for cerebral
ischemia/reperfusion damage in experimental animal: A meta-analysis
and systematic review. Oxid Med Cell Longev.
2022(7650438)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu Y, Liu N, Liu Y, He H, Luo Z, Liu W,
Song N and Ju M: Ginsenoside Rb1 reduces D-GalN/LPS-induced acute
liver injury by regulating TLR4/NF-κB signaling and NLRP3
inflammasome. J Clin Transl Hepatol. 10:474–485. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xue X, Liu Y, Qu L, Fan C, Ma X, Ouyang P
and Fan D: Ginsenoside Rh3 inhibits lung cancer metastasis by
targeting extracellular signal-regulated kinase: A network
pharmacology study. Pharmaceuticals (Basel). 15(758)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Song B, Ding L, Zhang H, Chu Y, Chang Z,
Yu Y, Guo D, Zhang S and Liu X: Ginsenoside Rb1 increases insulin
sensitivity through suppressing 11β-hydroxysteroid dehydrogenase
type I. Am J Transl Res. 9:1049–1057. 2017.PubMed/NCBI
|
|
21
|
Song M, Cui Y, Wang Q, Zhang X, Zhang J,
Liu M and Li Y: Ginsenoside Rg3 alleviates aluminum
chloride-induced bone impairment in rats by activating the
TGF-β1/Smad signaling pathway. J Agric Food Chem. 69:12634–12644.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu HL, Chen GH, Wu YT, Xie LP, Tan ZB, Liu
B, Fan HJ, Chen HM, Huang GQ, Liu M and Zhou YC: Ginsenoside Ro, an
oleanolic saponin of Panax ginseng, exerts an
anti-inflammatory effect by direct inhibiting toll-like receptor 4
signaling pathway. J Ginseng Res. 46:156–166. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wu Z, Yang Z, Liu L and Xiao Y: Natural
compounds protect against the pathogenesis of osteoarthritis by
mediating the NRF2/ARE signaling. Front Pharmacol.
14(1188215)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Scanzello CR and Goldring SR: The role of
synovitis in osteoarthritis pathogenesis. Bone. 51:249–257.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Di Nicola V: Degenerative osteoarthritis a
reversible chronic disease. Regen Ther. 15:149–160. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014(561459)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chow YY and Chin KY: The role of
inflammation in the pathogenesis of osteoarthritis. Mediators
Inflamm. 2020(8293921)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zahan OM, Serban O, Gherman C and Fodor D:
The evaluation of oxidative stress in osteoarthritis. Med Pharm
Rep. 93:12–22. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang H, Liao R, Tang W, Su W, Zeng M, Yang
J, Fan X, Xie J and Hu Y: Dietary inflammation index and
osteoarthritis in the elderly: Is there a mediating role of
physical activity? Br J Nutr. 128:2258–2266. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zarezadeh M, Mahmoudinezhad M, Hosseini B,
Khorraminezhad L, Razaghi M, Alvandi E and Saedisomeolia A: Dietary
pattern in autism increases the need for probiotic supplementation:
A comprehensive narrative and systematic review on oxidative stress
hypothesis. Clin Nutr. 42:1330–1358. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ott M, Gogvadze V, Orrenius S and
Zhivotovsky B: Mitochondria, oxidative stress and cell death.
Apoptosis. 12:913–922. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sadasivam N, Kim YJ, Radhakrishnan K and
Kim DK: Oxidative stress, genomic integrity, and liver diseases.
Molecules. 27(3159)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Park J, Lee J and Choi C: Mitochondrial
network determines intracellular ROS dynamics and sensitivity to
oxidative stress through switching inter-mitochondrial messengers.
PLoS One. 6(e23211)2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Youle RJ and Karbowski M: Mitochondrial
fission in apoptosis. Nat Rev Mol Cell Biol. 6:657–663.
2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sirše M: Effect of dietary polyphenols on
osteoarthritis-molecular mechanisms. Life (Basel).
12(436)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Baatar D, Siddiqi MZ, Im WT, Ul Khaliq N
and Hwang SG: Anti-inflammatory effect of ginsenoside
Rh2-Mix on lipopolysaccharide-stimulated RAW 264.7
murine macrophage cells. J Med Food. 21:951–960. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lee JH, Shehzad O, Ko SK, Kim YS and Kim
HP: Matrix metalloproteinase-13 downregulation and potential
cartilage protective action of the Korean red ginseng preparation.
J Ginseng Res. 39:54–60. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jhun JY, Na HS, Shin JW, Jung KA, Seo HB,
Ryu JY, Choi JW, Moon SJ, Park HJ, Oh SW, et al: Notoginseng Radix
and Rehmanniae Radix Preparata extract combination (YH23537)
reduces pain and cartilage degeneration in rats with monosodium
iodoacetate-induced osteoarthritis. J Med Food. 21:745–754.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xie JJ, Chen J, Guo SK, Gu YT, Yan YZ, Guo
WJ, Yao CL, Jin MY, Xie CL, Wang X, et al: Panax quinquefolium
saponin inhibits endoplasmic reticulum stress-induced apoptosis and
the associated inflammatory response in chondrocytes and attenuates
the progression of osteoarthritis in rat. Biomed Pharmacother.
97:886–894. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang Y, Cai W, Han G, Zhou S, Li J, Chen
M and Li H: Panax notoginseng saponins prevent senescence and
inhibit apoptosis by regulating the PI3K-AKT-mTOR pathway in
osteoarthritic chondrocytes. Int J Mol Med. 45:1225–1236.
2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Seo SK, Hong Y, Yun BH, Chon SJ, Jung YS,
Park JH, Cho S, Choi YS and Lee BS: Antioxidative effects of Korean
red ginseng in postmenopausal women: A double-blind randomized
controlled trial. J Ethnopharmacol. 154:753–757. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kim HI, Chon SJ, Seon KE, Seo SK and Choi
YR: Clinical effects of Korean red ginseng in postmenopausal women
with hand osteoarthritis: A double-blind, randomized controlled
trial. Front Pharmacol. 12(745568)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhu DC, Wang YH, Lin JH, Miao ZM, Xu JJ
and Wu YS: Maltol inhibits the progression of osteoarthritis via
the nuclear factor-erythroid 2-related factor-2/heme oxygenase-1
signal pathway in vitro and in vivo. Food Funct. 12:1327–1337.
2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lu H, Fu C, Kong S, Wang X, Sun L, Lin Z,
Luo P and Jin H: Maltol prevents the progression of osteoarthritis
by targeting PI3K/Akt/NF-κB pathway: In vitro and in vivo studies.
J Cell Mol Med. 25:499–509. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yang SM, Ka SM, Hua KF, Wu TH, Chuang YP,
Lin YW, Yang FL, Wu SH, Yang SS, Lin SH, et al: Antroquinonol
mitigates an accelerated and progressive IgA nephropathy model in
mice by activating the Nrf2 pathway and inhibiting T cells and
NLRP3 inflammasome. Free Radic Biol Med. 61:285–297.
2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yi YS: Roles of ginsenosides in
inflammasome activation. J Ginseng Res. 43:172–178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kim J, Ahn H, Han BC, Lee SH, Cho YW, Kim
CH, Hong EJ, An BS, Jeung EB and Lee GS: Korean red ginseng
extracts inhibit NLRP3 and AIM2 inflammasome activation. Immunol
Lett. 158:143–150. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Gao Y, Li J, Wang J, Li X, Li J, Chu S, Li
L, Chen N and Zhang L: Ginsenoside Rg1 prevent and treat
inflammatory diseases: A review. Int Immunopharmacol.
87(106805)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Cheng W, Jing J, Wang Z, Wu D and Huang Y:
Chondroprotective effects of ginsenoside Rg1 in human
osteoarthritis chondrocytes and a rat model of anterior cruciate
ligament transection. Nutrients. 9(263)2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Cho JY, Yoo ES, Baik KU, Park MH and Han
BH: In vitro inhibitory effect of protopanaxadiol ginsenosides on
tumor necrosis factor (TNF)-alpha production and its modulation by
known TNF-alpha antagonists. Planta Med. 67:213–218.
2001.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Cheng W, Wu D, Zuo Q, Wang Z and Fan W:
Ginsenoside Rb1 prevents interleukin-1 beta induced inflammation
and apoptosis in human articular chondrocytes. Int Orthop.
37:2065–2070. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kim MK, Kang H, Baek CW, Jung YH, Woo YC,
Choi GJ, Shin HY and Kim KS: Antinociceptive and anti-inflammatory
effects of ginsenoside Rf in a rat model of incisional pain. J
Ginseng Res. 42:183–191. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Aravinthan A, Hossain MA, Kim B, Kang CW,
Kim NS, Hwang KC and Kim JH: Ginsenoside Rb1 inhibits
monoiodoacetate-induced osteoarthritis in postmenopausal rats
through prevention of cartilage degradation. J Ginseng Res.
45:287–294. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hung IH, Schoenwolf GC, Lewandoski M and
Ornitz DM: A combined series of Fgf9 and Fgf18 mutant alleles
identifies unique and redundant roles in skeletal development. Dev
Biol. 411:72–84. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ellsworth JL, Berry J, Bukowski T, Claus
J, Feldhaus A, Holderman S, Holdren MS, Lum KD, Moore EE, Raymond
F, et al: Fibroblast growth factor-18 is a trophic factor for
mature chondrocytes and their progenitors. Osteoarthritis
Cartilage. 10:308–320. 2002.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Luan J, Che G, Man G and Xiao F:
Ginsenoside Rb1 from Panax ginseng attenuates
monoiodoacetate-induced osteoarthritis by inhibiting
miR-21-5p/FGF18-mediated inflammation. J Food Biochem.
46(e14340)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Yu Q, Zeng KW, Ma XL, Jiang Y, Tu PF and
Wang XM: Ginsenoside Rk1 suppresses pro-inflammatory responses in
lipopolysaccharide-stimulated RAW264.7 cells by inhibiting the
Jak2/Stat3 pathway. Chin J Nat Med. 15:751–757. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Saba E, Jeong D, Irfan M, Lee YY, Park SJ,
Park CK and Rhee MH: Anti-inflammatory activity of Rg3-enriched
korean red ginseng extract in murine model of sepsis. Evid Based
Complement Alternat Med. 2018(6874692)2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Olivotto E, Borzi RM, Vitellozzi R, Pagani
S, Facchini A, Battistelli M, Penzo M, Li X, Flamigni F, Li J, et
al: Differential requirements for IKKalpha and IKKbeta in the
differentiation of primary human osteoarthritic chondrocytes.
Arthritis Rheum. 58:227–239. 2008.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Xing L, Jiang M, Dong L, Gao J, Hou Y, Bai
G and Luo G: Cardioprotective effects of the YiQiFuMai injection
and isolated compounds on attenuating chronic heart failure via
NF-κB inactivation and cytokine suppression. J Ethnopharmacol.
148:239–245. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhang XH, Xu XX and Xu T: Ginsenoside Ro
suppresses interleukin-1β-induced apoptosis and inflammation in rat
chondrocytes by inhibiting NF-κB. Chin J Nat Med. 13:283–289.
2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Braicu C, Buse M, Busuioc C, Drula R,
Gulei D, Raduly L, Rusu A, Irimie A, Atanasov AG, Slaby O, et al: A
comprehensive review on MAPK: A promising therapeutic target in
cancer. Cancers (Basel). 11(1618)2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Chen Y, Shou K, Gong C, Yang H, Yang Y and
Bao T: Anti-inflammatory effect of geniposide on osteoarthritis by
suppressing the activation of p38 MAPK signaling pathway. Biomed
Res Int. 2018(8384576)2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Arafa EA, Refaey MS, Abd El-Ghafar OAM,
Hassanein EHM and Sayed AM: The promising therapeutic potentials of
ginsenosides mediated through p38 MAPK signaling inhibition.
Heliyon. 7(e08354)2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Joh EH, Lee IA, Jung IH and Kim DH:
Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1
activation-the key step of inflammation. Biochem Pharmacol.
82:278–286. 2011.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Hossain MA, Alam MJ, Kim B, Kang CW and
Kim JH: Ginsenoside-Rb1 prevents bone cartilage destruction through
down-regulation of p-Akt, p-P38, and p-P65 signaling in rabbit.
Phytomedicine. 100(154039)2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Cui T, Lan Y, Lu Y, Yu F, Lin S, Fu Y, Qiu
J and Niu G: Isoorientin ameliorates
H2O2-induced apoptosis and oxidative stress
in chondrocytes by regulating MAPK and PI3K/Akt pathways. Aging
(Albany NY). 15:4861–4874. 2023.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Nuttall ME, Nadeau DP, Fisher PW, Wang F,
Keller PM, DeWolf WE Jr, Goldring MB, Badger AM, Lee D, Levy MA, et
al: Inhibition of caspase-3-like activity prevents apoptosis while
retaining functionality of human chondrocytes in vitro. J Orthop
Res. 18:356–363. 2000.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Na JY, Kim S, Song K, Lim KH, Shin GW, Kim
JH, Kim B, Kwon YB and Kwon J: Anti-apoptotic activity of
ginsenoside Rb1 in hydrogen peroxide-treated chondrocytes:
Stabilization of mitochondria and the inhibition of caspase-3. J
Ginseng Res. 36:242–247. 2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Kim S, Na JY, Song KB, Choi DS, Kim JH,
Kwon YB and Kwon J: Protective effect of ginsenoside rb1 on
hydrogen peroxide-induced oxidative stress in rat articular
chondrocytes. J Ginseng Res. 36:161–168. 2012.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Huang Y, Wu D and Fan W: Protection of
ginsenoside Rg1 on chondrocyte from IL-1β-induced
mitochondria-activated apoptosis through PI3K/Akt signaling. Mol
Cell Biochem. 392:249–257. 2014.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Xu Z, Li X, Shen G, Zou Y, Zhang H, Yang K
and Zhu Y: The protective effect of ginsenoside Rg1 on apoptosis in
human ankle joint traumatic arthritis chondrocytes. Evid Based
Complement Alternat Med. 2022(6798377)2022.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Ma CH, Chou WC, Wu CH, Jou IM, Tu YK,
Hsieh PL and Tsai KL: Ginsenoside Rg3 attenuates TNF-α-induced
damage in chondrocytes through regulating SIRT1-mediated
anti-apoptotic and anti-inflammatory mechanisms. Antioxidants
(Basel). 10(1972)2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Zhang J, Xu HX, Zhu JQ, Dou YX, Xian YF
and Lin ZX: Natural Nrf2 inhibitors: A review of their potential
for cancer treatment. Int J Biol Sci. 19:3029–3041. 2023.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Bellezza I, Giambanco I, Minelli A and
Donato R: Nrf2-Keap1 signaling in oxidative and reductive stress.
Biochim Biophys Acta Mol Cell Res. 1865:721–733. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Dong C, Liu P, Wang H, Dong M, Li G and Li
Y: Ginsenoside Rb1 attenuates diabetic retinopathy in
streptozotocin-induced diabetic rats1. Acta Cir Bras.
34(e201900201)2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Yang Q, Lin J, Zhang H, Liu Y, Kan M, Xiu
Z, Chen X, Lan X, Li X, Shi X, et al: Ginsenoside compound K
regulates amyloid β via the Nrf2/Keap1 signaling pathway in mice
with scopolamine hydrobromide-induced memory impairments. J Mol
Neurosci. 67:62–71. 2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Hwang YP and Jeong HG: Ginsenoside Rb1
protects against 6-hydroxydopamine-induced oxidative stress by
increasing heme oxygenase-1 expression through an estrogen
receptor-related PI3K/Akt/Nrf2-dependent pathway in human
dopaminergic cells. Toxicol Appl Pharmacol. 242:18–28.
2010.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Vincent TL, McClurg O and Troeberg L: The
extracellular matrix of articular cartilage controls the
bioavailability of pericellular matrix-bound growth factors to
drive tissue homeostasis and repair. Int J Mol Sci.
23(6003)2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Hu Q and Ecker M: Overview of MMP-13 as a
promising target for the treatment of osteoarthritis. Int J Mol
Sci. 22(1742)2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Yang CY, Chanalaris A and Troeberg L:
ADAMTS and ADAM metalloproteinases in osteoarthritis-looking beyond
the ‘usual suspects’. Osteoarthritis Cartilage. 25:1000–1009.
2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Lee JH, Lim H, Shehzad O, Kim YS and Kim
HP: Ginsenosides from Korean red ginseng inhibit matrix
metalloproteinase-13 expression in articular chondrocytes and
prevent cartilage degradation. Eur J Pharmacol. 724:145–151.
2014.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Shin JS, Park N, Ra J, Kim Y, Shin M, Hong
M, Kim SH, Kwon HJ, Hong SP, Kim J and Bae H: Panax ginseng C.A.
Meyer modulates the levels of MMP3 in S12 murine articular
cartilage cell line. J Ethnopharmacol. 124:397–403. 2009.PubMed/NCBI View Article : Google Scholar
|
|
85
|
So MW, Lee EJ, Lee HS, Koo BS, Kim YG, Lee
CK and Yoo B: Protective effects of ginsenoside Rg3 on human
osteoarthritic chondrocytes. Mod Rheumatol. 23:104–111.
2013.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Chen Y, Lin S, Sun Y, Pan X, Xiao L, Zou
L, Ho KW and Li G: Translational potential of ginsenoside Rb1 in
managing progression of osteoarthritis. J Orthop Translat. 6:27–33.
2016.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Lee SY: Anti-metastatic and
anti-inflammatory effects of matrix metalloproteinase inhibition by
ginsenosides. Biomedicines. 9(198)2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Lee SY: Ginsenoside Rg1 drives
stimulations of timosaponin AIII-induced anticancer effects in
human osteosarcoma cells. Evid Based Complement Alternat Med.
2020(8980124)2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Young DA, Barter MJ and Wilkinson DJ:
Recent advances in understanding the regulation of
metalloproteinases. F1000Res. 8(F1000 Faculty
Rev-195)2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Zhang P: Ginsenoside-Rg5 treatment
inhibits apoptosis of chondrocytes and degradation of cartilage
matrix in a rat model of osteoarthritis. Oncol Rep. 37:1497–1502.
2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Deshotels L, Safa FM and Saba NS: NOTCH
signaling in mantle cell lymphoma: Biological and clinical
implications. Int J Mol Sci. 24(10280)2023.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Wang H, Tian Y, Wang J, Phillips KLE,
Binch ALA, Dunn S, Cross A, Chiverton N, Zheng Z, Shapiro IM, et
al: Inflammatory cytokines induce NOTCH signaling in nucleus
pulposus cells: Implications in intervertebral disc degeneration. J
Biol Chem. 288:16761–16774. 2013.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Wang W, Zeng L, Wang ZM, Zhang S, Rong XF
and Li RH: Ginsenoside Rb1 inhibits matrix metalloproteinase 13
through down-regulating Notch signaling pathway in osteoarthritis.
Exp Biol Med (Maywood). 240:1614–1621. 2015.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Jallali N, Ridha H, Thrasivoulou C,
Underwood C, Butler PEM and Cowen T: Vulnerability to ROS-induced
cell death in ageing articular cartilage: The role of antioxidant
enzyme activity. Osteoarthritis Cartilage. 13:614–622.
2005.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Kim JK, Choi MS, Jeung W, Ra J, Yoo HH and
Kim DH: Effects of gut microbiota on the pharmacokinetics of
protopanaxadiol ginsenosides Rd, Rg3, F2, and compound K in healthy
volunteers treated orally with red ginseng. J Ginseng Res.
44:611–618. 2020.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Kim DH: Gut microbiota-mediated
pharmacokinetics of ginseng saponins. J Ginseng Res. 42:255–263.
2018.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Kim HJ, Oh TK, Kim YH, Lee J, Moon JM,
Park YS and Sung CM: Pharmacokinetics of ginsenoside Rb1, Rg3, Rk1,
Rg5, F2, and compound K from red ginseng extract in healthy korean
volunteers. Evid Based Complement Alternat Med.
2022(8427519)2022.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Wang W, Wang GJ, Xie HT, Sun JG, Zhao S,
Jiang XL, Li H, Lv H, Xu MJ and Wang R: Determination of
ginsenoside Rd in dog plasma by liquid chromatography-mass
spectrometry after solid-phase extraction and its application in
dog pharmacokinetics studies. J Chromatogr B Analyt Technol Biomed
Life Sci. 852:8–14. 2007.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Zeng X, Deng Y, Feng Y, Liu Y, Yang L,
Huang Y, Sun J, Liang W and Guan Y: Pharmacokinetics and safety of
ginsenoside Rd following a single or multiple intravenous dose in
healthy Chinese volunteers. J Clin Pharmacol. 50:285–292.
2010.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Gao XY, Liu GC, Zhang JX, Wang LH, Xu C,
Yan ZA, Wang A, Su YF, Lee JJ, Piao GC and Yuan HD: Pharmacological
properties of ginsenoside Re. Front Pharmacol.
13(754191)2022.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Joo KM, Lee JH, Jeon HY, Park CW, Hong DK,
Jeong HJ, Lee SJ, Lee SY and Lim KM: Pharmacokinetic study of
ginsenoside Re with pure ginsenoside Re and ginseng berry extracts
in mouse using ultra performance liquid chromatography/mass
spectrometric method. J Pharm Biomed Anal. 51:278–283.
2010.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Zhao J, Su C, Yang C, Liu M, Tang L, Su W
and Liu Z: Determination of ginsenosides Rb1, Rb2, and Rb3 in rat
plasma by a rapid and sensitive liquid chromatography tandem mass
spectrometry method: Application in a pharmacokinetic study. J
Pharm Biomed Anal. 64-65:94–97. 2012.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Miao L, Yang Y, Li Z, Fang Z, Zhang Y and
Han CC: Ginsenoside Rb2: A review of pharmacokinetics and
pharmacological effects. J Ginseng Res. 46:206–213. 2022.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Xie F, Li S, Cheng Z, Liu X, Zhang H, Li
P, Liu Z, Guo X and Yu P: Determination of 20(S)-protopanaxadiol in
human plasma by HPLC-MS/MS: Application to a pharmacokinetic study.
Acta Pharmaceutica Sinica B. 3:385–391. 2013.
|
|
105
|
Li L, Chen X, Li D and Zhong D:
Identification of 20(S)-protopanaxadiol metabolites in human liver
microsomes and human hepatocytes. Drug Metab Dispos. 39:472–483.
2011.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Ren HC, Sun JG, Wang GJ, A JY, Xie HT, Zha
WB, Yan B, Sun FZ, Hao HP, Gu SH, et al: Sensitive determination of
20(S)-protopanaxadiol in rat plasma using HPLC-APCI-MS: Application
of pharmacokinetic study in rats. J Pharm Biomed Anal.
48:1476–1480. 2008.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Kim H, Lee JH, Kim JE, Kim YS, Ryu CH, Lee
HJ, Kim HM, Jeon H, Won HJ, Lee JY and Lee J: Micro-/nano-sized
delivery systems of ginsenosides for improved systemic
bioavailability. J Ginseng Res. 42:361–369. 2018.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Wang H, Zheng Y, Sun Q, Zhang Z, Zhao M,
Peng C and Shi S: Ginsenosides emerging as both bifunctional drugs
and nanocarriers for enhanced antitumor therapies. J
Nanobiotechnology. 19(322)2021.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Yang L, Zhang Z, Hou J, Jin X, Ke Z, Liu
D, Du M, Jia X and Lv H: Targeted delivery of ginsenoside compound
K using TPGS/PEG-PCL mixed micelles for effective treatment of lung
cancer. Int J Nanomedicine. 12:7653–7667. 2017.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Dong Y, Fu R, Yang J, Ma P, Liang L, Mi Y
and Fan D: Folic acid-modified ginsenoside Rg5-loaded bovine serum
albumin nanoparticles for targeted cancer therapy in vitro and in
vivo. Int J Nanomedicine. 14:6971–6988. 2019.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Liu Y, Zhu H, Zhou W and Ye Q:
Anti-inflammatory and anti-gouty-arthritic effect of free
ginsenoside Rb1 and nano ginsenoside Rb1 against MSU induced gouty
arthritis in experimental animals. Chem Biol Interact.
332(109285)2020.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Singh H, Du J, Singh P, Mavlonov GT and Yi
TH: Development of superparamagnetic iron oxide nanoparticles via
direct conjugation with ginsenosides and its in-vitro study. J
Photochem Photobiol B. 185:100–110. 2018.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Liu X, Tang I, Wainberg ZA and Meng H:
Safety considerations of cancer nanomedicine-A key step toward
translation. Small. 16(e2000673)2020.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Wu B, Yang L, Chen L, Ma L and Guo Y:
Traditional Chinese medicine therapies for patients with knee
osteoarthritis: A protocol for systematic review and network
meta-analysis. Medicine (Baltimore). 101(e29404)2022.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Ju L, Hu P, Chen P, Xue X, Li Z, He F, Qiu
Z, Cheng J and Huang F: Huoxuezhitong capsule ameliorates
MIA-induced osteoarthritis of rats through suppressing
PI3K/Akt/NF-κB pathway. Biomed Pharmacother.
129(110471)2020.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Liu T, Zhu L and Wang L: A narrative
review of the pharmacology of ginsenoside compound K. Ann Transl
Med. 10(234)2022.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Chen L, Zhou L, Wang Y, Yang G, Huang J,
Tan Z, Wang Y, Zhou G, Liao J and Ouyang D: Food and sex-related
impacts on the pharmacokinetics of a single-dose of ginsenoside
compound K in healthy subjects. Front Pharmacol.
8(636)2017.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Liu Y, Perumalsamy H, Kang CH, Kim SH,
Hwang JS, Koh SC, Yi TH and Kim YJ: Intracellular synthesis of gold
nanoparticles by Gluconacetobacter liquefaciens for delivery of
peptide CopA3 and ginsenoside and anti-inflammatory effect on
lipopolysaccharide-activated macrophages. Artif Cells Nanomed
Biotechnol. 48:777–788. 2020.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Kang S, Siddiqi MH, Yoon SJ, Ahn S, Noh
HY, Kumar NS, Kim YJ and Yang DC: Therapeutic potential of compound
K as an IKK inhibitor with implications for osteoarthritis
prevention: An in silico and in vitro study. In Vitro Cell Dev Biol
Anim. 52:895–905. 2016.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Hassamal S: Chronic stress,
neuroinflammation, and depression: An overview of
pathophysiological mechanisms and emerging anti-inflammatories.
Front Psychiatry. 14(1130989)2023.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Schaaf MJ and Cidlowski JA: Molecular
mechanisms of glucocorticoid action and resistance. J Steroid
Biochem Mol Biol. 83:37–48. 2002.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Bannuru RR, Osani MC, Vaysbrot EE, Arden
NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott
JH, Bhandari M, et al: OARSI guidelines for the non-surgical
management of knee, hip, and polyarticular osteoarthritis.
Osteoarthritis Cartilage. 27:1578–1589. 2019.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Guermazi A, Neogi T, Katz JN, Kwoh CK,
Conaghan PG, Felson DT and Roemer FW: Intra-articular
corticosteroid injections for the treatment of hip and knee
osteoarthritis-related pain: Considerations and controversies with
a focus on imaging-radiology scientific expert panel. Radiology.
297:503–512. 2020.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Li J, Du J, Liu D, Cheng B, Fang F, Weng
L, Wang C and Ling C: Ginsenoside Rh1 potentiates dexamethasone's
anti-inflammatory effects for chronic inflammatory disease by
reversing dexamethasone-induced resistance. Arthritis Res Ther.
16(R106)2014.PubMed/NCBI View
Article : Google Scholar
|
|
125
|
Barnes PJ and Adcock IM: Glucocorticoid
resistance in inflammatory diseases. Lancet. 373:1905–1917.
2009.PubMed/NCBI View Article : Google Scholar
|