Introduction
Acute myocardial infarction (AMI) is one of the
deadliest diseases in the world (1,2).
Although the extensive application of percutaneous coronary
intervention (PCI) has improved the prognosis of AMI in recent
years, abrupt reperfusion in the ischemic myocardium can lead to
ischemia/reperfusion (I/R) injury in cardiomyocytes (3,4).
However, the unclear mechanism of I/R injury limits the development
of therapeutic strategies.
Studies have shown that the bromodomain
(BRD)-containing protein family, including BRD2, BRD3, BRD4, BRD7
and BRD9, and the bromodomain, testis-specific (BRDT) protein
(5), is associated with a variety
of cardiovascular diseases. For instance, the upregulation of
citrate cycle genes by BRD2 may facilitate cardiac hypertrophy
(6). BRD4 is involved in
myocardial infarction (7), cardiac
hypertrophy (8) and even cardiac
remodeling and functions (9,10).
In addition, the myocardial infarction-induced myocardial injury is
regulated by BRD7 through activating Wnt/β-catenin signaling
(11). However, few studies have
reported the role of the BRD family in myocardial I/R injury.
Thus, the objective of the present study was to
investigate the role of the BRD family in myocardial I/R injury.
Given that myocardial I/R injury is a condition involving a complex
interplay of molecular mechanisms, such as intracellular calcium
overload, oxidative stress, disturbance of energy metabolism,
apoptosis, vascular endothelial injury and neutrophil infiltration
(12-15),
proteomics was performed to investigate the changes of all proteins
in a certain type of cell at the protein level and reveal the
biological function of BRDs more accurately than transcriptome
analysis (16). The present study
also used the tandem mass tag (TMT) labelling quantitative
technique combined with liquid chromatography-tandem mass
spectrometry (LC-MS/MS) to demonstrate the changes of all plasma
proteins in myocardial cells during I/R injury. It is hoped that
the present study could provide potential therapeutic targets by
determining a possible relationship between the expression of BRDs
and myocardial I/R injury. Understanding the relationship between
BRD expression and cardiac damage can pave the way for the
development of targeted interventions to protect cardiomyocytes
following AMI. These insights may lead to novel treatment
strategies aimed at improving patient outcomes in AMI.
Materials and methods
Cell lines and culture
Shanghai Cell Bank provided H9C2 cardiomyocytes with
the serial number GNR 5. Cells were grown in At 37˚C with 5%
CO2 in DMEM augmented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 10% streptomycin, and 1% penicillin (Thermo
Fisher Scientific, Inc.).
Establishment of the cardiomyocyte
hypoxia/reoxygenation model and experimental grouping
A hypoxia/reoxygenation (H/R) model was created by
filling a Billups-Rothenberg hypoxic/anaerobic incubator with a
mixture of 95% N2 and 5% CO2 (Chongqing Ruike
Gas Co. Ltd.). Prior to the cells were treated with hypoxia, they
were cultured in a medium (2 ml of DMEM) devoid of sugar and serum
for 3 h with 5% CO2 at 37˚C. The H/R and hypoxia groups
were then transferred to an anaerobic incubator containing a
hypoxia gas mixture. The control group was also subjected to
standard incubation conditions consisting of 5% CO2 and
37˚C. The six-well plates were taken away from the hypoxia for 4 h,
and their original medium was swapped out for the sugared medium.
After that, the cells spent time in a 5% CO2 incubator
at 37˚C for 2, 4, and 8 h to re-oxygenate. The abbreviation ‘H4’
was used to represent hypoxia for 4 h, and the abbreviations ‘H/R2’
‘H/R4’ and ‘H/R8’ used to represent hypoxia for 4 h and then
reoxygenation for 2, 4, and 8 h, respectively.
Detection of the cell survival rate by
CCK8
After cells were adhered for a day, both groups were
cultured normally in a 5% CO2 cell incubator, but the
experimental group also received H/R treatment as described above.
Cells in each group were treated with CCK-8 reaction solution
following hypoxia and H/R, and incubated at 37˚C for 1 h, in
accordance with the instructions provided in the CCK-8 test kit.
The OD values were determined by analyzing the microplates at 450
nm.
LDH release from H9C2 cardiomyocytes
was analyzed using LDH release assays
Following the establishment of the H/R paradigm, the
cell supernatants were collected, and LDH in cardiomyocytes was
quantified as directed by the manufacturer of the LDH kit (Nanjing
Jian Cheng Bioengineering Institute; cat. no. A020-2).
Detection of apoptosis by flow
cytometry
Following the establishment of a suitable H/R model,
the cell cycle analysis was performed on a fluorescence-activated
cell sorting flow cytometer (BD FACSCalibur; BD Biosciences). The
cells were resuspended in DMEM. Annexin V FITC (20 g/ml) and
propidium (PI) (50 µg/ml) in 100 µl Annexin V binding buffer were
added to the cells and incubated for 15 min at 4˚C in the dark. The
flow cytometer was used to analyze the samples for apoptosis.
FlowJo v10.0 (FlowJo LLC) and fluorescence-activated cell sorting
were used to analyze the data and calculate the proportion of
apoptotic cells. The total apoptotic rate was calculated as the
early apoptotic rate plus the late apoptotic rate.
Tandem mass tag (TMT) isobaric
labelling
After the cardiomyocytes were washed with PBS, they
were lysed using RIPA buffer. The protein concentration was
measured by a BCA protein assay kit. Filter-aided sample
preparation was used to digest 100 µg of proteins. The control
group, hypoxia for 4 h and reoxygenation for 2, 4, and 8 h groups
were labelled with 126, 127, 128, 129, and 130 Th, respectively,
and the reoxygenation for 4 h group was labelled again with 131 Th.
The samples were separated into eight fractions by pH12
reversed-phase chromatography. An Easy-nanoLC1000 liquid
chromatograph and an LTQ Orbitrap Velos mass spectrometer (Thermo
Fisher Scientific, Inc.) were used for proteomic analyses. The
Swiss-Prorat database's differential proteins (Homo sapiens,
release 2020_06; www.uniprot.org) were compared and screened using
Protein Discovery Software 1.4 (Thermo Fisher Scientific,
Inc.).
The Z-test was utilized to identify proteins with
statistically significant changes in abundance (P<0.05) and then
the significance of the hit was used to filter out proteins with
1.2-fold changes. Proteomics findings were uploaded to STRING
(https://string-db.org/). Kyoto Encyclopedia of
Genes and Genomes (KEGG) 2.4.1 (www.genome.jp/kegg/) was used to discover protein
pathways. The identified proteins were evaluated using Gene
Ontology (GO) terms in Metascape (https://metascape.org/).
Analysis of the cell proteome
The Proteome Discoverer 21 (Thermo Fisher
Scientific, Inc.) program transformed the proteome data, which was
then loaded into Mascot 2.2.0. algorithm (Matrix Science Ltd.) for
retrieval. After exporting the data, the expression matrix
containing all genes and phenotypes of the samples was subjected to
Gene Set Enrichment Analysis (GSEA) software (version 4.0.3, Broad
Institute) for the analysis of GO enrichment. Additionally, for a
functional-level analysis of the differentially expressed genes,
the present study utilized the Metascape database (https://metascape.org), an online tool that
facilitates the annotation, visualization, and integrated discovery
of gene lists (17). Through this
tool, it was possible to gain functional insights into the GO
enrichment and KEGG pathway analysis for the differentially
expressed genes.
Western blotting
After observing a significant decrease in cell
viability, an increase in LDH release and higher apoptotic rates in
the H/R4 group compared to the other groups, the present study
mainly concentrated on examining the expression of BRD2 in the H/R4
group. Total protein of H/R4 group was recovered from isolated
cardiomyocytes using standard methods and RIPA buffer. The protein
concentration was determined with a BCA protein kit. Each lane
contained 40 µg of protein that had been separated using 10%
SDS-PAGE and then transferred to a polyvinylidene fluoride membrane
(MilliporeSigma). The membranes were blocked for 2 h of room
temperature with Tris-buffered saline containing 0.1% Tween 20,
including 5% nonfat milk (TBST). Finally, the membranes were
treated with anti-BRD2 (1:1,000; cat. no. 139690; Abcam) and
anti-nuclear factor erythroid 2-related factor 2 (Nrf2) (1:1,000;
cat. no. 13901; Cell Signaling Technology) and anti-Vinculin
(1:1,000; cat. no. 16396-1-AP; Proteintech Group, Inc.) at 4˚C
overnight. Membranes were incubated with an HRP-conjugated goat
anti-rabbit or anti-mouse IgG secondary antibody for 1 h at room
temperature after the primary antibody incubation (1:500; Cell
Signaling Technology). The detection was made using a Bio-Rad
ChemiDoc MP Imaging System (Bio-Rad Laboratories, Inc.).
Determination of the effective
concentration of dBET1
dBET1 is a proteolysis-targeting chimera that can
result in selective degradation of BET proteins (18). In order to delve deeper into the
regulatory pathway indicated in the proteomics of BRD2, the
expression of haem oxygenase-1 (HO-1) and Nrf2 before and after the
administration of dBET1 was investigated. For the
concentration-response experiment, 2x105 cells were
cultured in 2 ml DMEM containing 20 nM dBET1 (Selleck Chemicals).
The cells were cultivated in an incubator containing 5%
CO2 at 37˚C for 48 h and oxygen-deprived for 4 h in a
Billups-Rothenberg hypoxic/anaerobic incubator containing a mixture
of gases (95% N2, 5% CO2). Then, western
blotting was used to determine the BRD2 protein expression. At a
dose of 20 nM, the expression of BRD2 was markedly suppressed. This
concentration was used for the subsequent experiment. Furthermore,
the cell viability, LDH release, and apoptotic rates in the H/R4
group after the administration of dBET1 were also detected, using
the same previously mentioned methods.
Statistical analysis
All statistical analyses were performed with SPSS
21.0 (IBM Corp.). The figures indicate the mean and standard
deviation of the mean (SD). Differences among three groups were
analyzed using one-way analysis of variance (ANOVA) followed by
Tukey's post-hoc test using GraphPad Prism software, as specified
in the figure legends. P<0.05 was considered to indicate a
statistically significant difference.
Results
Establishment of the (H/R) model
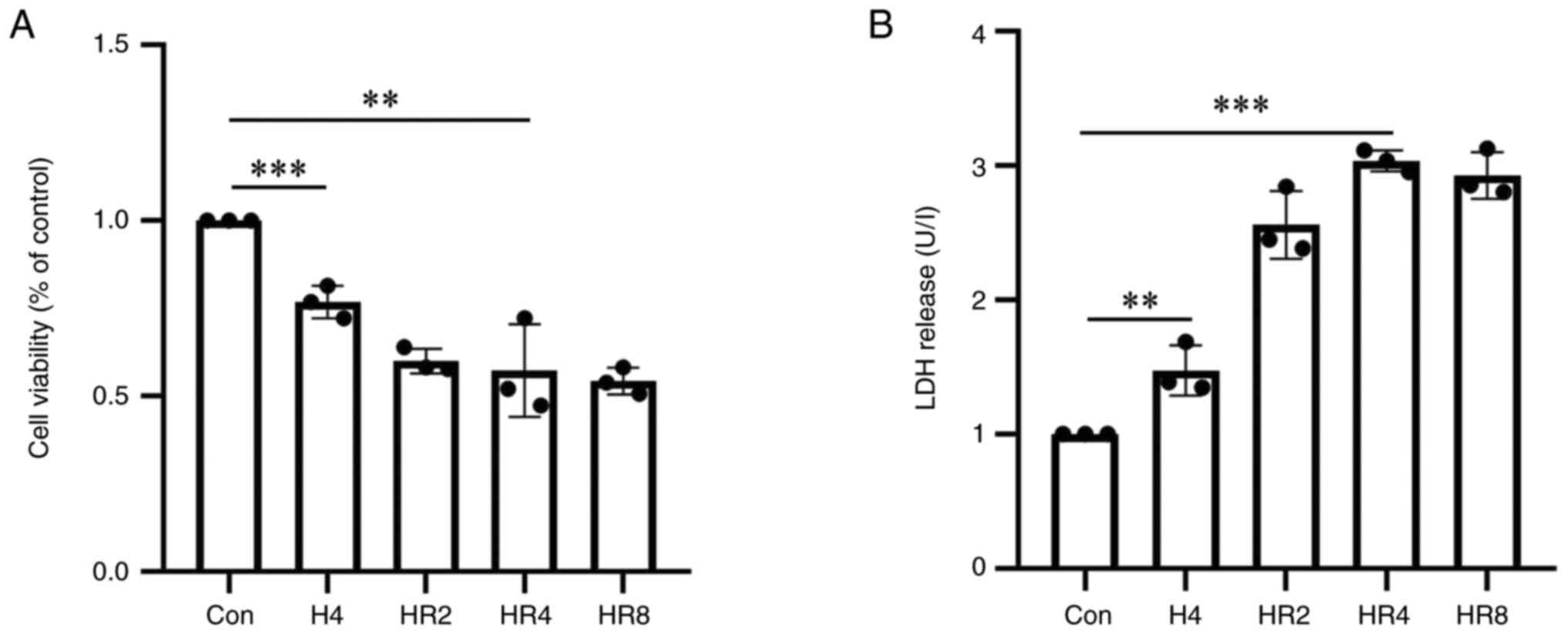
The CCK-8 results revealed a reduction in cell
survival as reoxygenation time increased (Fig. 1A). The difference in cell survival
rates between the control group and the 4 h reoxygenation group was
statistically significant (P<0.01). The LDH results indicated
that as reoxygenation time increased, cell damage was exacerbated.
In terms of cell damage, the difference between the 4 h
reoxygenation group and the control group was statistically
significant (P<0.001; Fig. 1B).
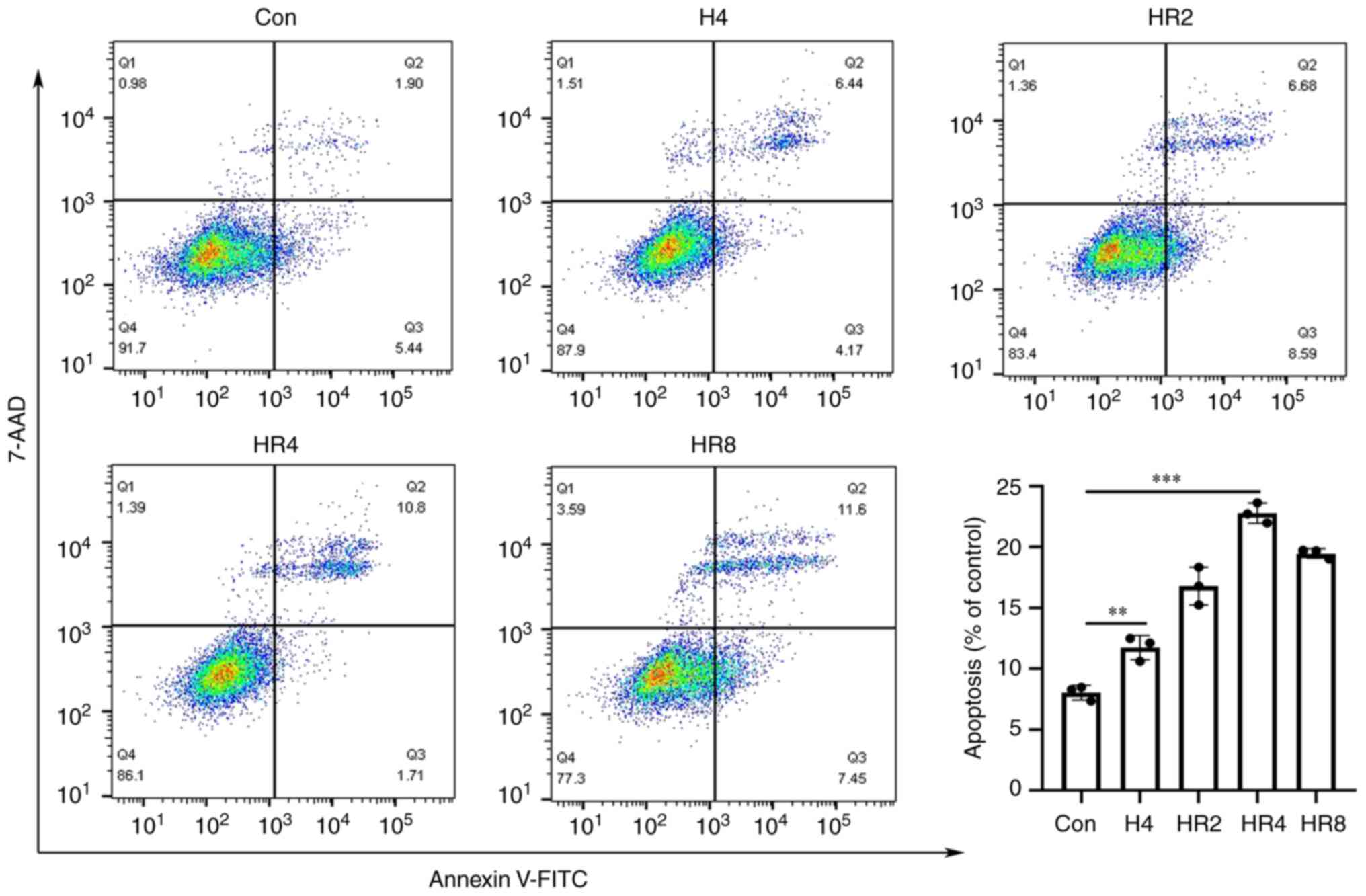
Flow cytometry demonstrated that the apoptosis rate of the 4 h
hypoxia group was significantly higher than that of the control
group (P<0.01; Fig. 2) and that
the apoptotic rate rose as reoxygenation time increased. The
difference in the apoptotic rate between the 4 h reoxygenation
group and the control group was statistically significant
(P<0.001; Fig. 2). It was
determined that 4 h of hypoxia and 4 h of reoxygenation were ideal
for the development of cardiomyocytes.
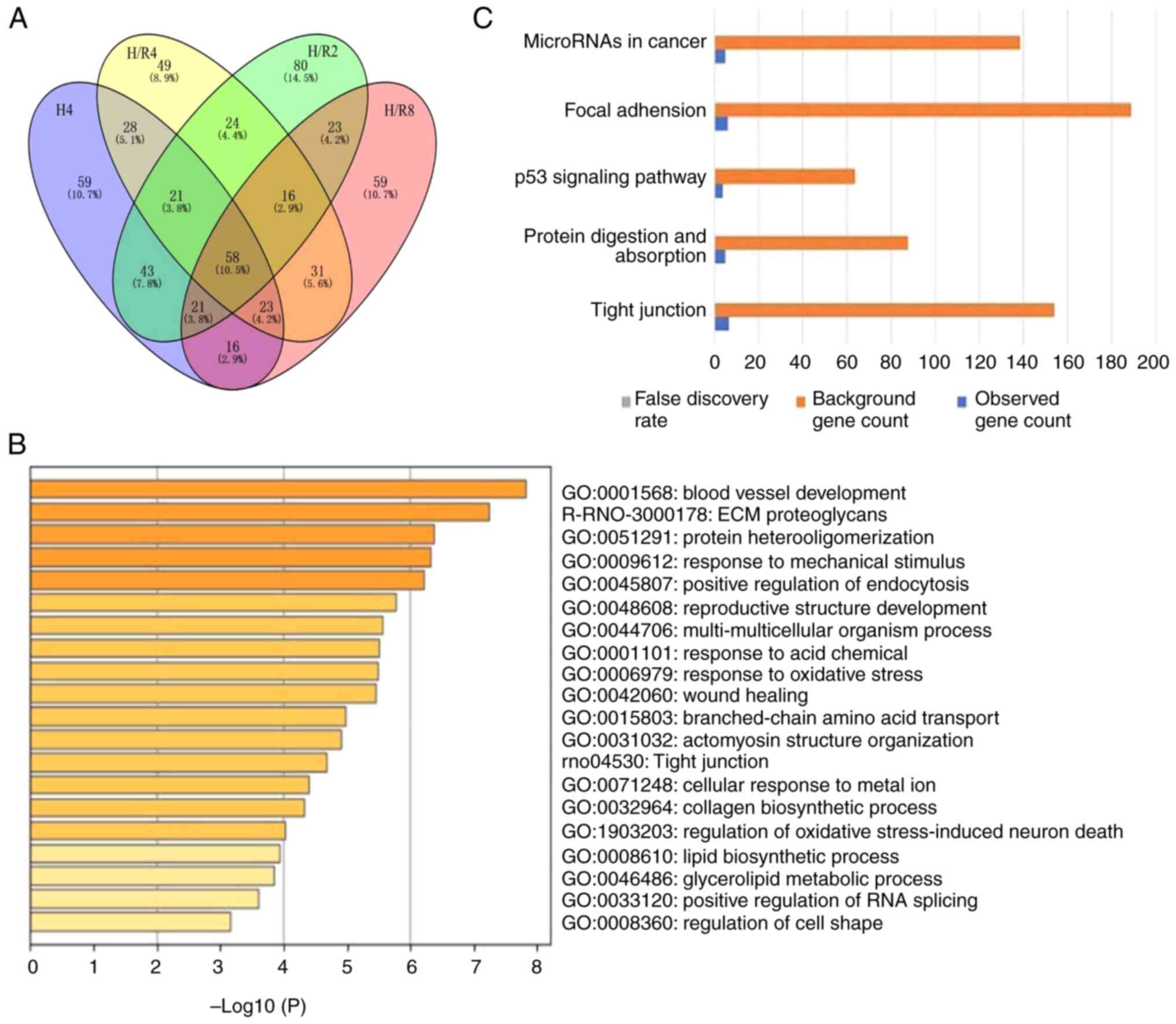
Screening and global analysis of
significantly different proteins in the H/R4 group
A total of 2,325 significantly differentially
expressed proteins were identified by TMT labelling combined with
reverse-phase liquid chromatography (RP-HPLC). Using a
bioinformatics method, the proteins of each experimental group were
screened according to the standard of fold change >1.2 or
<0.8 and P-value (t-test) <0.05. As shown in Fig. 3A, compared with the normal control
group, there were 267 significantly different proteins under
hypoxia for 4 h, 286 significantly different proteins under H/R2,
250 significantly different proteins under H/R4, and 247
significantly different proteins under H/R8. Among them, 119
proteins were involved in the process of hypoxia and reoxygenation.
A total of 49 proteins appeared in H/R4 but were not involved in
the process of simple hypoxia for 4 h.
Analysis of the effects of the
significant differentially expressed proteins in the H/R4
group
The present study identified 250 significantly
differentially expressed proteins (Fig. 3B and C) in the H/R4 group concerning the GO and
KEGG databases. The GO analysis results were as follows: Among
biological processes, the differentially expressed protein
functions were involved in regulating nucleic acid metabolism,
vascular development, lipid metabolism and metal ion transport
among others. Among molecular functions, the protein functions were
mainly focused on regulating enzyme molecular functions. In the
cellular component category, these differentially expressed
proteins were mainly distributed in the cytoplasm, endometrium
system, and extracellular gap. KEGG analysis showed that
differentially expressed proteins resulting from myocardial injury
induced by H/R were correlated with tight junctions, protein
degradation pathways and signaling pathways among others.
Cluster analysis of differentially
expressed proteins in the H/R4 group
The protein cluster analysis findings (Fig. 4A and B) revealed that the histone family
proteins H2AFZ, H2AFJ and H2AFY increased during hypoxia and
reduced following reoxygenation. Histone H3 (H3F3B) expression also
rose during hypoxia and dropped following reoxygenation, while the
expression levels of Ptsg2, Serprine1 and Junb proteins fell during
hypoxia for 4 h and increased following reoxygenation for 4 h. In
the heat map analysis, which resembled the volcano plot of the
protein screening, protein clustering was evident. The protein
interaction network map (Fig. 4C)
revealed that in H/R4, the H3F3B expression was dramatically
reduced and the BRD2 expression was significantly enhanced compared
to the control group. The increased protein Glyr was consistent
with the volcano plot.
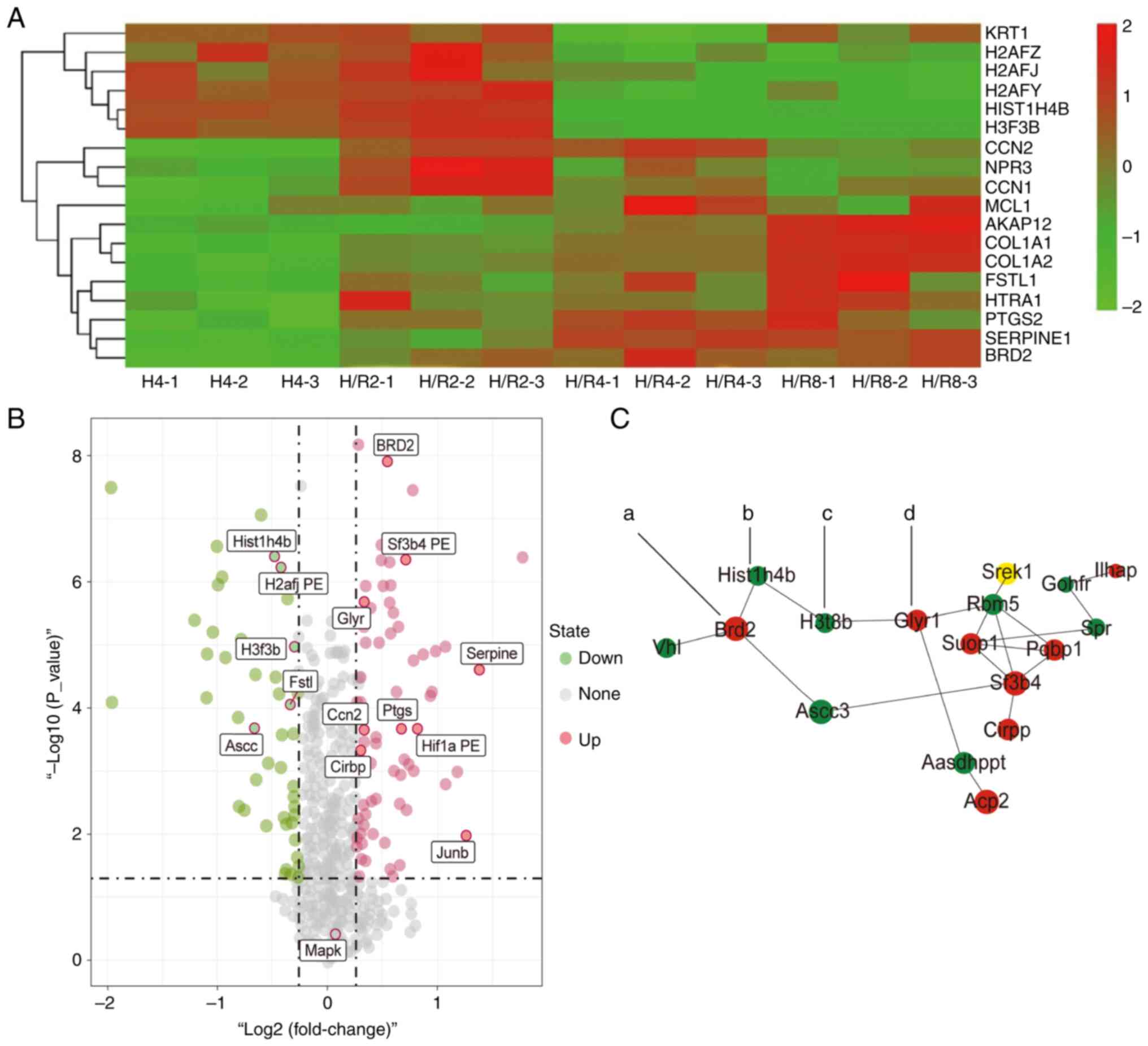 | Figure 4Cluster analysis of core
differentially expressed proteins in hypoxia-reoxygenation for 4 h
by heat map and volcano plot. (A) Heat map analysis of the TMT
abundance data for 18 representative proteins identified in the
whole proteome. (B) Volcano plot of differentially expressed
proteins in each group. The signal detection results show the
multiple regression (log 2 reporting odds ratio, x-axis) and
significance (−log 10 adjusted p-value, y-axis) for differentially
expressed proteins. Red dots denote repressed proteins, green dots
indicate activated proteins, and grey dots represent proteins that
were not differentially expressed. The dotted lines indicate that
the adjusted P-value=0.05. (C) Core differentially expressed
protein interaction network diagram (a, BRD2; b, Hist1h4b; c,
H3F3B; d, Gly). TMT, tandem mass tag; H4, hypoxia for 4 h; H/R2,
H/R4 and H/R8, hypoxia/reoxygenation for 2, 4 or 8 h. |
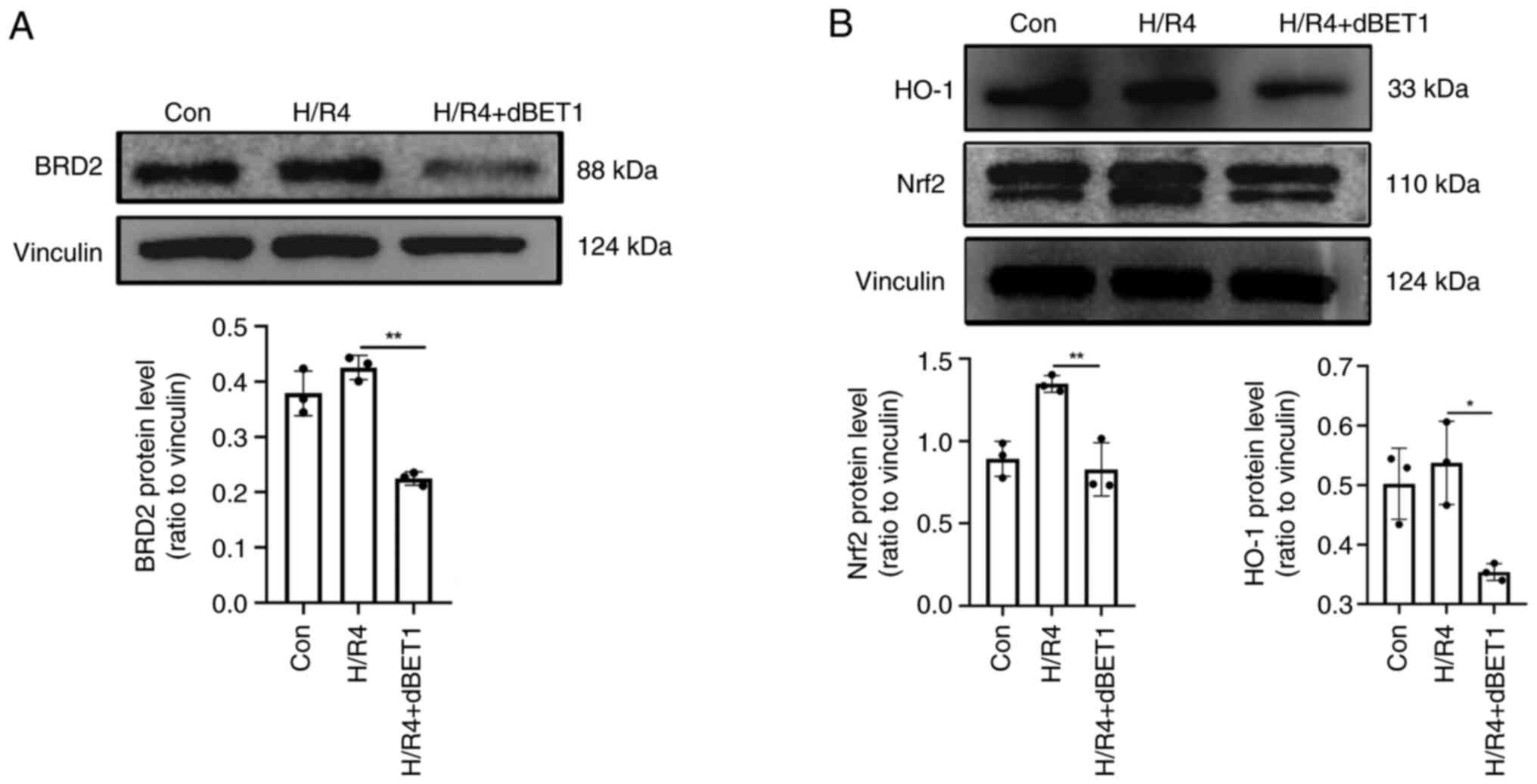
Overexpression of BRD2 regulated by
Nrf2/HO-1 signaling pathway in H/R4 group
Western blot analysis revealed that the expression
of BRD2 was dramatically increased after 4 h of exposure to hypoxia
and reoxygenation, corroborating the protein cluster analysis. In
addition, when the BRD protein inhibitor dBET1 was added, the
expression of BRD2 fell dramatically (P<0.01; Fig. 5A). This H/R mechanism was related
to the Nrf2/HO-1 signaling pathway, and dBET1 reversed the
upregulation (Fig. 5B).
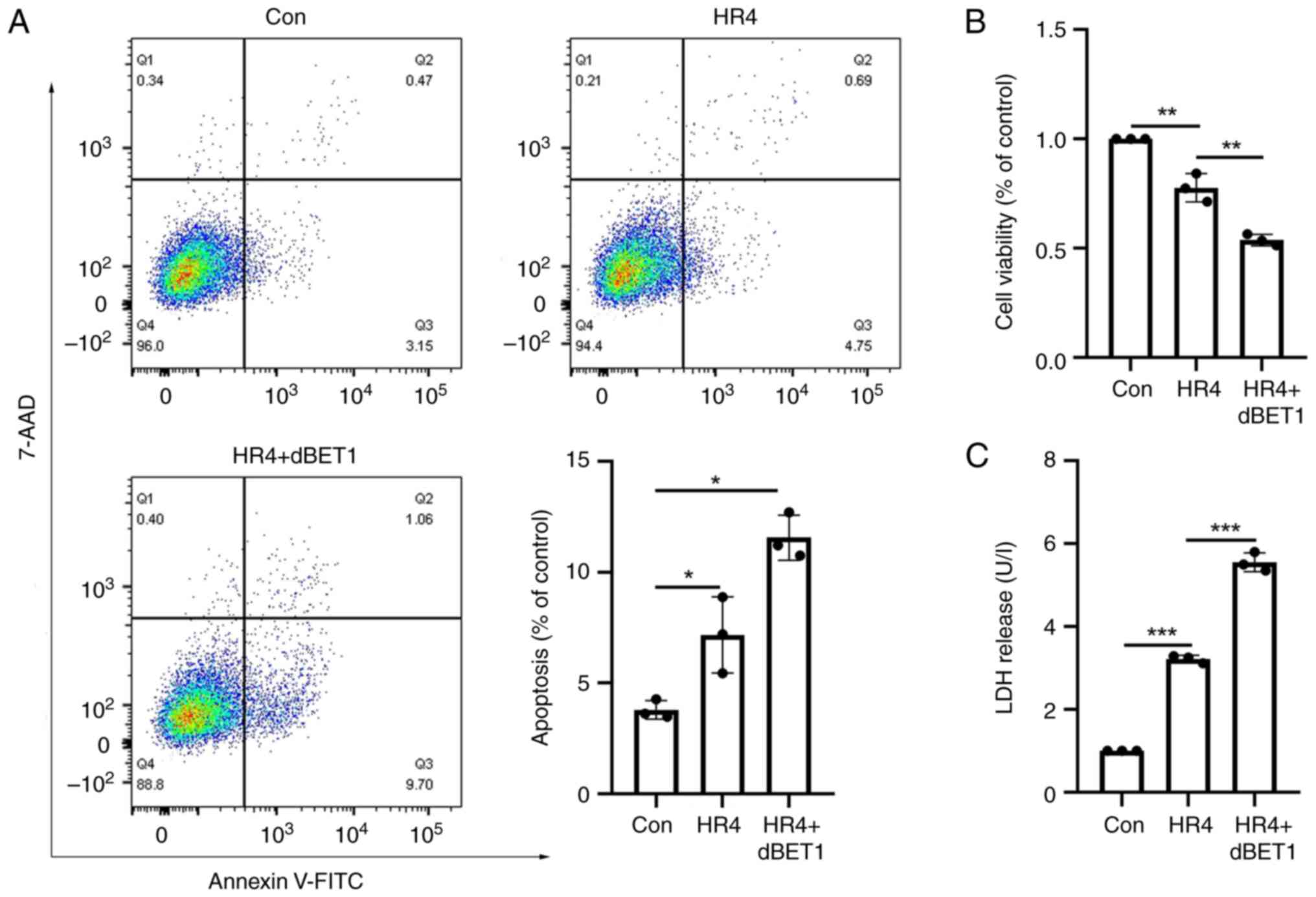
Effects of BRD2 protein inhibition on
cardiomyocyte's apoptosis, cell viability, and release of LDH
Flow cytometry analysis revealed that, relative to
the control group, cardiomyocyte apoptosis was elevated in the H/R4
group and in the H/R4 group following administration of the BRD2
inhibitor dBET1 (P<0.05; Fig.
6A). Using the CCK-8 assay, it was also determined that the
addition of dBET1 significantly lowered cell viability (P<0.01;
Fig. 6B). After addition of dBET1,
the release of LDH was enhanced (P<0.001; Fig. 6C).
Discussion
To the best of the authors' knowledge, this is the
first study to demonstrate that BRD2 can protect cardiomyocytes
from H/R-induced damage by targeting the Nrf2/HO-1 signaling
pathway. In addition, the present study enhanced the existing
knowledge of the mechanism of myocardial I/R damage from the
viewpoint of proteomics and revealed the relevance of BRD2 as a
possible therapeutic target.
Multiple signaling pathways have been linked to the
process of myocardial I/R damage (19-22),
but there are still no suitable interventions to alleviate I/R
injury during cardiac surgery due to the poor understanding of
proteome changes caused by the lack of high-throughput protein
detection technology. Given that the pathophysiological process of
H/R damage in cardiomyocytes is comparable with that of myocardial
I/R injury, a cardiomyocyte H/R model was developed to replicate
the myocardial I/R process. TMT labeling in conjunction with HPLC
was used to identify alterations in the proteome of H9C2
cardiomyocytes and a total of 2325 proteins with differential
expression were discovered. Through a proteomic analysis, Li et
al (19) demonstrated that
when human cardiac microvascular endothelial cells were subjected
to I/R injuries, the expression of 30 proteins changed,
highlighting their potential roles in cell proliferation, stress
response, and regulation of metabolic process upon I/R injury. The
present study also conducted in-depth information mining of
proteins related to intimal system changes with the development of
vesicle transport and exosome technology, such as Junb, Fstl1,
Kert1, H3f3b, Ptgs2, Hif1α, Hist1h4b, BRD2, Serpine1, and Glyr. The
GO functional analysis revealed that these proteins can regulate
vascular development, metal ion transport, and LDL lipid
metabolism, suggesting their potential targets for reducing
myocardial I/R injury (23-25).
These results were consistent with the previous research. For
instance, it has been demonstrated that Fstl1 is a cardioprotective
factor regulated by Akt to reduce the myocardial I/R injury
(26). In addition, inhibition of
Fstl1 expression can aggravate cardiomyocyte apoptosis in
myocardial H/R injury (27).
The BRD family serves as epigenetic ‘readers’ by
recognizing acetylated histones and recruiting transcriptional
regulator complexes to chromatin in order to regulate gene
transcription (28). Due to the
extensive role of the BRD family in cardiovascular diseases, the
present study explored the role of BRD2 in H/R4. When the BRD2
protein inhibitor dBET1 was performed in the H/R4 group, a decrease
was found in cell viability and an increased level in LDH release
and apoptosis in cardiomyocytes, indicating BRD2 may play a
protective role in myocardial I/R injury. A previous study also
found that the absence of BRD2 can decrease heart function and
increase the mortality of conditional knock-out mice after pressure
overload (29). In addition, the
expression of BRD2 is lower in the adult heart than in the fetal
heart, indicating its role in heart development and heart disease
(7).
In addition, the present study discovered that
proteins in the Nrf2/HO-1 signaling pathway reduced following the
addition of dBET1, indicating that BRD2 plays a significant
protective function through this pathway. Numerous studies have
also established that this pathway plays a crucial role in the
control of homeostasis by modifying calcium levels to inhibit
autophagy, ferroptosis and clockophagy (30-32).
Regarding cardiovascular illnesses, activation of the Nrf2/HO-1
pathway might enhance the prognosis following a myocardial
infarction damage through nuclear translocation and overexpression
of antioxidative genes (33). In
addition, arrhythmias may be alleviated by activating the NO route
through the Nrf2/HO-1 signaling axis (34). The activation of this pathway plays
a crucial role in myocardial remodeling (35). The method by which BRD2 contributes
to cardiac H/R damage through stimulation of the Nrf2/HO-1
signaling pathway may thus present a unique therapeutic target for
the treatment of I/R injury in cardiomyocytes, as shown by the
present findings.
Nevertheless, there were a few shortcomings
associated with the present study. First, it was conducted in
isolated cell experiments and therefore limits interpretations of
the results. Second, BRD2-related agonists have not been found so
far, so the role of the overexpression of BRD2 in I/R injury of
cardiomyocytes has remained to be fully elucidated. Further
pharmacological studies and animal studies are still warranted to
validate the conclusions of the present study.
The present study was the first to show, to the best
of the authors' knowledge, the sequential proteome alterations that
occur in cardiomyocytes throughout the H/R process. It discovered a
critical protective function for BRD2 in I/R damage to
cardiomyocytes and demonstrated the connection to the Nrf2/HO-1
signaling pathway. It represented a new target for the treatment of
I/R injuries of cardiomyocytes and provides an opportunity to
initiate timely interventions following PCI procedures.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Chongqing (grant no. cstc2014jcyjA10071), the Science
Foundation of Southwest Hospital (grant no. SWH2016JCYB-57) and the
National Science Foundation of China (grant no. 81370446).
Availability of data and materials
All datasets generated or analyzed during the
present study are available from the corresponding author upon
reasonable request.
Authors' contributions
YL and LS conceived the present study. YL performed
data curation. Conception and design was the responsibility of YL,
YF and XX. YF and XX were responsible for the analysis and
interpretation of data. LS and GT were responsible for supervision
and project administration, and writing, reviewing and editing the
manuscript. Visualization was the responsibility of XX. YL wrote
the original draft of the manuscript. LS was responsible for
funding acquisition. YL, GT and LS confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reed GW, Rossi JE and Cannon CP: Acute
myocardial infarction. Lancet. 389:197–210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lv J, Zhao Q, Yang J, Gao X, Zhang X, Ye
Y, Dong Q, Fu R, Sun H, Yan X, et al: Length of stay and short-term
outcomes in patients with ST-Segment elevation myocardial
infarction after primary percutaneous coronary intervention:
Insights from the China acute myocardial infarction registry. Int J
Gen Med. 14:5981–5991. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Algoet M, Janssens S, Himmelreich U, Gsell
W, Pusovnik M, Van den Eynde J and Oosterlinck W: Myocardial
ischemia-reperfusion injury and the influence of inflammation.
Trends Cardiovasc Med. 33:357–366. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen X, Rong C, Qi P, Bai W, Yao W, Zhang
Y and Dang Y: LDL-C and total stent length are independent
predictors of periprocedural myocardial injury and infarction for
unstable angina patients undergoing elective percutaneous coronary
intervention. Int J Gen Med. 14:1357–1365. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fujisawa T and Filippakopoulos P:
Functions of bromodomain-containing proteins and their roles in
homeostasis and cancer. Nat Rev Mol Cell Biol. 18:246–262.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lin Z and Li Z, Guo Z, Cao Y, Li J, Liu P
and Li Z: Epigenetic reader bromodomain containing protein 2
facilitates pathological cardiac hypertrophy via regulating the
expression of citrate cycle genes. Front Pharmacol.
13(887991)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sun Y, Xie Y, Du L, Sun J and Liu Z:
Inhibition of BRD4 attenuates cardiomyocyte apoptosis via NF-κB
pathway in a rat model of myocardial infarction. Cardiovasc Ther.
36:2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Q, Sun Y, Li T, Liu L, Zhao Y, Li L,
Zhang L and Meng Y: Function of BRD4 in the pathogenesis of high
glucose-induced cardiac hypertrophy. Mol Med Rep. 19:499–507.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim SY, Zhang X, Schiattarella GG,
Altamirano F, Ramos TAR, French KM, Jiang N, Szweda PA, Evers BM,
May HI, et al: Epigenetic Reader BRD4 (Bromodomain-Containing
Protein 4) governs nucleus-encoded mitochondrial transcriptome to
regulate cardiac function. Circulation. 142:2356–2370.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Padmanabhan A, Alexanian M,
Linares-Saldana R, González-Terán B, Andreoletti G, Huang Y,
Connolly AJ, Kim W, Hsu A, Duan Q, et al: BRD4
(Bromodomain-Containing Protein 4) Interacts with GATA4 (GATA
Binding Protein 4) to govern mitochondrial homeostasis in adult
cardiomyocytes. Circulation. 142:2338–2355. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chi X, Shan L, Hu Y, Zhang Y, Mao Y and
Wang X: Bromodomain-containing protein 7 contributes to myocardial
infarction-induced myocardial injury through activating
Wnt/β-catenin signaling. Ann Palliat Med. 10:10756–10767.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schirone L, Forte M, D'Ambrosio L, Valenti
V, Vecchio D, Schiavon S, Spinosa G, Sarto G, Petrozza V, Frati G
and Sciarretta S: An overview of the molecular mechanisms
associated with myocardial ischemic injury: State of the art and
translational perspectives. Cells. 11(1165)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen R, Zhou M and Zhu F: Immune
checkpoint inhibitors related to cardiotoxicity. J Cardiovasc Dev
Dis. 9(378)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pertiwi KR, Hillman RM, Scott CA and
Chilton EL: Ischemia reperfusion injury produces, and ischemic
preconditioning prevents, rat cardiac fibroblast differentiation:
Role of KATP channels. J Cardiovasc Dev Dis.
6(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xie LH, Fefelova N, Pamarthi SH and
Gwathmey JK: Molecular mechanisms of ferroptosis and relevance to
cardiovascular disease. Cells. 11(2726)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kustatscher G, Collins T, Gingras AC, Guo
T, Hermjakob H, Ideker T, Lilley KS, Lundberg E, Marcotte EM,
Ralser M and Rappsilber J: Understudied proteins: Opportunities and
challenges for functional proteomics. Nat Methods. 19:774–779.
2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou Y, Zhou B, Pache L, Chang M,
Khodabakhshi AH, Tanaseichuk O, Benner C and Chanda SK: Metascape
provides a biologist-oriented resource for the analysis of
systems-level datasets. Nat Commun. 10(1523)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
DeMars KM, Yang C, Castro-Rivera CI and
Candelario-Jalil E: Selective degradation of BET proteins with
dBET1, a proteolysis-targeting chimera, potently reduces
pro-inflammatory responses in lipopolysaccharide-activated
microglia. Biochem Biophys Res Commun. 497:410–415. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Q, Cui HH, Yang YJ, Li XD, Chen GH,
Tian XQ, Jin C, Dong QT, Huang PS and Xu J: Quantitative proteomics
analysis of ischemia/reperfusion injury-modulated proteins in
cardiac microvascular endothelial cells and the protective role of
tongxinluo. Cell Physiol Biochem. 41:1503–1518. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Anzell AR, Maizy R, Przyklenk K and
Sanderson TH: Mitochondrial quality control and disease: Insights
into ischemia-reperfusion injury. Mol Neurobiol. 55:2547–2564.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Davidson SM, Adameová A, Barile L,
Cabrera-Fuentes HA, Lazou A, Pagliaro P, Stensløkken KO and
Garcia-Dorado D: EU-CARDIOPROTECTION COST Action (CA16225).
Mitochondrial and mitochondrial-independent pathways of myocardial
cell death during ischaemia and reperfusion injury. J Cell Mol Med.
24:3795–3806. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bacci MR, Vasconcelos LY, Murad N, Chagas
ACP, Capuano AC, Alves BC, Pereira EC, Azzalis LA, Junqueira VB and
Fonseca F: Remote ischemic preconditioning in myocardial protection
in hemodialysis patients. Int J Gen Med. 11:175–178.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang L, Zhang Y, Zhu M, Zhang Q, Wang X,
Wang Y, Zhang J, Li J, Yang L, Liu J, et al: Resveratrol attenuates
myocardial ischemia/reperfusion injury through up-regulation of
vascular endothelial growth factor B. Free Radic Biol Med. 101:1–9.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li W, Feng G, Gauthier JM, Lokshina I,
Higashikubo R, Evans S, Liu X, Hassan A, Tanaka S, Cicka M, et al:
Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil
recruitment after heart transplantation. J Clin Invest.
129:2293–2304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lenarczyk M, Su J, Haworth ST, Komorowski
R, Fish BL, Migrino RQ, Harmann L, Hopewell JW, Kronenberg A, Patel
S, et al: Simvastatin mitigates increases in risk factors for and
the occurrence of cardiac disease following 10 Gy total body
irradiation. Pharmacol Res Perspect. 3(e00145)2015.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Oshima Y, Ouchi N, Sato K, Izumiya Y,
Pimentel DR and Walsh K: Follistatin-like 1 is an Akt-regulated
cardioprotective factor that is secreted by the heart. Circulation.
117:3099–3108. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li KS, Jiang WP, Li QC, Zhang HW, Bai Y,
Zhang X and Li HY: MiR-29a in mesenchymal stem cells inhibits FSTL1
secretion and promotes cardiac myocyte apoptosis in
hypoxia-reoxygenation injury. Cardiovasc Pathol.
46(107180)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gokani S and Bhatt LK: Bromodomains: A
novel target for the anticancer therapy. Eur J Pharmacol.
911(174523)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lbik D, Khadjeh S, Mohamed BA, Fischer A,
Hasenfuß G and Toischer K: Abstract 20724: The absence of the
chromatin reader Brd2 decreases heart function and increases
mortality after pressure overload. Circulation.
136(A20724)2017.
|
|
30
|
Zhao Y, Liu X, Fu X, Mo Z, Jiang Y and Yan
Y: Protective effects of epigallocatechin gallate against ischemia
reperfusion injury in rat skeletal muscle via activating Nrf2/HO-1
signaling pathway. Life Sci. 239(117014)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang J, Zhang Q, Liu G and Zhang N:
Therapeutic potentials and mechanisms of the Chinese traditional
medicine Danshensu. Eur J Pharmacol. 864(172710)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang X, Yu Y, Lei H, Cai Y, Shen J, Zhu
P, He Q and Zhao M: The Nrf-2/HO-1 signaling axis: A ray of hope in
cardiovascular diseases. Cardiol Res Pract.
2020(5695723)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang M, Zhu J, Qin X, Zhou M, Zhang X,
Gao Y, Zhang T, Xiao D, Cui W and Cai X: Cardioprotection of
tetrahedral DNA nanostructures in myocardial ischemia-reperfusion
injury. ACS Appl Mater Interfaces. 11:30631–30639. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Enayati A, Yassa N, Mazaheri Z, Rajaei M,
Pourabouk M, Ghorghanlu S, Basiri S and Khori V: Cardioprotective
and anti-apoptotic effects of Potentilla reptans L. root via Nrf2
pathway in an isolated rat heart ischemia/reperfusion model. Life
Sci. 215:216–226. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang P, Luo L, Shen Q, Shi G, Mohammed A,
Ni S and Wu X: Rosuvastatin improves myocardial hypertrophy after
hemodynamic pressure overload via regulating the crosstalk of
Nrf2/ARE and TGF-β/smads pathways in rat heart. Eur J Pharmacol.
820:173–182. 2018.PubMed/NCBI View Article : Google Scholar
|