|
1
|
Reed GW, Rossi JE and Cannon CP: Acute
myocardial infarction. Lancet. 389:197–210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lv J, Zhao Q, Yang J, Gao X, Zhang X, Ye
Y, Dong Q, Fu R, Sun H, Yan X, et al: Length of stay and short-term
outcomes in patients with ST-Segment elevation myocardial
infarction after primary percutaneous coronary intervention:
Insights from the China acute myocardial infarction registry. Int J
Gen Med. 14:5981–5991. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Algoet M, Janssens S, Himmelreich U, Gsell
W, Pusovnik M, Van den Eynde J and Oosterlinck W: Myocardial
ischemia-reperfusion injury and the influence of inflammation.
Trends Cardiovasc Med. 33:357–366. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen X, Rong C, Qi P, Bai W, Yao W, Zhang
Y and Dang Y: LDL-C and total stent length are independent
predictors of periprocedural myocardial injury and infarction for
unstable angina patients undergoing elective percutaneous coronary
intervention. Int J Gen Med. 14:1357–1365. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fujisawa T and Filippakopoulos P:
Functions of bromodomain-containing proteins and their roles in
homeostasis and cancer. Nat Rev Mol Cell Biol. 18:246–262.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lin Z and Li Z, Guo Z, Cao Y, Li J, Liu P
and Li Z: Epigenetic reader bromodomain containing protein 2
facilitates pathological cardiac hypertrophy via regulating the
expression of citrate cycle genes. Front Pharmacol.
13(887991)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sun Y, Xie Y, Du L, Sun J and Liu Z:
Inhibition of BRD4 attenuates cardiomyocyte apoptosis via NF-κB
pathway in a rat model of myocardial infarction. Cardiovasc Ther.
36:2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Q, Sun Y, Li T, Liu L, Zhao Y, Li L,
Zhang L and Meng Y: Function of BRD4 in the pathogenesis of high
glucose-induced cardiac hypertrophy. Mol Med Rep. 19:499–507.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim SY, Zhang X, Schiattarella GG,
Altamirano F, Ramos TAR, French KM, Jiang N, Szweda PA, Evers BM,
May HI, et al: Epigenetic Reader BRD4 (Bromodomain-Containing
Protein 4) governs nucleus-encoded mitochondrial transcriptome to
regulate cardiac function. Circulation. 142:2356–2370.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Padmanabhan A, Alexanian M,
Linares-Saldana R, González-Terán B, Andreoletti G, Huang Y,
Connolly AJ, Kim W, Hsu A, Duan Q, et al: BRD4
(Bromodomain-Containing Protein 4) Interacts with GATA4 (GATA
Binding Protein 4) to govern mitochondrial homeostasis in adult
cardiomyocytes. Circulation. 142:2338–2355. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chi X, Shan L, Hu Y, Zhang Y, Mao Y and
Wang X: Bromodomain-containing protein 7 contributes to myocardial
infarction-induced myocardial injury through activating
Wnt/β-catenin signaling. Ann Palliat Med. 10:10756–10767.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schirone L, Forte M, D'Ambrosio L, Valenti
V, Vecchio D, Schiavon S, Spinosa G, Sarto G, Petrozza V, Frati G
and Sciarretta S: An overview of the molecular mechanisms
associated with myocardial ischemic injury: State of the art and
translational perspectives. Cells. 11(1165)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen R, Zhou M and Zhu F: Immune
checkpoint inhibitors related to cardiotoxicity. J Cardiovasc Dev
Dis. 9(378)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pertiwi KR, Hillman RM, Scott CA and
Chilton EL: Ischemia reperfusion injury produces, and ischemic
preconditioning prevents, rat cardiac fibroblast differentiation:
Role of KATP channels. J Cardiovasc Dev Dis.
6(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xie LH, Fefelova N, Pamarthi SH and
Gwathmey JK: Molecular mechanisms of ferroptosis and relevance to
cardiovascular disease. Cells. 11(2726)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kustatscher G, Collins T, Gingras AC, Guo
T, Hermjakob H, Ideker T, Lilley KS, Lundberg E, Marcotte EM,
Ralser M and Rappsilber J: Understudied proteins: Opportunities and
challenges for functional proteomics. Nat Methods. 19:774–779.
2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou Y, Zhou B, Pache L, Chang M,
Khodabakhshi AH, Tanaseichuk O, Benner C and Chanda SK: Metascape
provides a biologist-oriented resource for the analysis of
systems-level datasets. Nat Commun. 10(1523)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
DeMars KM, Yang C, Castro-Rivera CI and
Candelario-Jalil E: Selective degradation of BET proteins with
dBET1, a proteolysis-targeting chimera, potently reduces
pro-inflammatory responses in lipopolysaccharide-activated
microglia. Biochem Biophys Res Commun. 497:410–415. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Q, Cui HH, Yang YJ, Li XD, Chen GH,
Tian XQ, Jin C, Dong QT, Huang PS and Xu J: Quantitative proteomics
analysis of ischemia/reperfusion injury-modulated proteins in
cardiac microvascular endothelial cells and the protective role of
tongxinluo. Cell Physiol Biochem. 41:1503–1518. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Anzell AR, Maizy R, Przyklenk K and
Sanderson TH: Mitochondrial quality control and disease: Insights
into ischemia-reperfusion injury. Mol Neurobiol. 55:2547–2564.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Davidson SM, Adameová A, Barile L,
Cabrera-Fuentes HA, Lazou A, Pagliaro P, Stensløkken KO and
Garcia-Dorado D: EU-CARDIOPROTECTION COST Action (CA16225).
Mitochondrial and mitochondrial-independent pathways of myocardial
cell death during ischaemia and reperfusion injury. J Cell Mol Med.
24:3795–3806. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bacci MR, Vasconcelos LY, Murad N, Chagas
ACP, Capuano AC, Alves BC, Pereira EC, Azzalis LA, Junqueira VB and
Fonseca F: Remote ischemic preconditioning in myocardial protection
in hemodialysis patients. Int J Gen Med. 11:175–178.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang L, Zhang Y, Zhu M, Zhang Q, Wang X,
Wang Y, Zhang J, Li J, Yang L, Liu J, et al: Resveratrol attenuates
myocardial ischemia/reperfusion injury through up-regulation of
vascular endothelial growth factor B. Free Radic Biol Med. 101:1–9.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li W, Feng G, Gauthier JM, Lokshina I,
Higashikubo R, Evans S, Liu X, Hassan A, Tanaka S, Cicka M, et al:
Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil
recruitment after heart transplantation. J Clin Invest.
129:2293–2304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lenarczyk M, Su J, Haworth ST, Komorowski
R, Fish BL, Migrino RQ, Harmann L, Hopewell JW, Kronenberg A, Patel
S, et al: Simvastatin mitigates increases in risk factors for and
the occurrence of cardiac disease following 10 Gy total body
irradiation. Pharmacol Res Perspect. 3(e00145)2015.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Oshima Y, Ouchi N, Sato K, Izumiya Y,
Pimentel DR and Walsh K: Follistatin-like 1 is an Akt-regulated
cardioprotective factor that is secreted by the heart. Circulation.
117:3099–3108. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li KS, Jiang WP, Li QC, Zhang HW, Bai Y,
Zhang X and Li HY: MiR-29a in mesenchymal stem cells inhibits FSTL1
secretion and promotes cardiac myocyte apoptosis in
hypoxia-reoxygenation injury. Cardiovasc Pathol.
46(107180)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gokani S and Bhatt LK: Bromodomains: A
novel target for the anticancer therapy. Eur J Pharmacol.
911(174523)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lbik D, Khadjeh S, Mohamed BA, Fischer A,
Hasenfuß G and Toischer K: Abstract 20724: The absence of the
chromatin reader Brd2 decreases heart function and increases
mortality after pressure overload. Circulation.
136(A20724)2017.
|
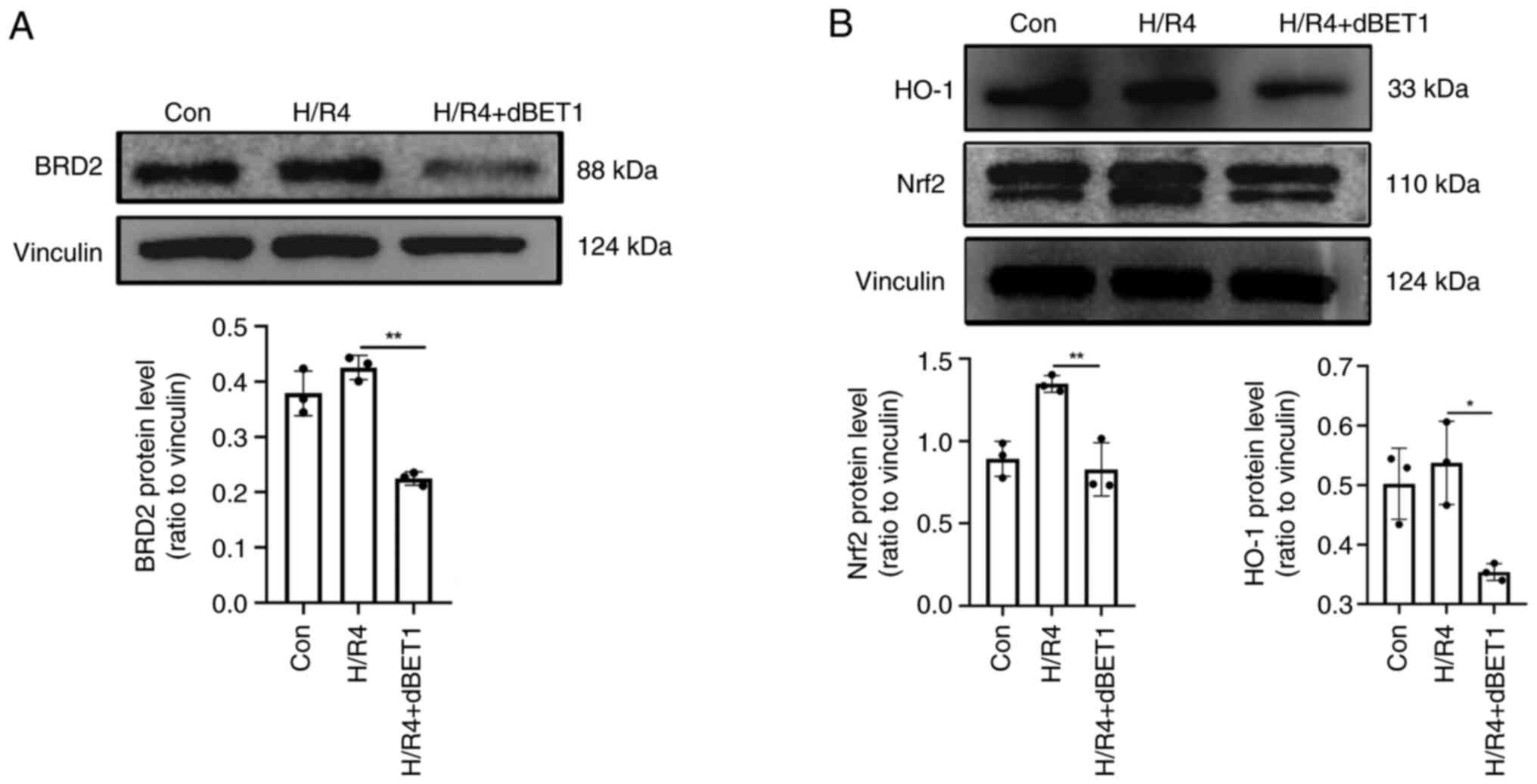
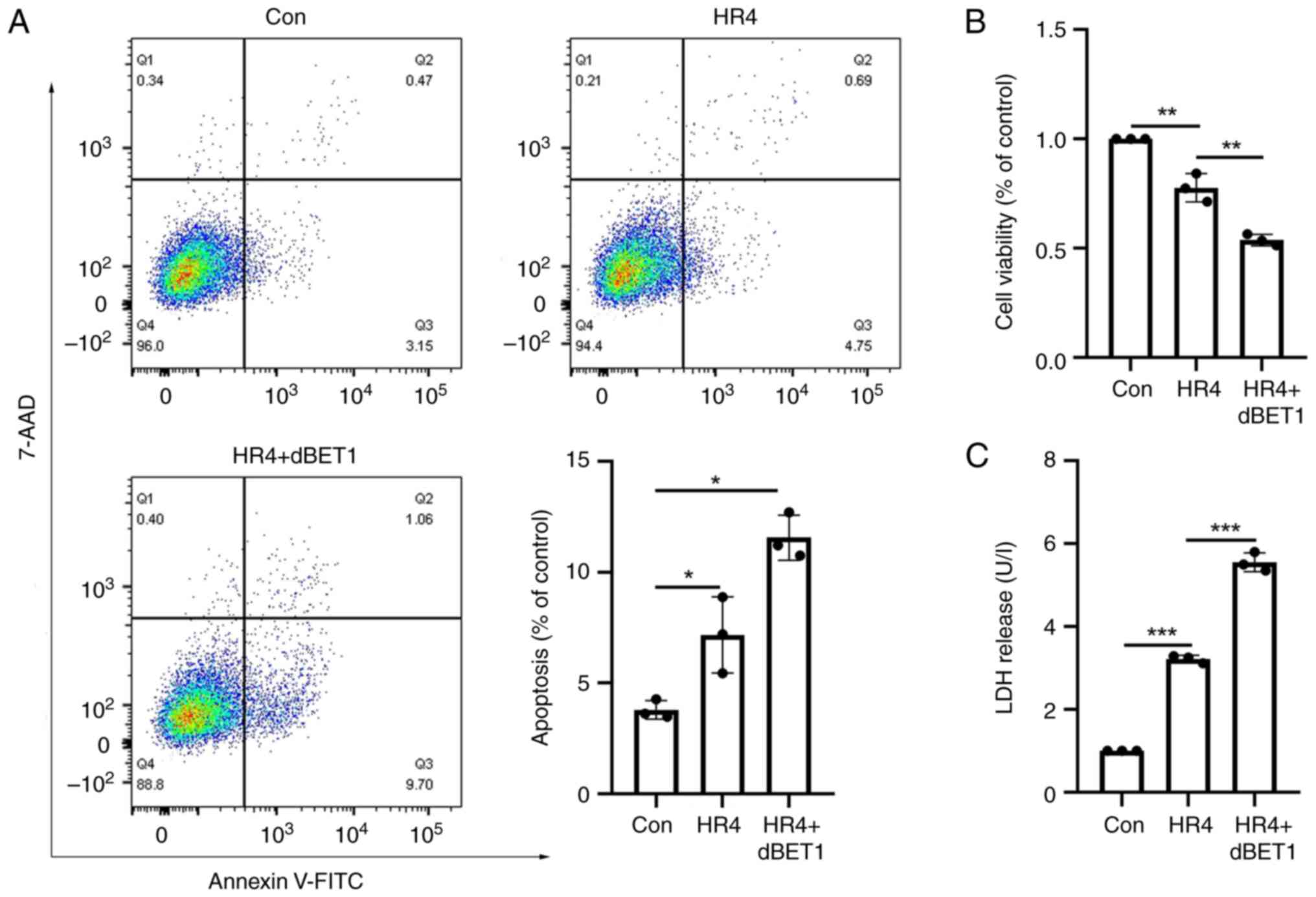
|
30
|
Zhao Y, Liu X, Fu X, Mo Z, Jiang Y and Yan
Y: Protective effects of epigallocatechin gallate against ischemia
reperfusion injury in rat skeletal muscle via activating Nrf2/HO-1
signaling pathway. Life Sci. 239(117014)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang J, Zhang Q, Liu G and Zhang N:
Therapeutic potentials and mechanisms of the Chinese traditional
medicine Danshensu. Eur J Pharmacol. 864(172710)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang X, Yu Y, Lei H, Cai Y, Shen J, Zhu
P, He Q and Zhao M: The Nrf-2/HO-1 signaling axis: A ray of hope in
cardiovascular diseases. Cardiol Res Pract.
2020(5695723)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang M, Zhu J, Qin X, Zhou M, Zhang X,
Gao Y, Zhang T, Xiao D, Cui W and Cai X: Cardioprotection of
tetrahedral DNA nanostructures in myocardial ischemia-reperfusion
injury. ACS Appl Mater Interfaces. 11:30631–30639. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Enayati A, Yassa N, Mazaheri Z, Rajaei M,
Pourabouk M, Ghorghanlu S, Basiri S and Khori V: Cardioprotective
and anti-apoptotic effects of Potentilla reptans L. root via Nrf2
pathway in an isolated rat heart ischemia/reperfusion model. Life
Sci. 215:216–226. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang P, Luo L, Shen Q, Shi G, Mohammed A,
Ni S and Wu X: Rosuvastatin improves myocardial hypertrophy after
hemodynamic pressure overload via regulating the crosstalk of
Nrf2/ARE and TGF-β/smads pathways in rat heart. Eur J Pharmacol.
820:173–182. 2018.PubMed/NCBI View Article : Google Scholar
|