Introduction
It is almost 100 years since Dr Henry Pancoast first
described the clinical and radiographic findings associated with
superior sulcus tumors (1), which
were later named after the author. Superior sulcus tumors are a
relatively rare subtype of non-small cell lung cancer (NSCLC),
accounting for <5% of all bronchogenic carcinomas. This tumor
occurs in the apex of the upper lobe of the lung and often involves
the first rib, brachial plexus, subclavian vessels, sympathetic
chain, stellate ganglion or vertebrae. Clinically, a superior
sulcus tumor consists of a constellation of characteristic
symptoms, including arm and shoulder pain or Horner's syndrome
(2,3).
In the 1930s to 1950s, superior sulcus tumors of the
lung were considered inoperable and incurable and radiation therapy
was predominantly used to alleviate the condition. In the 1950s,
Chardack and MacCallum (4)
reported the first successful surgical resection and postoperative
radiotherapy for upper sulcus tumors; the patient was alive and
disease-free 5 years later. In 1956, Shaw introduced a new
treatment model: Preoperative radiotherapy combined with radical
resection surgery. Patients receiving this treatment achieved good
results (5) and within 40 years,
bimodal therapy (radiotherapy plus surgery) became the standard
treatment method for upper sulcus tumors (6). However, survival did not
significantly improve with this treatment and the 5-year overall
survival remained ~30%. Since the 1990s, increasing experience and
research data in combination therapy has led to the introduction of
radical surgical resection after induced chemoradiotherapy as a new
treatment standard for superior sulcus tumors, with significantly
improved results (7-14).
The present study reported a patient with an
epidermal growth factor receptor (EGFR)-mutated superior sulcus
tumor who underwent surgical resection following neoadjuvant
targeted therapy and achieved good curative effect, with no
recurrence to date during follow-up.
Case Report
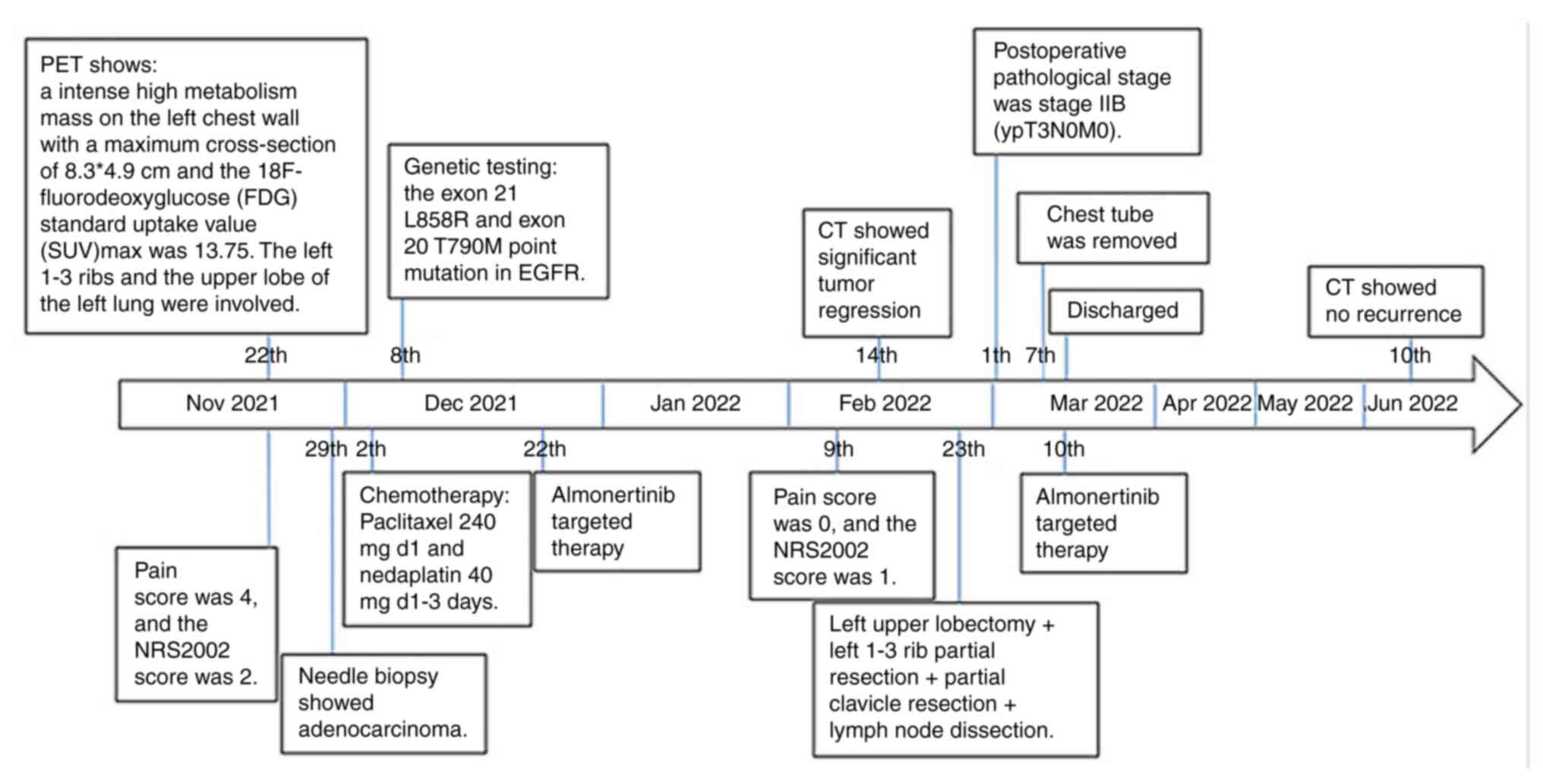
A 52-year-old male patient was from a rural area and
had a history of smoking and alcohol consumption. In the past
month, he had repeatedly experienced left chest pain, which he was
able to bear most of the time, so he did not go to the hospital;
occasionally, he needed to take painkillers to alleviate pain
symptoms. The left chest pain had gradually worsened over the
previous week, accompanied by persistent left shoulder pain. This
pain prevented the patient placing their left thoracic back and
shoulders on the back of a chair and the surface of a bed.
Therefore, the patient sought medical treatment at the Songzi
People's Hospital (Jingzhou, China) in November 2021 and the doctor
prescribed non-steroidal anti-inflammatory drugs for pain relief;
however, these drugs did not relieve the left chest and shoulder
pain. Simultaneously, chest computed tomography (CT) examination
revealed a lump in the left lung of the patient. As the exact
diagnosis was unclear, the patient visited the thoracic surgery
clinic of Huazhong University of Science and Technology Tongji
Medical College Affiliated Hubei Cancer Hospital (Wuhan, China) in
November 2021.
The patient was unable to rest flat on the ward bed
upon admission and the pain was grade 4 (Pain was scored on a 0-10
numerical rating scale, ranging from 0 (no pain) to 10 (worst pain
imaginable) or categorized (none, mild, moderate, severe or very
severe/horrible). (15-17),
with a Nutritional Risk Screening 2002 (NRS2002) score of 2 (The
NRS2002 system evaluates patients based on their nutritional status
and disease severity, and gives a total score of 0-6 based on
whether they are absent, mild, moderate, or severe) (18). The patient had no family history of
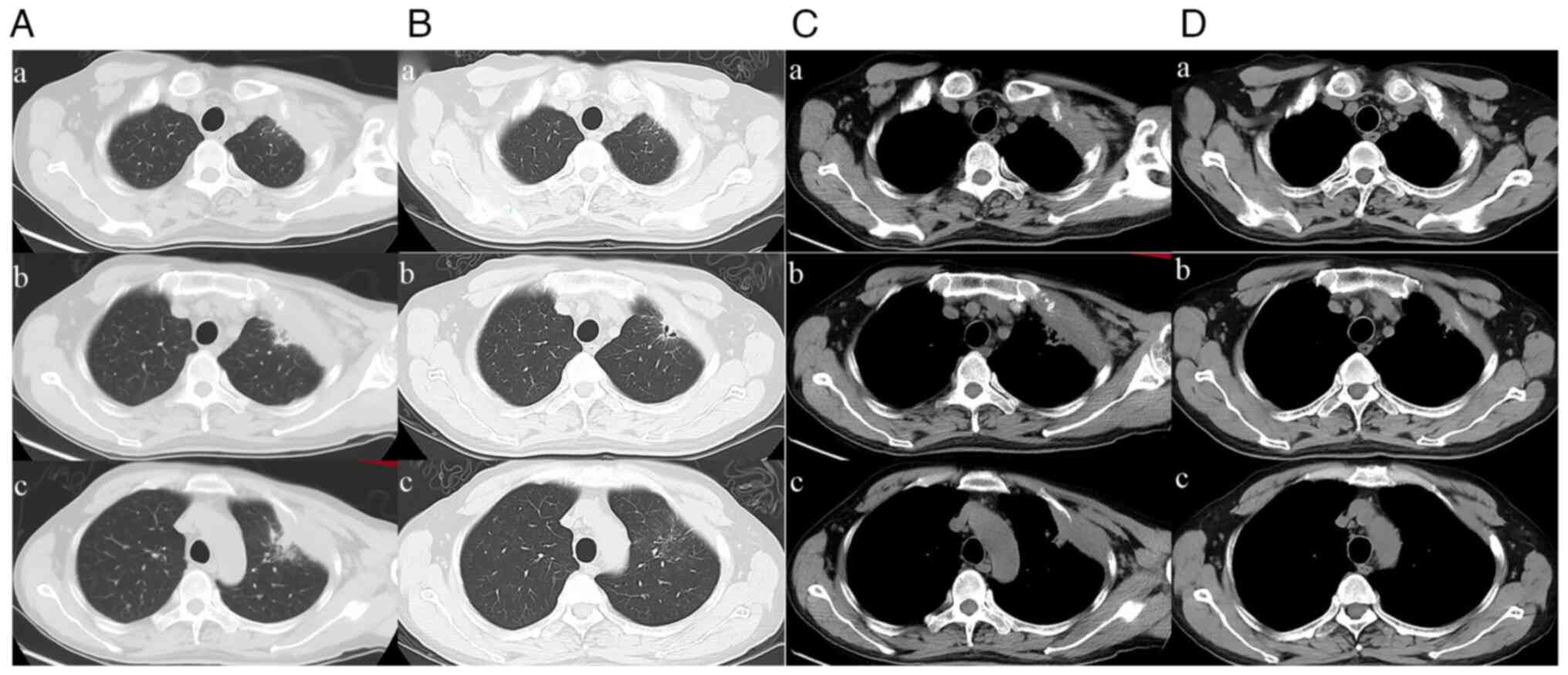
oncologic diseases. A CT scan revealed an 81x43 mm mass of the left
upper chest wall, which invaded the first, second and third
anterior ribs (Figs. 1 and
2A). Considering that the
patient's mass was located at the left lung apex, compression of
the mass itself onto the left brachial plexus might have been the
cause of the severe pain. Consequently, a powerful opioid analgesic
drug (morphine; initial dose 20 mg q12 h) was administered to the
patient for pain relief. After maintaining the initial dose for 12
days, in order to achieve superior pain relief, the patient's daily
dose of morphine was increased to 30 mg q12 h and continued to be
used for ~1 month thereafter. Upon admission on November 23, 2021,
blood routine and biochemical examination revealed the following: a
white blood cell count of 21.2 (normal range:
4-10x109/l), a platelet count of 440 (normal range:
100-300x109/l), hemoglobin of 133 (normal range: 110-170
g/l) and carbon dioxide binding capacity 20.6 (normal range: 21-31
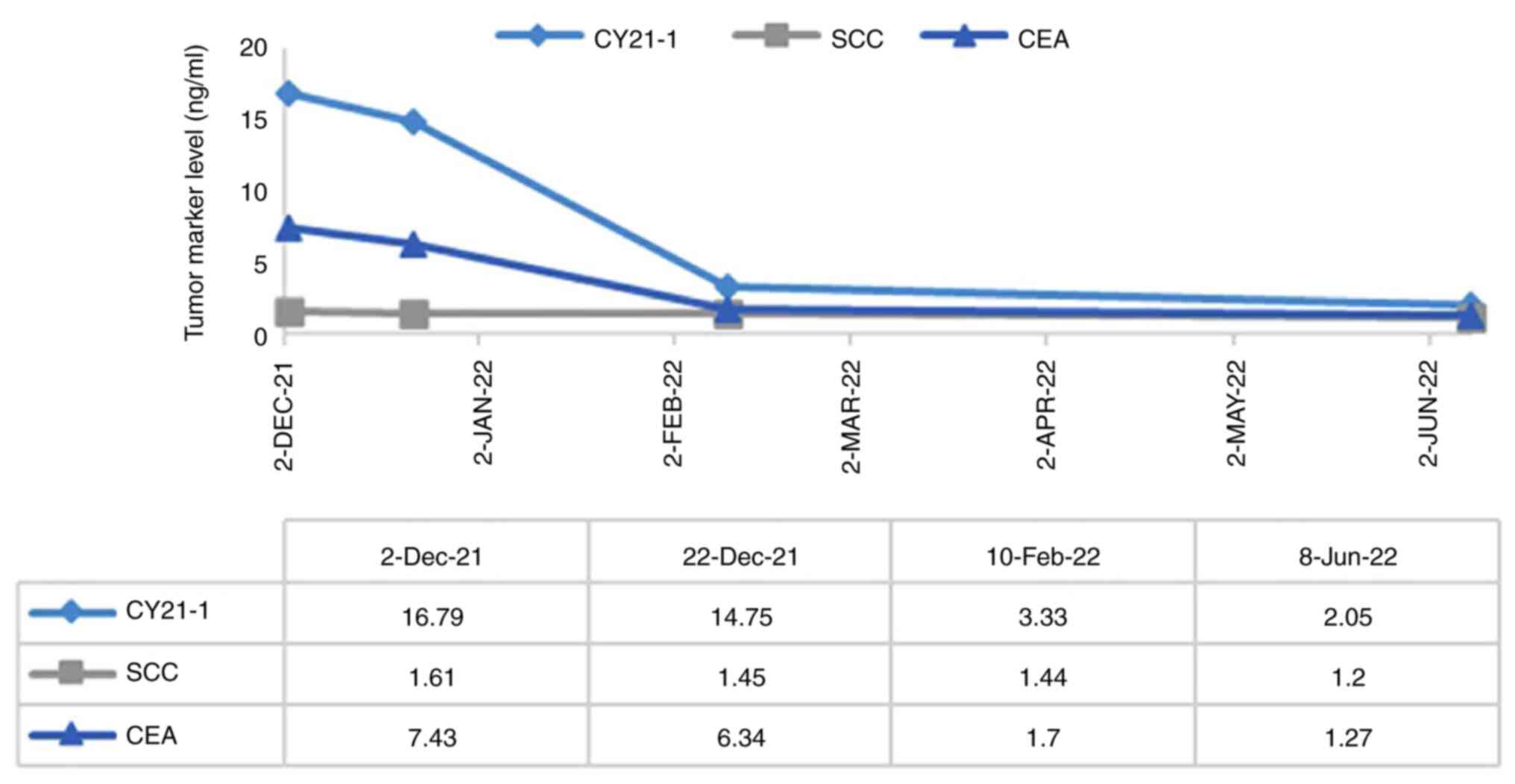
mmol/l). Tumor marker results showed cancer antigen 125 180 U/ml
(normal range, <35 U/ml), cytokeratin 19 fragment (CYFRA 21-1)
16.79 ng/ml (normal range, <3.3 ng/ml), neuroson-specific
enolase 17.53 ng/ml (normal range, 0-16.3 ng/ml), carcinoembryonic
antigen 7.43 ng/ml (normal range, <5 ng/ml) and SCC 1.61 ng/ml
(normal range, <2.7 ng/ml). Positron emission tomography CT
revealed intense high metabolism of the mass, with a size of 83x49
mm, an 18F-fluorodeoxyglucose standard uptake value maximum of
13.75 and involvement of the left 1-3 ribs and the upper lobe of
the left lung. Needle biopsy of the mass showed poorly
differentiated adenocarcinoma; therefore, the patient was diagnosed
with stage cT4N2M0, ⅢA lung cancer and TNM classification was
performed according to the criteria of the 8th edition staging of
the TNM classification (19). The
mutation of EGFR was detected by DNA sequencing. DNA sequencing was
performed using reverse transcription-polymerase chain reaction,
which was carried out by the Pathology Department of Hubei Cancer
Hospital. The AmoyDx EGFR 29 Mutations Detection Kit (Amoy
Diagnostics Co., Ltd.) was used. The AmoyDx EGFR 29 Mutations
Detection Kit is a real-time PCR assay for qualitative detection of
29 somatic mutation in exons 18, 19, 20 and 21 of EGFR gene in
human genomic DNA extracted from formalin-fixed paraffin-embedded
tumor tissue. The kit adopts amplification refractory mutation
system technology which comprises specific primers and fluorescent
probes to detect gene mutations in reverse transcription PCR assay.
During the nucleic acid amplification, the targeted mutant DNA is
matched with the bases at the 3' end of the primer, amplified
selectively and efficiently, then the mutant amplicon is detected
by fluorescent probes labeled with fluorescein amidite. Where the
wild-type DNA cannot be matched with specific primers, no
amplification occurs. The kit is composed of EGFR Reaction Mix
strips, EGFR Enzyme Mix (contains Taq DNA polymerase for PCR
amplification and uracil-N-glycosylase which works at room
temperature to prevent PCR amplicon carryover contamination) and
EGFR Positive Control. The thermocycling conditions are shown in
Table I. The results showed that
there were exon 21 L858R and exon 20 T790M point mutations in
EGFR.
 | Table ICycling parameters. |
Table I
Cycling parameters.
| Stage | Cycles | Temperature | Time | Data collection |
|---|
| 1 | 1 | 95˚C | 5 min | / |
| | | 95˚C | 25 sec | / |
| 2 | 15 | 64˚C | 20 sec | / |
| | | 72˚C | 20 sec | / |
| | | 93˚C | 25 sec | / |
| 3 | 31 | 60˚C | 35 sec | FAM and HEX/VIC |
| | | 72˚C | 20 sec | / |
During the period of examination following
admission, the painkillers were effective, allowing the patient to
briefly rest flat on the ward bed. The patient was offered targeted
therapy (almonertinib; 110 mg qd) from the second neoadjuvant
cycle. After 6 weeks of almonertinib targeted therapy, the
patient's chest pain was significantly relieved, the tumor marker
CYFRA 21-1 was 3.33 ng/ml, markedly lower than that before
treatment and other lung adenocarcinoma-related tumor markers had
decreased to normal ranges (Fig.
3). In addition, the blood routine test results reached normal
standards: white blood cell count was 4.3 (normal range:
4-10x109/l), platelet count was 268 (normal range:
100-300x109/l) and hemoglobin was 109 (normal range:
110-170 g/l). Chest enhanced CT showed significant tumor regression
compared to the chest CT results on November 22, 2021; the target
lesion diameter was reduced by 36.75% (Fig. 2B). According to the Response
Evaluation Criteria in Solid Tumors (RECIST) methodology, the
efficacy evaluation was partial response (PR). The clinical stage
reduced to stage ⅡB (T3N0M0) (19).
The patient underwent a thoracotomy on February 23,
2022. A combined incision of the anterolateral incision and the
superior median sternotomy was used and tumor-free margins obtained
in a section of the chest wall (ribs 1, 2 and 3), which included
1-3 parts of the anterior rib and a segment of the clavicle. Next,
a lobectomy and systematic lymph node dissection was performed
according to the surgical treatment principle of lung cancer.
Finally, the chest wall was reconstructed with artificial mesh
after placing a chest tube. The whole operation took 5 h and the
intraoperative bleeding totaled ~600 ml.
Postoperative histopathological examination was
carried out by the Pathology Department of Hubei Cancer Hospital,
and the pathological results were moderately differentiated and
invasive adenocarcinoma in the left upper lobe of the lung [large
area of inflammatory and fibrotic components (83%), ~15% of tumor
cells and 2% of tumor cell necrosis], with visceral pleural
invasion (PL3+) and rib invasion. The lymph nodes and cutting edge
were negative, that is, R0 resection. The pathological stage was
ypT3N0M0, ⅡB (19).
The patient had postoperative chylous pleural
effusion and recovered after receiving total parenteral nutrition
therapy for 3 days without any serious in-hospital complications.
The chest tube was removed on postoperative day 12 and the patient
was discharged 15 days after surgery. The targeted therapy with
almonertinib was continued postoperatively. No disease recurrence
has been detected during one year of follow-up.
Discussion
It is not satisfactory to treat superior sulcus
tumors with a single form of treatment. Preoperative irradiation,
which aims to shrink tumors, appears to improve tumor resectability
and yields satisfactory palliative results when combined with
surgical resection. In 1961, Shaw et al (5) first described the advantages of
preoperative radiotherapy for superior sulcus tumors.
Since the 1990s, concurrent chemoradiotherapy
combined with surgery has been applied to the treatment of superior
sulcus tumors and a series of studies have confirmed the
effectiveness of this method of treatment (10,12,20,21).
The principle of adding concurrent chemotherapy on a base of
preoperative radiotherapy is adding systemic treatment on a base of
local treatment to control occult systemic disease and limit the
risk of distant relapse (10).
Induction concurrent chemoradiotherapy followed by radical surgical
resection has become the current standard of care in patients with
superior sulcus tumors.
The clinical stage of our patient was T4N0M0 and the
radiotherapy plan could not be implemented because of the patient's
pain and inability to lie down. Therefore, paclitaxel combined with
platinum-based chemotherapy was administered and genetic testing
performed. Considering the efficacy of chemotherapy for resectable
NSCLC, the 5-year survival benefit is only 5-6% (22-24).
Subsequently, targeted therapy was administered after learning of
the EGFR gene mutation. After 4-8 weeks of treatment, imaging
showed that this patient had a good response to treatment, with
significant tumor volume reduction. This may indicate an efficacy
advantage of almonertinib in the neoadjuvant phase of therapy.
Likewise, the EMERGING-CTONG 1103 study (25) revealed that there is a tendency
toward an improved overall response rate, lymph node step-down,
major pathological response and R0 resection rate with the
neoadjuvant erlotinib, in comparison with a neoadjuvant
chemotherapy for patients who suffered from EGFRm NSCLC and chronic
progression-free survival to a great extent. According to the
indications from case reports and other reported clinical tests,
EGFR-tyrosine kinase inhibitor (EGFR-TKI) therapy before surgery
can achieve a certain effect for patients with resectable NSCLC
(26-36),
which indicates that neoadjuvant targeted therapy can also become a
clinical option. Based on superior sulcus tumors exhibiting the
same biological behavior as other lung cancers (37), we consider that neoadjuvant
targeted therapy may be a consideration for superior sulcus
tumors.
Compared with the efficacy of the pathologic
complete response (pCR) obtained after neoadjuvant immunotherapy
combined with chemotherapy reported in Tang et al (38), our patient still had viable tumor
cells after neoadjuvant targeted therapy. However, the small
proportion of tumor cells and the large number of inflammatory
responses suggest that our neoadjuvant targeted therapy is
effective. Furthermore, it is not known whether the patient can
achieve superior treatment results or even achieve pCR if the
duration of preoperative induction targeted therapy is prolonged
(for example, up to 8 weeks). In addition, the timing of surgery
after neoadjuvant targeted therapy should be considered because it
also may affect the overall oncologic outcomes (31,39).
For example, in a retrospective study of patients with stage IIIa
NSCLC, after preoperative EGFR-TKI neoadjuvant therapy, 1- and
3-year survival were significantly decreased in the short-delay
group compared with the long-delay group (40). However, there is no unified
conclusion on the surgical intervention time of neoadjuvant
targeted therapy. We consider that choosing to perform surgical
intervention after 2 weeks of drug withdrawal can reduce the impact
of drug side effects and increase the safety of surgery.
The present study is the first case report of
neoadjuvant targeted therapy for superior sulcus tumors. In this
case, almonertinib achieved good efficacy and safety and this has
reference significance for guiding perioperative targeted therapy
for EGFR-mutated superior sulcus tumors. In summary, the present
study provided real-world evidence that neoadjuvant targeted
therapy enables patients to achieve surgical R0 resection and
obtain superior results. In the future, following confirmation by
more studies in this field, neoadjuvant targeted therapy may become
a standard treatment option for superior sulcus tumors after
neoadjuvant chemoradiotherapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JK and FX conceived and designed the study. SC
collected data and images. KK and ZJ wrote the manuscript. KK, SC
and JK confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient.
Patient consent for publication
The patient provided written informed consent
regarding the publication of the case details and any associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nikolaos P, Vasilios L, Efstratios K,
Panagiotis A, Christos P, Nikolaos B, Antonios H, Tsakiridis K,
Zarogoulidis P, Zarogoulidis K, et al: Therapeutic modalities for
Pancoast tumors. J Thorac Dis. 6 (Suppl 1):S180–S193.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Detterbeck FC: Changes in the treatment of
Pancoast tumors. Ann Thorac Surg. 75:1990–1997. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Marulli G, Battistella L, Mammana M,
Calabrese F and Rea F: Superior sulcus tumors (Pancoast tumors).
Ann Transl Med. 4(239)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chardack WM and Maccallum JD: Pancoast
tumor; five-year survival without recurrence or metastases
following radical resection and postoperative irradiation. J Thorac
Surg. 31:535–542. 1956.PubMed/NCBI
|
|
5
|
Shaw RR, Paulson DL and Kee JL: Treatment
of superior sulcus tumor by irradiation followed by resection. Ann
Surg. 154:29–40. 1961.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shaw RR: Pancoast's tumor. Ann Thorac
Surg. 37:343–345. 1984.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Martínez-Monge R, Herreros J, Aristu JJ,
Aramendía JM and Azinovic I: Combined treatment in superior sulcus
tumors. Am J Clin Oncol. 17:317–322. 1994.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Attar S, Krasna MJ, Sonett JR, Hankins JR,
Slawson RG, Suter CM and McLaughlin JS: Superior sulcus (Pancoast)
tumor: Experience with 105 patients. Ann Thorac Surg. 66:193–198.
1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barnes JB, Johnson SB, Dahiya RS, Temes
RT, Herman TS and Thomas CR Jr: Concomitant weekly cisplatin and
thoracic radiotherapy for Pancoast tumors of the lung: Pilot
experience of the San Antonio cancer institute. Am J Clin Oncol.
25:90–92. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rusch VW, Giroux DJ, Kraut MJ, Crowley J,
Hazuka M, Winton T, Johnson DH, Shulman L, Shepherd F, Deschamps C,
et al: Induction chemoradiation and surgical resection for superior
sulcus non-small-cell lung carcinomas: Long-term results of
Southwest oncology group trial 9416 (Intergroup Trial 0160). J Clin
Oncol. 25:313–318. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pourel N, Santelmo N, Naafa N, Serre A,
Hilgers W, Mineur L, Molinari N and Reboul F: Concurrent
cisplatin/etoposide plus 3D-conformal radiotherapy followed by
surgery for stage IIB (superior sulcus T3N0)/III non-small cell
lung cancer yields a high rate of pathological complete response.
Eur J Cardiothorac Surg. 33:829–836. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kunitoh H, Kato H, Tsuboi M, Shibata T,
Asamura H, Ichinose Y, Katakami N, Nagai K, Mitsudomi T, Matsumura
A, et al: Phase II trial of preoperative chemoradiotherapy followed
by surgical resection in patients with superior sulcus
non-small-cell lung cancers: Report of Japan clinical oncology
group trial 9806. J Clin Oncol. 26:644–649. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kappers I, Belderbos JS, Burgers JA, van
Zandwijk N, Groen HJ and Klomp HM: Non-small cell lung carcinoma of
the superior sulcus: Favourable outcomes of combined modality
treatment in carefully selected patients. Lung Cancer. 59:385–390.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Albain KS, Rusch VW, Crowley JJ, Rice TW,
Turrisi AT III, Weick JK, Lonchyna VA, Presant CA, McKenna RJ and
Gandara DR: Concurrent cisplatin/etoposide plus chest radiotherapy
followed by surgery for stages IIIA (N2) and IIIB non-small-cell
lung cancer: Mature results of Southwest oncology group phase II
study 8805. J Clin Oncol. 13:1880–1892. 1995.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jensen MP and Karoly P: Self-report scales
and procedures for assessing pain in adults. In: Turk DC, Melzack R
(eds). Handbook of Pain Assessment, 2nd edn. Guilford Publications:
New York: pp 15-34, 2001.
|
|
16
|
Kang Y and Demiris G: Self-report pain
assessment tools for cognitively intact older adults: Integrative
review. Int J Older People Nurs. 13(e12170)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Simmons SF, Schnelle JF, Saraf AA, Coelho
CC, Jacobsen JM, Kripalani S, Bell S, Mixon A and Vasilevskis EE:
Pain and satisfaction with pain management among older patients
during the transition from acute to skilled nursing care.
Gerontologist. 56:1138–1145. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kondrup J, Rasmussen HH, Hamberg O and
Stanga Z: Ad Hoc ESPEN Working Group. Nutritional risk screening
(NRS 2002): A new method based on an analysis of controlled
clinical trials. Clin Nutr. 22:321–336. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Felip E and Rosell R: New strategies in
the treatment of resectable non-small cell lung cancer. Expert Rev
Anticancer Ther. 1:224–228. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Waseda R, Klikovits T, Hoda MA,
Hoetzenecker K, Bertoglio P, Dieckmann K, Zöchbauer-Müller S,
Pirker R, Prosch H, Döme B and Klepetko W: Trimodality therapy for
Pancoast tumors: T4 is not a contraindication to radical surgery. J
Surg Oncol. 116:227–235. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Roth JA, Fossella F, Komaki R, Ryan MB,
Putnam JB Jr, Lee JS, Dhingra H, De Caro L, Chasen M and McGavran
M: A randomized trial comparing perioperative chemotherapy and
surgery with surgery alone in resectable stage IIIA non-small-cell
lung cancer. J Natl Cancer Inst. 86:673–680. 1994.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rosell R, Gómez-Codina J, Camps C, Maestre
J, Padille J, Cantó A, Mate JL, Li S, Roig J and Olazábal A: A
randomized trial comparing preoperative chemotherapy plus surgery
with surgery alone in patients with non-small-cell lung cancer. N
Engl J Med. 330:153–158. 1994.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pisters KM, Vallières E, Crowley JJ,
Franklin WA, Bunn PA Jr, Ginsberg RJ, Putnam JB Jr, Chansky K and
Gandara D: Surgery with or without preoperative paclitaxel and
carboplatin in early-stage non-small-cell lung cancer: Southwest
oncology group trial S9900, an intergroup, randomized, phase III
trial. J Clin Oncol. 28:1843–1849. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhong WZ, Chen KN, Chen C, Gu CD, Wang J,
Yang XN, Mao WM, Wang Q, Qiao GB, Cheng Y, et al: Erlotinib versus
gemcitabine plus cisplatin as neoadjuvant treatment of stage
IIIA-N2 EGFR-mutant non-small-cell lung cancer (EMERGING-CTONG
1103): A randomized phase II study. J Clin Oncol. 37:2235–2245.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hishida T, Nagai K, Mitsudomi T, Yokoi K,
Kondo H, Horinouchi H, Akiyama H, Nagayasu T and Tsuboi M: Japan
Clinical Oncology Group. Salvage surgery for advanced non-small
cell lung cancer after response to gefitinib. J Thorac Cardiovasc
Surg. 140:e69–e71. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lara-Guerra H, Chung CT, Schwock J,
Pintilie M, Hwang DM, Leighl NB, Waddell TK and Tsao MS:
Histopathological and immunohistochemical features associated with
clinical response to neoadjuvant gefitinib therapy in early stage
non-small cell lung cancer. Lung Cancer. 76:235–241.
2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Schaake EE, Kappers I, Codrington HE,
Olmos RA, Teertstra HJ, van Pel R, Burgers JA, van Tinteren H and
Klomp HM: Tumor response and toxicity of neoadjuvant erlotinib in
patients with early-stage non-small-cell lung cancer. J Clin Oncol.
30:2731–2738. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhong W, Yang X, Yan H, Zhang X, Su J,
Chen Z, Liao R, Nie Q, Dong S, Zhou Q, et al: Phase II study of
biomarker-guided neoadjuvant treatment strategy for IIIA-N2
non-small cell lung cancer based on epidermal growth factor
receptor mutation status. J Hematol Oncol. 8(54)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun L, Guo YJ, Song J, Wang YR, Zhang SL,
Huang LT, Zhao JZ, Jing W, Han CB and Ma JT: Neoadjuvant EGFR-TKI
therapy for EGFR-mutant NSCLC: A systematic review and pooled
analysis of five prospective clinical trials. Front Oncol.
10(586596)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cansouline X, Lipan B, Sizaret D, Tallet
A, Vandier C, Carmier D and Legras A: EGFR-mutant non-small-cell
lung cancer at surgical stages: What is the place for tyrosine
kinase inhibitors? Cancers (Basel). 14(2257)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xiong L, Lou Y, Bai H, Li R, Xia J, Fang
W, Zhang J, Han-Zhang H, Lizaso A, Li B, et al: Efficacy of
erlotinib as neoadjuvant regimen in EGFR-mutant locally advanced
non-small cell lung cancer patients. J Int Med Res.
48(300060519887275)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang Y, Fu F, Hu H, Wang S, Li Y, Hu H
and Chen H: Gefitinib as neoadjuvant therapy for resectable stage
II-IIIA non-small cell lung cancer: A phase II study. J Thorac
Cardiovasc Surg. 161:434–442.e2. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen WQ, Li P, Wang Q, Zhang YJ, Li HY,
Jin XT, Yan S, Kou GF, Cai SL and Liu G: A randomized controlled
study of erlotinib versus pemetrexed combined with cisplatin in
neoadjuvant therapy of stage A EGFR-mutant lung adenocarcinoma.
Zhonghua Zhong Liu Za Zhi. 40:133–137. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
35
|
Liu M, Jiang G, He W, Zhang P and Song N:
Surgical resection of locally advanced pulmonary adenocarcinoma
after gefitinib therapy. Ann Thorac Surg. 92:e11–e12.
2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Feng S, Qiang Z, Wanwan C, Zhaozhun Z,
Yuewu X and Shencun F: Case report: Aumolertinib as neoadjuvant
therapy for patients with unresectable Stage III non-small cell
lung cancer with activated EGFR mutation: Case series. Front Oncol.
12(872225)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Arcasoy SM and Jett JR: Superior pulmonary
sulcus tumors and Pancoast's syndrome. N Engl J Med. 337:1370–1376.
1997.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tang WF, Xu W, Huang WZ, Lin GN, Zeng YM,
Lin JS, Wu M, Bao H, Peng JW, Jiang HM, et al: Pathologic complete
response after neoadjuvant tislelizumab and chemotherapy for
Pancoast tumor: A case report. Thorac Cancer. 12:1256–1259.
2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Wei
YC, Liu YY, Chen C, Cheng Y, Yin R, et al: Gefitinib versus
vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA
(N1-N2) EGFR-mutant NSCLC: Final overall survival analysis of
CTONG1104 phase III trial. J Clin Oncol. 39:713–722.
2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rice JD, Heidel J, Trivedi JR and van
Berkel VH: Optimal surgical timing after neoadjuvant therapy for
Stage IIIa non-small cell lung cancer. Ann Thorac Surg.
109:842–847. 2020.PubMed/NCBI View Article : Google Scholar
|