Introduction
Folic acid metabolism requires the conversion of
5-methylene tetrahydrofolic acid to 5-methyltetrahydrofolic acid by
methylenetetrahydrofolate reductase (MTHFR) (1). The gene polymorphism of MTHFR has
been linked to an increased risk of colorectal cancer (2,3).
MTHFR may have a significant role in folic acid metabolism,
converting 5,10-methylene tetrahydrofolic acid to
5-methyltetrahydrofolic acid, which supplies methyl groups for the
conversion of homocysteine to methionine (4). The mutation at the MTHFR C677T
location decreases MTHFR activity, resulting in normal
hypomethylation of the gene, which in turn results in aberrant
methylation (5). By contrast,
5-methylene tetrahydrofolic acid is necessary for the conversion of
deoxyuric acid to thymic acid. A decrease in MTHFR enzyme activity
results in aberrant thymidylate production, which in turn results
in the incorrect binding of uracil to DNA, which damages DNA and
causes single- and double-strand breaks (6,7). DNA
damage has an essential role in the development and incidence of
cancer (8). Patients with
colorectal cancer are in a state of hypercoagulability, which means
they have an increased tendency to form blood clots. This is due to
a number of factors associated with the cancer itself, including
the release of pro-coagulant factors by the tumor cells and the
activation of the coagulation system by the body in response to the
cancer (9).
In addition, previous research has revealed that the
MTHFR gene polymorphism is associated with thrombosis (10). The mutation generates an imbalance
between vasodilator and contractile factors, which increases the
risk of developing deep vein thrombosis (DVT) (11). The MTHFR C677T mutation results in
the conversion of alanine to valine, which decreases the active
site of MTHFR and causes homocysteine to be converted to methionine
(5). Within the blood, a chemical
called homocysteine is formed when the amino acid methionine is
naturally broken down (metabolized) to be excreted in the urine.
During this breakdown process, homocysteine may be recycled by the
body to be used to build other proteins. Vitamins B12, B6 and
folate are required to do this recycling and the MTHFR enzyme is
needed for recycling to be efficient (12). Homocysteine cannot be efficiently
recycled if an individual does not have sufficient vitamin B12, B6
or folate. Mutations of the gene responsible for the MTHFR enzyme
may result in an enzyme that is not optimally active and,
consequently, homocysteine levels are increased. Mutations of the
gene responsible for the MTHFR enzyme may result in an enzyme that
is not optimally active and, consequently, homocysteine levels are
increased (13). Continuous
accumulation of homocysteine generates excessive oxygen free
radicals that harm the structure and function of vascular
endothelial cells, leading to a reduction in the release of the
endothelial relaxation factor and prostaglandins, as well as to
thrombosis (14).
Patients with colorectal tumors face the
contradictory risks of thrombus and bleeding after surgery and a
surgeon must find a balance between both. One patient at our
department suffered an acute pulmonary embolism (PE) after
undergoing surgery for colorectal cancer. In this case, the rescue
failed and the patient died as a result. Due to this event, the
present study was conceived, focusing on the management of the
postoperative thrombus risk in patients with colorectal cancer. Due
to being hypercoagulable and bedridden for the majority of the time
after surgery, patients with colorectal cancer are more likely to
have thrombosis and experience acute PEs (15).
However, the connection between MTHFR gene
polymorphism and the risk of thrombosis in patients with colorectal
cancer remains uncertain. In the present study, the MTHFR gene
polymorphism of 591 patients with colorectal cancer who were
pathologically diagnosed after surgery was analyzed. The aim of the
present study was to investigate the association between MTHFR gene
polymorphisms and the risk of venous thrombosis in patients with
colorectal cancer and to provide a theoretical basis for early
intervention in patients with a high risk of thrombosis after
surgery for colorectal cancer.
Patients and methods
Patients and data gathering
Patients underwent laparoscopic or da Vinci robotic
surgery for colorectal cancer at the Department of Colorectal
Surgery [Sir Run Run Shaw Hospital (SRRSH), Zhejiang University
School of Medicine, Hangzhou, China], between January 2020 and
December 2021. Written informed consent and informed consent for
biospecimen collection were obtained, which was approved by the
ethics committee of SRRSH (Zhejiang University School of Medicine,
Hangzhou, China) and the patients had provided basic clinical data,
including age, gender and body mass index (BMI). The present study
excluded patients undergoing emergency surgery, non-minimally
invasive colorectal cancer surgery and non-oncology surgery.
Measuring the parameters of D-dimer
(DDi), DVT, PE and thromboelastography (Detailed in below)
DDi and thromboelastography were determined by
collecting venous blood from patients on the first day after the
operation, following the manufacturers' protocols using the
STA-Liatest D-Di assay (automatic coagulation analyzer for DDi
test; STA R MAX; STAGO; Diagnostica), thromboelastography kaolin
detection kit (coagulation method; no. 20212400053; Changshu
ChangJiang Biotechnology Co. Ltd) and thromboelastography (for
thromboelastography test; TCA-6000; no. 20142220297; Zhejiang
Shengyu Medical Technology Co. Ltd). In order to monitor the
occurrence of DVT events, not all patients underwent color Doppler
ultrasonography (IE33; Philips Medical Systems) of both lower
extremities and lung CT pulmonary angiogram (CTPA). A significant
increase in DDi was generally associated with obvious clinical
symptoms (16), such as bilateral
lower limb edema, chest tightness and shortness of breath, and a
significant decrease in blood oxygen saturation was examined
further using arterial blood gas analysis, color Doppler
ultrasonography of both lower extremities or lung CTPA determined
by clinicians.
Identification of MTHFR
polymorphisms
On the first day of routine postoperative blood
testing, 2 ml of peripheral blood was collected in EDTA
anticoagulant tubes, DNA was extracted and purified using a DNA
extraction kit (cat. no. 20160167; Changsha Sanji Bio) and PCR
primers were designed using the whole gene sequence of MTHFR as a
template in the conserved region of the C677T locus (17). The primer sequences used were as
follows: MTHFR-forward primer, 5'-TGTCATCCCTATTGGCAGGTTAC-3';
MTHFR-reverse primer, 5'-GCCTTCACAAAGCGGAAGAAT-3'; and
MTHFR-sequencing primer, 5'-TGCGTGATGATGAAAT-3'. The PCR
amplification (Changsha Sanji Biotechnology Co. Ltd) was followed
by pyrophosphate sequencing using PyroMark Q24 (QIAGEN GmbH). The
associated analysis software (PyroMark Q24 software v2.0.8) was
used to examine the data and the sequences of the genes were
identified.
Statistical analysis
SPSS (version 24; IBM Corp.) was utilized for
statistical data analysis. The Chi-square test was used to analyze
categorical variables, one-way ANOVA (with Tukey's post-hoc test
and the least-significant difference post-hoc test) was used to
analyze continuous variables between multiple groups with a normal
distribution (expressed as mean ± standard deviation) and the
Kruskal-Wallis test (with the Bonferroni-Dunn's post-hoc test) was
used to analyze continuous variables between multiple groups with a
non-normal distribution (expressed as the median ± interquartile
range). P<0.05 was considered to indicate a statistically
significant difference.
Results
Association of MTHFR gene variants
with the clinical features of patients with colorectal cancer
With the approval of the ethics committee of SRRSH
affiliated to the Medical College of Zhejiang University (Hangzhou,
China), 591 patients who underwent surgery for colorectal cancer
between January 2020 and December 2021 were enrolled, including 361
males and 230 females, with a mean age of 64.39 years and a mean
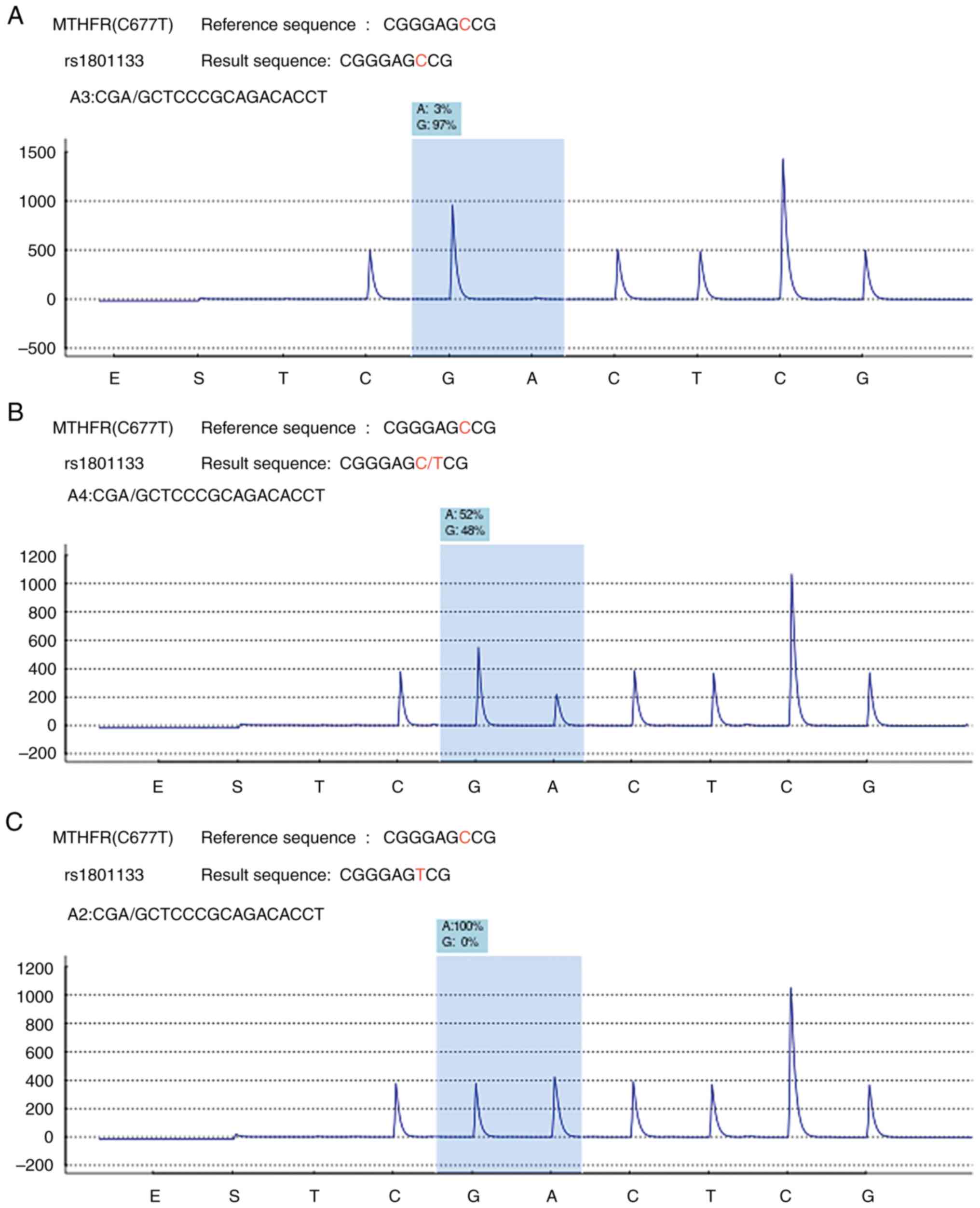
BMI of 23.11 kg/m2. As indicated in Table I, the distribution of the MTHFR
gene among the patients enrolled in the entire observation and
study cycle was the CC type in 219 (37.1%) cases, CT type in 289
(48.9%) cases and TT type in 83 (14%) cases. The representative
chromatograms from the pyrosequencing of the CC, CT and TT variants
are presented in Fig. 1. The
association between the MTHFR gene polymorphism genotype
distribution and the age, sex and BMI of the patients was
investigated. According to the data, there was no significant
difference in the age (P=0.229), sex (P=0.283) or BMI (P=0.908) of
the patients (Table I).
 | Table IAssociation of MTHFR gene variants
with the clinical features of patients with colorectal cancer. |
Table I
Association of MTHFR gene variants
with the clinical features of patients with colorectal cancer.
| | MTHFR genotype | |
|---|
| Characteristic | CC (n=219) | CT (n=289) | TT (n=83) | P-value |
|---|
| Sex | | | | 0.283 |
|
Female | 94 (15.9) | 104 (17.6) | 32 (5.4) | |
|
Male | 125 (21.2) | 185 (31.3) | 51 (8.6) | |
| Age, years | 63.83±11.92 | 65.2±11.61 | 63.05±11.53 | 0.229 |
| BMI, kg/m2 | 23.02±3.17 | 23.16±3.63 | 23.13±4.21 | 0.908 |
Association of MTHFR gene
polymorphisms with thrombotic events and the monitoring of dynamic
DDi
Clinically, the DDi in the peripheral blood of the
patients was screened on the first postoperative day and the test
was repeated as needed when DDi was >5 µg/ml. In addition, an
ultrasound of the deep veins in the lower legs and, if necessary
when obvious chest tightness, pulmonary CTPA was performed. From
the time of surgery until the time of discharge, patients were
observed to assess and document the incidence of thrombosis,
including DVT, and PE. DVT and PE was experienced in 48/591 and
4/591 cases, respectively. As presented in Table II, there was no statistically
significant difference between the proportion of patients with
different MTHFR genotypes and the proportion of patients that
experienced thrombotic events. In addition, there was no
statistically significant difference between the patients with
different MTHFR genotypes and DDi levels on the first postoperative
day. There was also no statistically significant association
between the highest postoperative DDi levels and the MTHFR
genotype.
 | Table IIAssociation of MTHFR gene
polymorphisms with thrombotic events and monitoring of the dynamic
D-dimer. |
Table II
Association of MTHFR gene
polymorphisms with thrombotic events and monitoring of the dynamic
D-dimer.
| | MTHFR genotype | |
|---|
| Characteristic | CC (n=219) | CT (n=289) | TT (n=83) | P-value |
|---|
| DVT | | | | 0.574 |
|
No | 201(34) | 268 (45.3) | 74 (12.5) | |
|
Yes | 18(3) | 21 (3.6) | 9 (1.5) | |
| PE | | | | 0.608 |
|
No | 217 (36.7) | 288 (48.7) | 82 (13.9) | |
|
Yes | 2 (0.3) | 1 (0.2) | 1 (0.2) | |
| DDi 1st day | 2.35±2.52 | 2.19±2.19 | 2.46±2.5 | 0.583 |
| DDi highest time | 2.97±3.45 | 2.65±2.65 | 3.38±3.34 | 0.146 |
Relationship between
thromboelastography and MTHFR genotype
On the first postoperative day, the
thromboelastogram of the peripheral blood of the patients was
examined, which included five indices: The creatine kinase (CK)
reaction time (CK-R) value, the CK kinetics (CK-K) value, the CK
angle of clot formation (CK-Angle), the CK maximum amplitude
(CK-MA) and the CK composite coagulation index (CK-CI). Each of
these indices were compared with the MTHFR genotype distribution to
determine whether there were any differences (Table III). Among the MTHFR CC genotype,
CT genotype and TT genotype, the CK-R values (mean ± standard
deviation) were 4.44±1.21, 4.46±0.97 and 4.40±0.99 min,
respectively. CK-K values were 1.36±0.44, 1.32±0.33 and 1.42±0.53
min for the MTHFR CC genotype, CT genotype and TT genotype
respectively. The CK-Angle values were 73.80±4.35, 74.09±3.58 and
73.39±4.92 degrees for the MTHFR CC genotype, CT genotype and TT
genotype, respectively. The CK-MA values were 64.18±5.71,
65.18±5.20 and 64.02±6.62 mm for the MTHFR CC genotype, CT genotype
and TT genotype, respectively. The mean ± standard deviation CK-CI
values were 2.25±1.59, 2.41±1.31 and 2.2±1.61 for the MTHFR CC
genotype, CT genotype and TT genotype, respectively. The analysis
revealed that there were no statistically significant differences
in the thromboelastography indexes among patients with colorectal
cancer and different MTHFR genotypes (all P>0.05), indicating
that differences in the MTHFR genotypes of patients with colorectal
cancer do not result in differences in their coagulation
function.
 | Table IIIRelationship between
thromboelastography and the MTHFR genotypes. |
Table III
Relationship between
thromboelastography and the MTHFR genotypes.
| | MTHFR genotype | |
|---|
| Characteristic | CC (n=219) | CT (n=289) | TT (n=83) | P-value |
|---|
| CK-R | 4.44±1.21 | 4.46±0.97 | 4.4±0.99 | 0.903 |
| CK-K | 1.36±0.44 | 1.32±0.33 | 1.42±0.53 | 0.149 |
| CK-Angle | 73.8±4.35 | 74.09±3.58 | 73.39±4.92 | 0.413 |
| CK-MA | 64.18±5.71 | 65.18±5.2 | 64.02±6.62 | 0.081 |
| CK-CI | 2.25±1.59 | 2.41±1.31 | 2.2±1.61 | 0.350 |
Discussion
MTHFR is an enzyme involved in the metabolism of
folate, a B vitamin essential to DNA synthesis and repair. MTHFR
enzymes are encoded by the MTHFR gene. Variations in this gene,
known as polymorphisms, may influence enzyme activity and folate
metabolism. The three most common polymorphisms have been
extensively studied. At position 677 in the MTHFR gene, the C677T
polymorphism changes cytosine to thymine, causing MTHFR enzyme
activity to be reduced (18).
Several health conditions have been associated with this MTHFR
polymorphism, including cardiovascular disease, neural tube defects
and certain types of cancer (19).
The A1298C polymorphism occurs at position 1,298 of the MTHFR gene,
which results in a less pronounced MTHFR enzyme activity reduction
compared with the C677T polymorphism. A previous study has
suggested that the A1298C polymorphism may increase the risk of
depression, schizophrenia and neural tube defects, but it has not
been extensively studied (20).
Caucasians are more likely to carry the A1298C polymorphism
compared with Hispanics and African Americans are more likely to
carry the C677T polymorphism (21). It is normal for MTHFR to metabolize
homocysteine. However, individuals with the C677T or A1298C
polymorphisms may accumulate homocysteine in the blood because of
reduced MTHFR enzyme activity. Several health conditions have been
linked to elevated homocysteine levels, including cardiovascular
disease, stroke and Alzheimer's disease. MTHFR polymorphisms C677T
and A1298C are relatively common (22); they accounted for 33.54% and may
affect MTHFR enzyme activity and folate metabolism, which may
increase health risks. Despite this, other genetic and
environmental factors may also have a role, so having these
polymorphisms does not necessarily mean an individual will develop
a particular health condition. Other less common MTHFR
polymorphisms have been identified and studied in addition to the
three aforementioned ones. Neural tube defects have been linked to
the R594Q and R653Q polymorphisms and cardiovascular disease has
been linked to the T1317C polymorphism (23).
Clinicians are paying a growing amount of attention
to the problem of postoperative DVT in patients with tumors.
Patients with tumors are in a condition of hypercoagulability; by
contrast, after major pelvic and abdominal surgery, patients with
tumors are susceptible to DVT episodes due to extended bed rest
(24). Consequently, the early
identification of patients at high risk of DVT and the appropriate
treatments are needed. In the present study, the MTHFR gene
polymorphisms of 591 patients who had surgery for colorectal cancer
were identified and linked with age, gender, BMI and other
variables. The results indicated that there was no significant
difference between the MTHFR genotype and the age, sex and BMI of
the patients. In addition, the expression level of DDi in the
different genotypes of these patients were examined on the first
postoperative day and during the perioperative period, and it was
revealed that there was no link between the DDi expression level
and the genotype distribution. In addition, the postoperative
thromboelastogram indices of patients with various genotypes were
evaluated and revealed that there were no significant
variations.
A meta-analysis of 30,650 individuals from 29
studies revealed that the MTHFR 677TT polymorphism may be
associated with a lower risk of colorectal cancer; however, this
may not apply to all populations (25). Results were positive for whites and
Asians but negative for blacks and Hispanics, demonstrating ethnic
disparities. There have been several studies investigating the
relationship between MTHFR polymorphism and the risk of thrombosis
in Asian populations. A meta-analysis of several studies conducted
in Asian populations also revealed an association between the MTHFR
C677T polymorphism and the risk of venous thrombus embolism (VTE),
particularly in East Asian populations (26). Another study revealed that the
MTHFR A1298C polymorphism was associated with an increased risk of
arterial thromboembolism in Taiwanese patients (27). Overall, while there may be a number
of ethnic/regional disparities in the relationship between MTHFR
polymorphism and the risk of thrombosis, there is still evidence
suggesting a significant association in Asian populations. Compared
with the 677CC gene, the 677TT gene is associated with a 20%
increased risk of venous thrombosis. However, in North America, the
677TT genotype did not have any effect on venous thrombosis, which
may explain the high consumption of folic acid and riboflavin in
the region (21). Another
investigation revealed that the MTHFR C677T polymorphism was not
connected with the incidence of venous thrombosis and concluded
that there was no clinical justification for measuring this
polymorphism (28). This is
consistent with the findings of the present study.
Of note, the present study had certain limitations.
To begin with, the limited sample size may limit the confidence of
the results. Increasingly, there is growing awareness of deep vein
thrombosis (DVT) events occurring in various clinical scenarios,
including admission VTE assessments, preoperative evaluations,
postoperative checks, and standardized VTE testing before patients
are discharged. In line with established guidelines, if ultrasound
of both lower extremities or pulmonary CTPA confirms the presence
of DVT or PE, appropriate anticoagulant treatment is administered.
However, the adoption of precautionary measures based on specific
MTHFR polymorphisms lacks substantial evidential support within
this proactive clinical approach. In addition to the introduction
of preoperative DVT education, the widespread use of thromboelastic
stockings, minimally invasive surgery to decrease trauma and
postoperative activities to encourage patients to get out of bed
earlier have effectively lowered the incidence of DVT episodes.
Even if the MTHFR gene is connected with the risk of venous
thrombosis, this association may have been largely obscured by
introduction of preoperative DVT education, the widespread use of
thromboelastic stockings, minimally invasive surgery to decrease
trauma and postoperative activities to encourage patients to get
out of bed earlier have effectively lowered the incidence of DVT
episodes.
In conclusion, even though the results are
‘negative’, the present study may help physicians realize that
MTHFR polymorphism detection is not necessary for therapeutic
purposes. Regarding the danger of venous thrombosis, patients
undergoing pelvic and abdominal surgery should pay attention to the
standardized procedural enforcement system for DVT prevention.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EC designed the study, performed the experiments,
analyzed and interpreted the data and wrote the manuscript. WZ and
LC collected samples and clinical data, analyzed and interpreted
the data and wrote the manuscript. GC and FW provided and analyzed
the samples and interpreted results and clinical data of the
patients. WZ and EC confirm the authenticity of all the raw data.
All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients before specimen collection. This study was approved by the
ethics committee of Sir Run Run Shaw Hospital, Zhejiang University
(Hangzhou, China; approval no. 20210622-8).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vidmar Golja M, Šmid A, Karas Kuželički N,
Trontelj J, Geršak K and Mlinarič-Raščan I: Folate insufficiency
due to MTHFR deficiency is bypassed by 5-methyltetrahydrofolate. J
Clin Med. 9(2836)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shannon B, Gnanasampanthan S, Beilby J and
Iacopetta B: A polymorphism in the methylenetetrahydrofolate
reductase gene predisposes to colorectal cancers with
microsatellite instability. Gut. 50:520–524. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shiao S and Yu C: Meta-prediction of MTHFR
gene polymorphism mutations and associated risk for colorectal
cancer. Biol Res Nurs. 18:357–369. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eaton AM, Sandler R, Carethers JM,
Millikan RC, Galanko J and Keku TO: 5,10-methylenetetrahydrofolate
reductase 677 and 1298 polymorphisms, folate intake, and
microsatellite instability in colon cancer. Cancer Epidemiol
Biomarkers Prev. 14:2023–2029. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sohn KJ, Jang H, Campan M, Weisenberger
DJ, Dickhout J, Wang YC, Cho RC, Yates Z, Lucock M, Chiang EP, et
al: The methylenetetrahydrofolate reductase C677T mutation induces
cell-specific changes in genomic DNA methylation and uracil
misincorporation: A possible molecular basis for the site-specific
cancer risk modification. Int J Cancer. 124:1999–2005.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Abbasi IHR, Abbasi F, Wang L, Abd El Hack
ME, Swelum AA, Hao R, Yao J and Cao Y: Folate promotes S-adenosyl
methionine reactions and the microbial methylation cycle and boosts
ruminants production and reproduction. AMB Express.
8(65)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Huang R and Zhou PK: DNA damage repair:
Historical perspectives, mechanistic pathways and clinical
translation for targeted cancer therapy. Signal Transduct Target
Ther. 6(254)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yousefzadeh M, Henpita C, Vyas R,
Soto-Palma C, Robbins P and Niedernhofer L: DNA damage-how and why
we age? eLife. 10(e62852)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hamza MS and Mousa SA: Cancer-associated
thrombosis: Risk factors, molecular mechanisms, future management.
Clin Appl Thromb Hemost. 26(1076029620954282)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Garakanidze S, Costa E, Bronze-Rocha E,
Santos-Silva A, Nikolaishvili G, Nakashidze I, Kakauridze N, Glonti
S, Khukhunaishvili R, Koridze M and Ahmad S:
Methylenetetrahydrofolate reductase gene polymorphism (C677T) as a
risk factor for arterial thrombosis in Georgian patients. Clin Appl
Thromb Hemost. 24:1061–1066. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Moll S and Varga EA: Homocysteine and
MTHFR mutations. Circulation. 132:e6–e9. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kumar A, Palfrey HA, Pathak R, Kadowitz
PJ, Gettys TW and Murthy SN: The metabolism and significance of
homocysteine in nutrition and health. Nutr Metab (Lond).
14(78)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Troesch B, Weber P and Mohajeri MH:
Potential links between impaired one-carbon metabolism due to
polymorphisms, inadequate B-vitamin status, and the development of
Alzheimer's disease. Nutrients. 8(803)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pushpakumar S, Kundu S and Sen U:
Endothelial dysfunction: The link between homocysteine and hydrogen
sulfide. Curr Med Chem. 21:3662–3672. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Donnellan E and Khorana AA: Cancer and
venous thromboembolic disease: A review. Oncologist. 22:199–207.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Erdinler IC, Ucer E, Eksik A, Akyol A and
Yazici S: Noncardiogenic pulmonary edema associated with
clopidogrel: A serious but unexpected side effect of clopidogrel.
Can J Cardiol. 23:478–480. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Antonaros F, Olivucci G, Cicchini E,
Ramacieri G, Pelleri MC, Vitale L, Strippoli P, Locatelli C, Cocchi
G, Piovesan A and Caracausi M: MTHFR C677T polymorphism analysis: A
simple, effective restriction enzyme-based method improving
previous protocols. Mol Genet Genomic Med. 7(e628)2019.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Raghubeer S and Matsha TE:
Methylenetetrahydrofolate (MTHFR), the one-carbon cycle, and
cardiovascular risks. Nutrients. 13(4562)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Petrone I, Bernardo PS, Dos Santos EC and
Abdelhay E: MTHFR C677T and A1298C polymorphisms in breast cancer,
gliomas and gastric cancer: A review. Genes (Basel).
12(587)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rai V, Yadav U, Kumar P, Yadav SK and
Gupta S: Methylenetetrahydrofolate reductase A1298C genetic
variant& risk of schizophrenia: A meta-analysis. Indian J Med
Res. 145:437–447. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Graydon JS, Claudio K, Baker S, Kocherla
M, Ferreira M, Roche-Lima A, Rodríguez-Maldonado J, Duconge J and
Ruaño G: Ethnogeographic prevalence and implications of the
677C>T and 1298A>C MTHFR polymorphisms in US primary care
populations. Biomark Med. 13:649–661. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nefic H, Mackic-Djurovic M and Eminovic I:
The frequency of the 677C>T and 1298A>C polymorphisms in the
methylenetetrahydrofolate reductase (MTHFR) gene in the population.
Med Arch. 72:164–169. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Brody LC, Conley M, Cox C, Kirke PN,
McKeever MP, Mills JL, Molloy AM, O'Leary VB, Parle-McDermott A,
Scott JM and Swanson DA: A polymorphism, R653Q, in the
trifunctional enzyme methylenetetrahydrofolate
dehydrogenase/methenyltetrahydrofolate
cyclohydrolase/formyltetrahydrofolate synthetase is a maternal
genetic risk factor for neural tube defects: Report of the Birth
Defects Research Group. Am J Hum Genet. 71:1207–1215.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Panteleimonitis S, Pickering O, Ahmad M,
Harper M, Qureshi T, Figueiredo N and Parvaiz A: Robotic rectal
cancer surgery: Results from a European multicentre case series of
240 resections and comparative analysis between cases performed
with the da Vinci Si and Xi systems. Laparosc Endosc Robot Surg.
3:6–11. 2020.
|
|
25
|
Hubner RA and Houlston RS: MTHFR C677T and
colorectal cancer risk: A meta-analysis of 25 populations. Int J
Cancer. 120:1027–1035. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang P, Gao X, Zhang Y, Hu Y, Ma H, Wang
W, Wang H, Zhang J, Xu H and Lu Z: Association between MTHFR C677T
polymorphism and venous thromboembolism risk in the Chinese
population: a meta-analysis of 24 case-controlled studies.
Angiology. 66:422–432. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jang MJ, Jeon YJ, Choi WI, Choi YS, Kim
SY, Chong SY, Oh D and Kim NK: The 677C>T mutation of the MTHFR
gene increases the risk of venous thromboembolism in Koreans and a
meta-analysis from Asian population. Clin Appl Thromb Hemost.
19:309–314. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hoffbrand AV: Postgraduate Haematology.
7th edition. John Wiley & Sons, Ltd, Hoboken, NJ, 2016.
|