Introduction
Systemic lupus erythematosus (SLE) is an autoimmune
disease of unknown etiology, characterized by systemic inflammation
and clinical heterogeneity, which typically presents in young women
of childbearing age (20-40 years old) (1). It can affect almost all organ
systems, with clinical manifestations ranging from skin involvement
to multisystem organ failure (1,2). The
heart is one of the most commonly affected organs in SLE.
Cardiovascular complications of SLE include pericarditis,
myocarditis, cardiomyopathy, endocarditis, heart block and coronary
artery disease (CAD) (3).
Cardiovascular disease (CVD) is the leading cause of morbidity and
mortality in patients with SLE (4). Patients with SLE exhibit features of
accelerated atherosclerosis and an increased risk of CAD that can
be clinically silent in the initial stages, or angina or acute
myocardial infarction (AMI) during disease progression (5). It has been reported that the risk of
developing AMI is 2.67-10.00 times higher among patients with SLE
compared with individuals without SLE (6).
Women, especially young women, are more susceptible
to SLE compared with men (7).
However, male and female patients with SLE have different clinical
profiles and outcomes. Studies have reported that male patients are
more likely to experience cardiovascular and renal complications
and have higher mortality rates compared with female patients
(8-10).
Males with SLE have been reported to have a higher prevalence of
CAD and myocardial infarction and poorer outcomes (9,11).
To date, few studies have investigated the cause of CAD in male
patients with SLE. Herein, the present study reported a rare case
of AMI in a male patient diagnosed with SLE.
Case report
A 29-year-old male with no previous cardiovascular
history was admitted to Qilu Hospital of Shandong University
(Jinan, China) in June 2019 with intermittent strangulation and
crushing of the chest for ~10 days. Accompanying symptoms included
shoulder and back pain, odynophagia and toothache. There was no
history of trauma, cough, fever, syncope or palpitations. The
patient was a nonsmoker with normal lipid levels. Physical
examination showed that the patient had no obvious positive signs
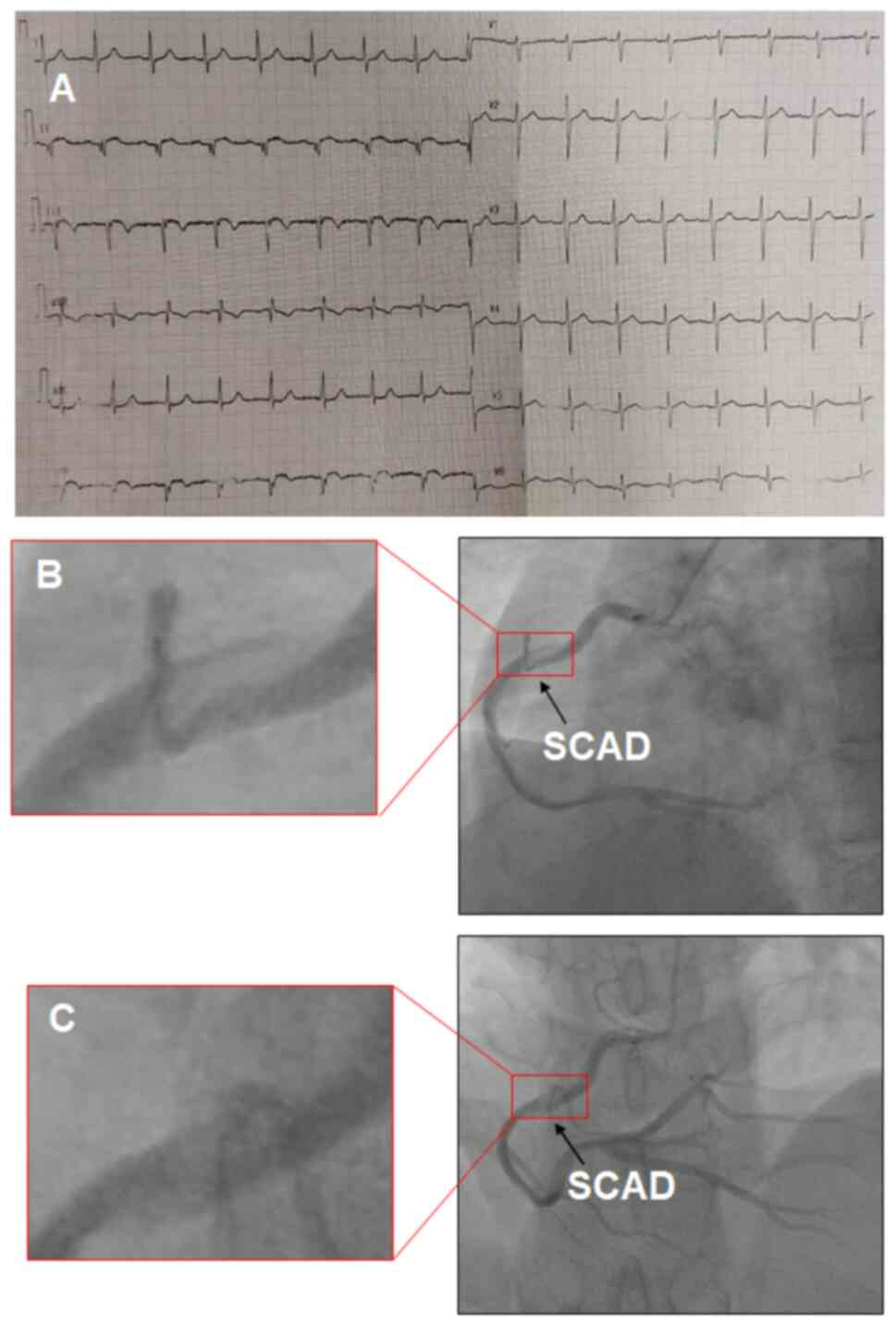
of other acute or chronic diseases. An electrocardiogram exhibited
ST-T abnormalities, including abnormal Q waves and a slight ST
elevation in the inferior (II, III and aVF) limb leads (Fig. 1A). The patient's blood pressure was
133/85 mmHg and serum cardiac troponin T level was 0.517 ng/ml
(normal range, 0.000-0.014 ng/ml). The left ventricular ejection
fraction, assessed using echocardiography, was 59%. The patient was
diagnosed with acute inferior myocardial infarction. After the
patient was admitted, their symptoms were relieved. Therefore,
emergency coronary angiography was not performed. During
hospitalization, the patient received medications, including
aspirin (100 mg/day), clopidogrel (75 mg/day), rosuvastatin (20
mg/day), metoprolol succinate (47.5 mg/day) and
low-molecular-weight heparin (5,000 IU) twice daily. At 5 days
post-admission, the patient underwent coronary angiography, which
showed a long, extended, spiral-shaped dissection of the right
coronary artery (RCA) (Fig. 1B and
1C). No significant stenosis was
observed in the left main artery, left anterior descending (LAD)
artery or left circumflex artery.
The treatment of spontaneous coronary artery
dissection (SCAD) with percutaneous coronary intervention (PCI) is
associated with high rates of technical failure related to passage
of coronary wire into the false lumen of the dissected vessel or
loss of coronary flow through propagation of dissection, and
complications. In a previous retrospective study of 189 patients
with SCAD, the PCI failure rate was 53% (12). Conservative therapy (nonoperative
treatment) is preferred for patients with SCAD (12). Considering the young age and
absence of atherosclerosis risk in the present patient, a coronary
stent was not implanted and the possible etiologies were
investigated. A detailed medical history and examination was
performed and laboratory tests showed an increased erythrocyte
sedimentation rate of 36.00 mm/h (normal range, 0.00-15.00 mm/h).
Immunological testing was performed and the autoimmune panel was
positive for antinuclear (titer, 1/1280), anti-nuclear
ribonucleoprotein/Smith and anti-Sjogren's syndrome A antibodies.
The patient was diagnosed with SLE and was administered prednisone
(50 mg/day), hydroxychloroquine (200 mg/twice daily) and calcium
carbonate (600 mg/day). The patient remained stable and
asymptomatic during treatment in the following 3 months.
In September 2019, a coronary angiography was
repeated and showed no dissection of the RCA (Fig. 2A and B). Intravascular ultrasound (IVUS) and
optical coherence tomography (OCT) were performed to evaluate the
morphology of the lumen and vessel walls. These tests showed an
isolated atherosclerotic lesion without arterial dissection in the
RCA (Fig. 2C-H). During drug
treatment, the patient experienced no further episodes of
intermittent chest strangulation or squeezing. In conjunction with
the clinical manifestations and coronary angiography, clopidogrel
was discontinued and the prednisone dose was reduced to 30 mg/day.
Calcium carbonate (600 mg/day) treatment was continued to prevent
osteoporosis and osteopenia.
After September 2019, the patient visited the
hospital every 3 months for follow-up. No other chest pain symptoms
were observed during follow-up. In December 2023, no major
complications of AMI or SLE had occurred. The patient was able to
perform daily activities normally under continuous long-term drug
therapy with hydroxychloroquine (200 mg/twice daily) and prednisone
(5 mg/day). The prognosis of the patient for SCAD is considered to
be favorable; however, the condition of SLE requires long-term
evaluation of clinical follow-up. In order to evaluate the
condition of the patient and adjust the medication according to the
condition, the patient has been asked to visit the Rheumatology
Department of Qilu Hospital every 3-6 months.
Discussion
SLE is associated with an increased prevalence of
CAD. The most common cause of CAD in patients with SLE is premature
coronary atherosclerosis, independent of traditional risk factors,
such as hypercholesterolemia, smoking and hypertension, for
cardiovascular disease (13). SCAD
is an uncommon condition that causes sudden coronary artery
occlusion and AMI, and SCAD is responsible for 1-4% of AMI cases as
the underlying cause (14,15). The cause of SCAD remains unclear
and may be associated with patient vulnerability, emotional and
physical stress, use of stimulant medications or illicit drugs and
hormonal triggers (16). It has
previously been reported that there may be an association between
SCAD and systemic inflammatory disorders, including SLE (14). To the best of our knowledge, the
present study is the first reported case of a male diagnosed with
SLE who presented with an AMI secondary to SCAD. Furthermore, the
present patient exhibited no other symptoms of SLE and AMI was the
primary presentation.
However, there are few reports on the prevalence of
SCAD caused by SLE (17-28).
Chest pain was the most common symptom and was the first
manifestation in two of the aforementioned cases (21,25).
Notably, only two cases involving male patients have been reported
(19,26). A young male developed persistent
chest tightness ~4 years after the diagnosis of SLE and coronary
angiography revealed a long dissection, as well as the presence of
a thrombus shadow originating from the diagonal branch of the LAD
(19). Huang et al
(19) reported that a young male
was hospitalized several times for acute pericarditis, acute
pleurisy, myocarditis, coronary arteritis and lupus nephritis.
Based on a comprehensive assessment of the aforementioned patient's
condition, a stent was implanted in the LAD lesion to prevent
further development of coronary dissection. In another case, a
young adolescent with SLE was reported as having SCAD (26). However, the study did not provide a
detailed report of this case.
SLE is a chronic autoimmune inflammatory disease
affecting multiple organs. Cardiac involvement is a major cause of
morbidity and mortality in patients with SLE (3). SLE accelerates the formation of
atherosclerotic plaques, with or without the presence of
traditional cardiac risk factors (29). Endothelial dysfunction is the
initial step in atherosclerosis and previous studies have reported
that vascular damage is accelerated in patients with SLE and
vascular repair mechanisms are ineffective (29,30).
On the one hand, the inflammatory response caused by SLE directly
damages the vascular endothelium. By contrast, antibodies such as
antiphospholipid, anti-oxidized low-density lipoprotein,
anti-apolipoprotein A-I and anti-double-stranded DNA can mediate
endothelial cell damage, which increases lipid deposition (31). In addition, studies have reported
that certain cytokines, such as IFN-α (32), INF-γ, TNF-α (33) and IL-17(34) can promote the development of
atherosclerosis.
SCAD is a rare cause of AMI. SCAD is characterized
by the rupture of the coronary intima and the formation of an
intramural hematoma, which leads to obstruction of the coronary
artery lumen and myocardial infarction (35). A number of mechanisms have been
proposed to explain the primary events of SCAD. The first states
that the primary event is the rupture of the intima of the coronary
artery, while the second states that there is spontaneous
hemorrhage originating from the vasa vasorum within the vessel wall
(36,37). Patients with SLE less frequently
exhibit traditional cardiovascular risk factors; however, coronary
disease in this population is deemed a cumulative outcome of
vasculitis, persistent vessel wall inflammation and an elevated
susceptibility to atherosclerosis (38). A previous report suggested that
specific proteases, such as tryptase and chymase, may promote
arterial dissection and thrombosis (39). In addition, the presence of
antiphospholipid antibodies (aPL) in patients with SCAD-SLE has
been reported in two cases, whereas data on the role of aPL in
promoting coronary artery disease in humans are inconsistent
(21,40). Therefore, more in-depth basic
research investigating the role of aPL in SCAD-SLE is required.
In the present report, the patient was diagnosed
with AMI and a coronary angiography showed a spiral dissection of
the RCA. The patient was 29 years of age with no history of
traditional cardiovascular risk factors. Therefore, a coronary
drug-eluting stent was not placed in the patient. Immunological
tests showed the presence of antibodies against SLE, which
indicated a diagnosis of SLE. Corticosteroids are used for the
treatment of SLE and they may control vascular inflammation of the
coronary arteries caused by SLE (41). There may be an association between
SCAD and systemic inflammatory disorders and, conversely,
inflammation may be involved in SCAD (16). Anti-inflammatory treatment may be
effective in patients with SCAD, especially those with
inflammation-related SCAD phenotypes, by promoting the healing of
arterial dissections (42).
In the present study, following a corticosteroid and
standard medication treatment regime for AMI for 3 months, the
coronary artery dissection of the RCA was resolved as demonstrated
by coronary angiography. IVUS and OCT further confirmed these
results. To the best of our knowledge, no study has adequately
reported the intravascular imaging characteristics of the coronary
arteries in patients with SLE and SCAD to date.
To the best of our knowledge, this is the first
report on SLE-related SCAD. SCAD should be considered in patients
with SLE and AMI, particularly in young patients without
cardiovascular risk factors. Coronary angiography should be
performed immediately and appropriate medical treatment should be
initiated. Clinical follow-up by specialist physicians should be
recommended. Considering that cardiovascular events may be
clinically silent during the initial stages of SLE, further studies
are required to explore the associations between SLE, AMI and SCAD.
Early diagnosis of SCAD is important for providing an appropriate
therapy, which differs from that for AMI caused by
atherosclerosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the grants of the
National Natural Science Foundation of China (grant no. 81970319)
and the Taishan Scholars Program of Shandong Province (grant no.
tsqn202103170).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL designed the study, analyzed and interpreted the
clinical data and patient symptoms after coronary angiography and
drafted the manuscript. QZ performed the clinical data aggregate
review. XM was responsible for the supervision, funding and
critical review. YL and XM confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committees of Qilu Hospital of Shandong University (Jinan, China;
approval no. 2021155).
Patient consent for publication
Written informed consent for publication of the
clinical details and images was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kiriakidou M and Ching CL: Systemic lupus
erythematosus. Ann Intern Med. 172:ITC81–ITC96. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Smith PP and Gordon C: Systemic lupus
erythematosus: Clinical presentations. Autoimmun Rev. 10:43–45.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Miner JJ and Kim AH: Cardiac
manifestations of systemic lupus erythematosus. Rheum Dis Clin
North Am. 40:51–60. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zen M, Salmaso L, Amidei CB, Fedeli U,
Bellio S, Iaccarino L, Doria A and Saia M: Mortality and causes of
death in systemic lupus erythematosus over the last decade: Data
from a large population-based study. Eur J Intern Med. 112:45–51.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zafar A, Mohib A, Syed H and Kumar S: Role
of cardiologists in the management of systemic lupus erythematosus:
First reported case of three-vessel disease in a young woman in
Pakistan. Cureus. 11(e5096)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wells DK and Ward MM: Nephritis and the
risk of acute myocardial infarction in patients with systemic lupus
erythematosus. Clin Exp Rheumatol. 28:223–229. 2010.PubMed/NCBI
|
|
7
|
Tian J, Zhang D, Yao X, Huang Y and Lu Q:
Global epidemiology of systemic lupus erythematosus: A
comprehensive systematic analysis and modelling study. Ann Rheum
Dis. 82:351–356. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Frutos AR, Casas I, Rúa-Figueroa I,
López-Longo FJ, Calvo-Alén J, Galindo M, Fernández-Nebro A,
Pego-Reigosa JM and Marqués AO: RELESSER Group, part of the Spanish
Society of Rheumatology Systemic Autoimmune Diseases Study Group
(EASSER). Systemic lupus erythematosus in Spanish males: A study of
the Spanish rheumatology society lupus registry (RELESSER) cohort.
Lupus. 26:698–706. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mihailovic J, Ribi C, Chizzolini C,
Trendelenburg M, Von Kempis J, Dahdal S and Huynh-Do U: Swiss
Systemic Lupus Erythematosus Cohort Study Group (SSCS). Worse
cardiovascular and renal outcome in male SLE patients. Sci Rep.
13(18628)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lai CC, Sun YS, Chen WS, Liao HT, Chen MH,
Tsai CY, Huang DF, Chou CT and Chang DM: Risk factors for mortality
in systemic lupus erythematosus patients: Analysis of adult and
pediatric cohorts in Taiwan. J Chin Med Assoc. 85:1044–1050.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vavlukis M, Pop-Gjorcevab D, Poposka L,
Sandevska E and Kedev S: Myocardial infarction in systemic lupus
erythematosus-the sex-specific risk profile. Curr Pharm Des.
27:3221–3228. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tweet MS, Eleid MF, Best PJ, Lennon RJ,
Lerman A, Rihal CS, Holmes DR Jr, Hayes SN and Gulati R:
Spontaneous coronary artery dissection: Revascularization versus
conservative therapy. Circ Cardiovasc Interv. 7:777–786.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Asanuma Y, Oeser A, Shintani AK, Turner E,
Olsen N, Fazio S, Linton MF, Raggi P and Stein CM: Premature
coronary-artery atherosclerosis in systemic lupus erythematosus. N
Engl J Med. 349:2407–2415. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mahmoud AN, Taduru SS, Mentias A, Mahtta
D, Barakat AF, Saad M, Elgendy AY, Mojadidi MK, Omer M, Abuzaid A,
et al: Trends of incidence, clinical presentation, and in-hospital
mortality among women with acute myocardial infarction with or
without spontaneous coronary artery dissection: A population-based
analysis. JACC Cardiovasc Interv. 11:80–90. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tweet MS, Hayes SN, Pitta SR, Simari RD,
Lerman A, Lennon RJ, Gersh BJ, Khambatta S, Best PJ, Rihal CS and
Gulati R: Clinical features, management, and prognosis of
spontaneous coronary artery dissection. Circulation. 126:579–588.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim ESH: Spontaneous coronary-artery
dissection. N Engl J Med. 383:2358–2370. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Álvarez-Lario B, Álvarez-Roy L,
Mayordomo-Gómez S and García-García JM: Spontaneous coronary artery
dissection in systemic lupus erythematosus: Case-based review.
Rheumatol Int. 39:1821–1827. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chaaban N and Kshatriya S: Spontaneous
coronary artery dissection with systemic lupus erythematosus.
Ochsner J. 22:353–355. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang H, Ma X, Xu L, Wang X, Shi D, Zhao F
and Zhang Y: Spontaneous coronary artery dissection and
atherosclerosis in a young man with systemic lupus erythematosus: A
case report and literature review. Front Cardiovasc Med.
9(951188)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kumanayaka DD, Hernandez I, Ahmad A and
Suleiman A: Systemic lupus erythematous associated with
multi-vessel spontaneous coronary artery dissection. Global Cardiol
Sci Pract. 2023(e202324)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Reddy S, Vaid T, Sanjeeva NC and Shetty
RK: Spontaneous coronary artery dissection as the first
presentation of systemic lupus erythematosus. BMJ Case Rep.
24(bcr2016216344)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Patel R, Patel R, Rahming H, Tian J and
Kandov R: Spontaneous coronary artery disease (SCAD) in a patient
with systemic lupus erythematosus (SLE). Cureus.
15(e43061)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rekik S, Lanfranchi P, Jacq L and
Bernasconi F: Spontaneous coronary artery dissection in a 35
year-old woman with systemic lupus erythematosus successfully
treated by angioplasty. Heart Lung Circ. 22:955–958.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Besinger BR and Gardner S: Spontaneous
coronary artery dissection in a 27-year-old woman. J Emerg Med.
44:e239–e242. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Aldoboni AH, Hamza EA, Majdi K, Ngibzadhe
M, Palasaidi S and Moayed DA: Spontaneous dissection of coronary
artery treated by primary stenting as the first presentation of
systemic lupus erythematosus. J Invasive Cardiol. 14:694–696.
2002.PubMed/NCBI
|
|
26
|
Kothari D, Ruygrok P, Gentles T and
Occleshaw C: Spontaneous coronary artery dissection in an
adolescent man with systemic lupus erythematosus. Intern Med J.
37:342–343. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
García-Sánchez M, Carrillo J, Montero Y
and Seniscal D: Spontaneous coronary dissection associated with
systemic lupus erythematosus. Archivos de cardiologia de Mexico.
91:114–120. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sharma AK, Farb A, Maniar P, Ajani AE,
Castagna M, Virmani R, Suddath W and Lindsay J: Spontaneous
coronary artery dissection in a patient with systemic lupus
erythematosis. Hawaii Med J. 62:248–253. 2003.PubMed/NCBI
|
|
29
|
Skaggs BJ, Hahn BH and McMahon M:
Accelerated atherosclerosis in patients with SLE-mechanisms and
management. Nat Rev Rheumatol. 8:214–223. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rajagopalan S, Somers EC, Brook RD, Kehrer
C, Pfenninger D, Lewis E, Chakrabarti A, Richardson BC, Shelden E,
McCune WJ and Kaplan MJ: Endothelial cell apoptosis in systemic
lupus erythematosus: A common pathway for abnormal vascular
function and thrombosis propensity. Blood. 103:3677–3683.
2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wigren M, Nilsson J and Kaplan MJ:
Pathogenic immunity in systemic lupus erythematosus and
atherosclerosis: Common mechanisms and possible targets for
intervention. J Intern Med. 278:494–506. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Buie JJ, Renaud LL, Muise-Helmericks R and
Oates JC: IFN-α negatively regulates the expression of endothelial
nitric oxide synthase and nitric oxide production: Implications for
systemic lupus erythematosus. J Immunol. 199:1979–1988.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rho YH, Chung CP, Oeser A, Solus J, Raggi
P, Gebretsadik T, Shintani A and Stein CM: Novel cardiovascular
risk factors in premature coronary atherosclerosis associated with
systemic lupus erythematosus. J Rheumatol. 35:1789–1794.
2008.PubMed/NCBI
|
|
34
|
von Vietinghoff S, Koltsova EK, Mestas J,
Diehl CJ, Witztum JL and Ley K: Mycophenolate mofetil decreases
atherosclerotic lesion size by depression of aortic T-lymphocyte
and interleukin-17-mediated macrophage accumulation. J Am Coll
Cardiol. 57:2194–2204. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hayes SN, Kim ESH, Saw J, Adlam D,
Arslanian-Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME,
Mieres JH, et al: Spontaneous coronary artery dissection: Current
state of the science: A scientific statement from the American
heart association. Circulation. 137:e523–e557. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Saw J, Mancini GB, Humphries K, Fung A,
Boone R, Starovoytov A and Aymong E: Angiographic appearance of
spontaneous coronary artery dissection with intramural hematoma
proven on intracoronary imaging. Catheter Cardiovasc Interv.
87:E54–E61. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kwon TG, Gulati R, Matsuzawa Y, Aoki T,
Guddeti RR, Herrmann J, Lennon RJ, Ritman EL, Lerman LO and Lerman
A: Proliferation of coronary adventitial vasa vasorum in patients
with spontaneous coronary artery dissection. JACC Cardiovasc
Imaging. 9:891–892. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Karrar A, Sequeira W and Block JA:
Coronary artery disease in systemic lupus erythematosus: A review
of the literature. Semin Arthritis Rheum. 30:436–443.
2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kounis NG, Koniari I, Velissaris D,
Soufras G and Hahalis G: Aortic aneurysm and dissection in systemic
lupus erythematosus-pathophysiologic and therapeutic
considerations. Eur J Rheumatol. 5:209–211. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nisar MK and Mya T: Spontaneous coronary
artery dissection in the context of positive anticardiolipin
antibodies and clinically undiagnosed systemic lupus erythematosus.
Lupus. 20:1436–1438. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chuang YW, Yu MC, Lin CL, Yu TM, Shu KH
and Kao CH: Risk of peripheral arterial occlusive disease in
patients with systemic lupus erythematosus: A nationwide
population-based cohort study. Medicine (Baltimore).
94(e2121)2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Krittanawong C, Saw J and Olin JW: Updates
in spontaneous coronary artery dissection. Curr Cardiol Rep.
22(123)2020.PubMed/NCBI View Article : Google Scholar
|