Introduction
Sepsis is a complex syndrome caused by
pathophysiological and biochemical dysregulation triggered by
autogenous factors in response to bacterial, viral, parasitic or
fungal infections (1,2). According to the 1997-2017 Global
Burden of Disease Study, there were 48.9 million sepsis cases and
~11 million sepsis-related deaths in the past two decades (3). Data from China demonstrated that in
2022 alone, a total of 25.5% of intensive care unit (ICU) patients
were diagnosed with sepsis and in ~13% of these cases sepsis
progressed to septic shock that is associated with severe
circulatory, cellular, coagulation and metabolic abnormalities that
lead to higher risk of mortality compared with uncomplicated sepsis
(4). Diagnosis of sepsis is
difficult due to the lack of signs and symptoms and absence of any
gold standard test (1,2). It is considered a major public health
concern, with high morbidity and mortality, and a heavy economic
burden on the healthcare system (5-7).
Catecholamines, such as norepinephrine, have been
widely used for restoring circulatory failure in sepsis. However,
their use is associated with several adverse effects, such as
non-compensatory tachycardia, insulin resistance and coagulopathy,
all of which may lead to poor outcomes for the patient (8,9).
Additionally, catecholamines may worsen hypermetabolism by causing
hyperglycemia and hyperlactatemia that may result in further
end-organ damage (10). Patients
with sepsis also have activated adrenergic system which can be
considered as an adaptive response to the disease (11). Recently, a concept of
‘decatecholaminization’ has been put forward for patients with
sepsis. It aims to improve patient outcomes by blocking
beta-adrenergic receptors, and limiting intrinsic adrenergic
response by delivery of exogenous catecholamines (12,13).
A previous randomized controlled trial (RCT) has shown that the use
of short-acting beta-blockers can significantly reduce mortality
rates in patients with sepsis (14). Another systematic review and
meta-analysis of seven RCTs demonstrated that the use of
beta-blockers in patients with sepsis indeed offers a significant
survival advantage and is associated with a reduction in 28-day
mortality (15). Therefore,
understanding the effect of premorbid use of beta-blockers on the
outcomes of septic patients is crucial. While several observational
studies attempted to assess the role of premorbid beta-blockers on
outcomes of sepsis, the results were inconclusive (16-18).
Moreover, a total of two prior meta-analyses included a limited
number of studies (11,19).
The present study aimed to conduct the most
comprehensive review on the effect of premorbid beta-blockers on
the outcomes of patients with sepsis.
Materials and methods
Literature search and inclusion
criteria
The present study was conducted following the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
guidelines (20), and the review
protocol was published on PROSPERO (https://www.crd.york.ac.uk/prospero/; protocol no.
CRD42023491920).
The authors collaborated with an experienced medical
librarian to search Embase (https://www.embase.com/search/quick), Scopus
(https://www.scopus.com/home.uri), Web of
Science (https://www.webofscience.com/wos/) and PubMed
(https://pubmed.ncbi.nlm.nih.gov) for
peer-reviewed articles or conference proceedings. The search
included studies from inception of databases to 15th December 2023.
A separate additional search was performed on Google Scholar
(https://scholar.google.com) for any
missed articles. All studies from the inception of these databases
to the last search date were eligible. The language was restricted
to English.
Literature was searched with different combinations
of the following key words: Beta blockers, adrenergic beta
antagonist, beta antagonist, beta-adrenoreceptor antagonist,
beta-adrenergic receptor antagonist, beta-adrenergic blocking
agent, adrenergic beta-1 receptor antagonists, sepsis, septic
shock, septicaemia and systemic inflammatory response syndrome.
Further details are provided in Table
SI.
After the initial search of the databases, all
search results were combined in a single reference manager software
(EndNote version 20; Clarivate). All duplicate entries were
removed. Two authors independently screened the studies based on
the following inclusion criteria:
i) Studies on adult patients with sepsis or septic
shock; ii) exposure was premorbid use of beta-blockers; iii)
comparison was no premorbid use of beta-blockers; iv) outcomes of
interest were mortality and the need for mechanical ventilation;
and v) all study types were eligible. Studies on beta-blocker use
after diagnosis of sepsis were excluded. Studies without a control
group, and studies reporting data on all antihypertensive agents
rather than on beta-blockers specifically were also not
eligible.
After initial screening, relevant studies were
identified and downloaded. Full texts of these studies were further
independently reviewed by the two authors, and all differences were
resolved by discussion with a third author. References of selected
articles were scrutinized to discover other relevant papers missed
by the primary search strategy.
Data extraction and study quality
assessment
Extracted data included author, study type,
location, sample size, sepsis definition, sample size, age and sex
details, lactate levels, Sequential Organ Failure Assessment score
(SOFA) score, septic shock, type of outcomes reported and
follow-up. The primary outcome was mortality and the secondary
outcome was the need for mechanical ventilation. Unadjusted and
adjusted data for mortality were extracted separately.
Studies were assessed for their methodological
quality by the two authors using the Newcastle Ottawa Scale (NOS)
(21). Points were awarded for the
representativeness of the study cohort, comparability of groups and
measurement of outcomes with each receiving a maximum of four, two
and three points respectively.
Statistical analysis
Continuous data were presented as the mean (standard
deviation) or median (interquartile range). Binary outcomes
(unadjusted data) were analyzed using the inverse-variance
random-effects meta-analysis. The effect size was reported as odds
ratios (ORs) with 95% confidence intervals (CIs). Adjusted data
were combined using the generic inverse variation function using
‘Review Manager’ (RevMan, v.5.3; The Cochrane Collaboration). To
quantify the inter-study variability, statistical heterogeneity was
checked using the χ2 test and I2 statistic.
P-value of <0.10 with the χ2 test or an I2
value of >50% was considered as substantial heterogeneity.
Publication bias for the primary outcome was checked by funnel
plots. The robustness of the meta-analysis for the primary outcome
was further verified by a sensitivity analysis. Individual studies
were excluded, and the final OR was recalculated.
Results
Search outcomes
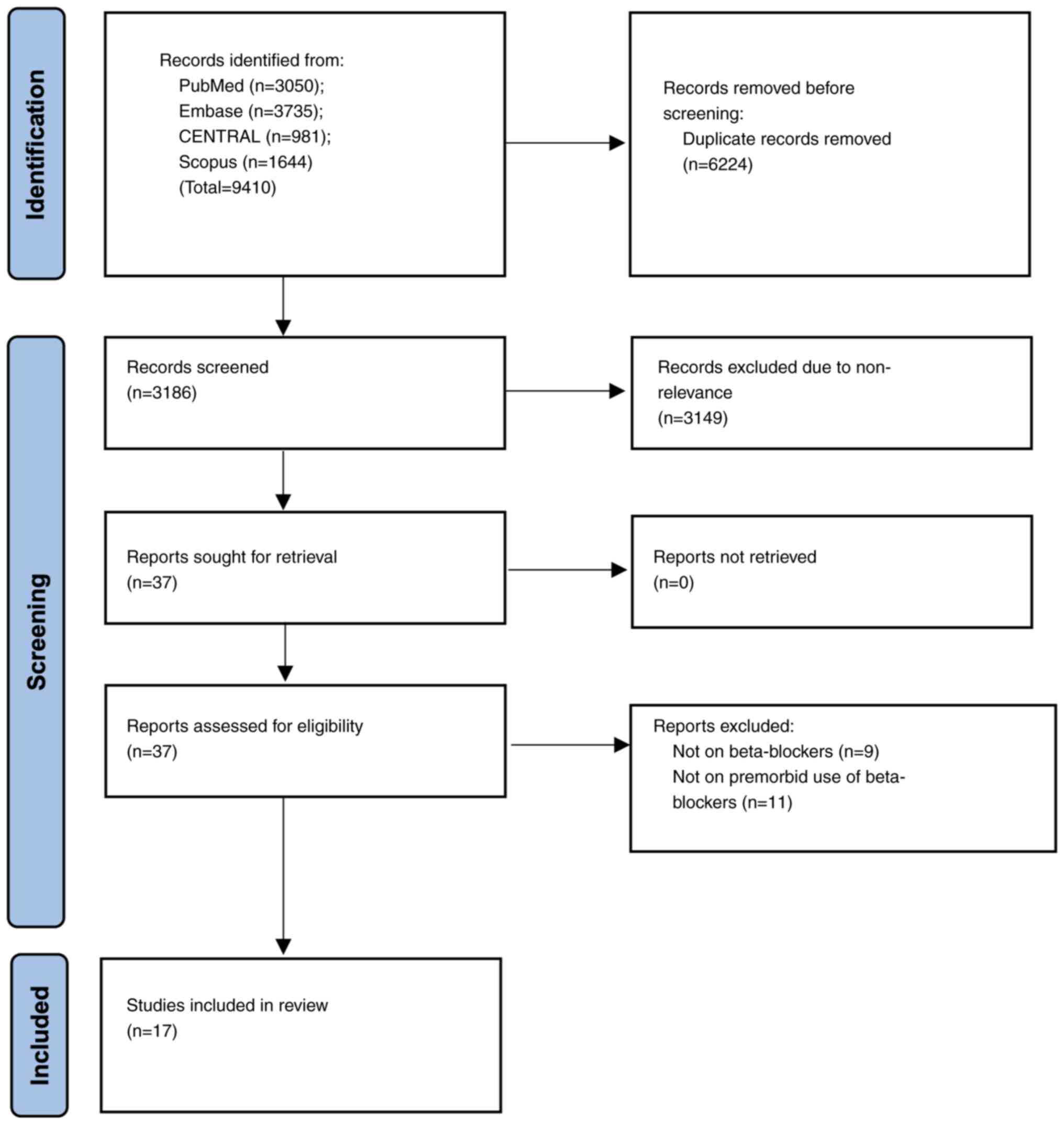
Systematic search across four databases identified
9,410 studies. After deduplication, a total of 3,186 articles
underwent the initial screening of titles and abstracts. Full texts
of the remaining 37 studies were selected for further analysis. Of
them, nine studies in total were excluded since they reported data
on other antihypertensive drugs and an additional number of ten
studies were excluded because they assessed the effect of ongoing
or newly prescribed beta-blockers on patients with sepsis. Finally,
a total of 17 studies (16-18,22-35),
comparing premorbid beta-blocker use with controls in patients with
sepsis, were selected for the analysis (Fig. 1).
Study characteristics
Data extracted by the authors are shown in Table I. A total of three studies were
prospective while 14 were retrospective. In total, four studies
(17,29-31)
were reported as conference proceedings. The included studies were
from the USA, France, Italy, Israel, Singapore, China, Taiwan,
India and Saudi Arabia, and were published between the years 2012
and 2023. ‘Sepsis-2’ and ‘Sepsis-3’ were the most accustomed
definitions in the included studies. The 17 studies included 64,586
patients. Of them, a total of 8,665 patients received premorbid
beta-blockers, and the 55,921 patients that were not treated by the
premorbid beta-blockers were used as a control group. The
mean/median age of patients was >60 years across studies. Most
studies reported a predominance of the male sex in both groups. The
percentage of patients with septic shock ranged from 3.4 to 100%.
In total, 16 studies reported unadjusted mortality rates, while one
study reported only adjusted mortality data. Mortality was reported
as the ICU or in-hospital mortality, or as 28- or 30-days
mortality. For the meta-analysis, ICU and in-hospital data were
pooled together as in-hospital mortality and 28- and 30-days
mortality was pooled together as one-month mortality. The total NOS
score of the studies was between 6 and 8.
 | Table IBaseline details of included
studies. |
Table I
Baseline details of included
studies.
| First author | Year | Type | Place | Definition of
sepsis | Groups | Sample size | Age (years) | Male sex (%) | Septic shock
(%) | Admission SOFA
score | Lactate levels
(mmol/l) | Outcomes | Follow-up | NOS score | (Refs.) |
|---|
| Kumar et
al | 2023 | P | India | Sepsis-3 | BB | 38 | NR | NR | 0 | 7.3(3) | 1.4 (1.1) | Mortality | 28 days | 6 | (28) |
| | | | | | No BB | 38 | | | 0 | 7.4 (3.3) | 1.5 (0.8) | | | | |
| Ma et
al | 2022 | R | China | Sepsis-3 | BB | 48 | 72.8 (12.3) | 52.1 | 64.6 | 9.9 (4.4) | NR | Mortality, MV | 28 days | 7 | (27) |
| | | | | | No BB | 180 | 64.3 (16.1) | 63.3 | 78.9 | 11.5 (4.6) | | | | | |
| Tan et
al | 2021 | R | Multi- | Sepsis-3/ | BB | 1556 | 70.3 (13.5) | 54.9 | 9.8 | 10.5 (3.6) | 1.5 (1-2.4) | Mortality, MV | In-hospital | 8 | (26) |
| | | | national | ICD-9 | No BB | 2530 | 63.9 (16.4) | 54.1 | 13.8 | 10.8 (3.8) | 1.6 (1.1-2.7) | | | | |
| Pham et
al | 2021 | R | Australia | Sepsis-2 | BB | 49 | 70.9 (NR) | 75.5 | 69.4 | NR | 2.6 (2.1-3) | Mortality | In-hospital | 6 | (25) |
| | | | | | No BB | 140 | 63.5 (NR) | 50 | 70 | | 3.6 (3.1-4.1) | | | | |
| Kuo et
al | 2021 | R | Taiwan | Sepsis-3 | BB | 209 | 71.3 (14.3) | 66.5 | 34.4 | NR | 2 (1.9) | Mortality | ICU | 6 | (18) |
| | | | | | No BB | 1053 | 68.9 (17.3) | 66.5 | 46.1 | | 1.8 (3.1) | | | | |
| Guz et
al | 2021 | P | Israel | Sepsis-2 | BB | 320 | 74 (62-82) | 45.9 | 3.4 | 2 (1-3) | NR | Mortality, MV | 30 days | 7 | (24) |
| | | | | | No BB | 866 | 72 (57-83) | 47.3 | 4.5 | 2 (0-3) | | | | | |
| Chan et
al | 2021 | P | Singapore | Sepsis-3 | BB | 70 | 77.5 (62-85) | 52.9 | NR | 3 (2-5) | 1.7 (1.5) | Mortality | 28 days | 6 | (35) |
| | | | | | No BB | 125 | 70 60-79) | 56 | | 2 (1-3) | 1.8 (1.7) | | | | |
| Hsieh et
al | 2019 | R | Taiwan | ICD-9 | BB | 1040 | NR | NR | NR | NR | NR | Mortality | In-hospital | 7 | (23) |
| | | | | | No BB | 33213 | | | | | | | | | |
| DeMott et
al | 2018 | R | USA | ICD-9 | BB | 46 | 67 (57-72) | 52.2 | 100 | 14 (10-16) | NR | Mortality, MV | In-hospital | 6 | (22) |
| | | | | | No BB | 51 | 62 (51-73) | 62.7 | 100 | 12 (9-16) | | | | | |
| Charles et
al | 2018 | R | France | NR | BB | 230 | 72.9 (61.5-80) | 66.1 | NR | 9 (6-12) | 1.75 (0.9-3.4) | Mortality, MV | In-hospital | NEa | (29) |
| | | | | | No BB | 708 | 66.9 (56-78) | 63 | NR | 9 (6-13) | 1.8 (0.8-4) | | | | |
| Arnautovic et
al | 2018 | R | USA | Septic shock
defined as patients requiring vasopressors to maintain 65 mmHg
despite adequate fluid resuscitation, as well as a serum lactate
level >2.0 mmol/l | BB | 49 | NR | NR | 100 | NR | NR | Mortality | In-hospital | 6 | (16) |
| | | | | | No BB | 60 | | | 100 | | | | | | |
| Singer et
al | 2017 | R | USA | ICD-9 | BB | 2838 | NR | 64.7 | NR | NR | NR | Mortality | 30 days | 7 | (34) |
| | | | | | No BB | 4001 | NR | 62.9 | | | | | | | |
| Alsolamy et
al | 2016 | R | Saudi | NR | BB | 623 | NR | NR | NR | NR | NR | Mortality | ICU | NEa | (30) |
| | | | Arabia | | No BB | 4006 | | | | | | | | | |
| Sharma et
al | 2015 | R | NR | NR | BB | 48 | 71 (NR) | 42 | NR | NR | NR | Mortality | In-hospital | NEa | (17) |
| | | | | | No BB | 75 | 65 (NR) | 51 | | | | | | | |
| Contenti et
al | 2015 | R | France | Sepsis-2 | BB | 65 | 78(11) | 62.9 | 30.8 | 5 (2.8) | 3.9 (2.3) | Mortality, MV | 28 days | 6 | (33) |
| | | | | | No BB | 195 | 75(16) | 54.4 | 32.3 | 5.3 (2.8) | 5.6 (3.6) | | | | |
| Al-Qadi et
al | 2014 | R | USA | NR | BB | 375 | NR | NR | NR | NR | NR | Mortality | In-hospital | NEa | (31) |
| | | | | | No BB | 276 | | | | | | | | | |
| Macchia et
al | 2012 | R | Italy | ICD-9 | BB | 1061 | 72 (12.8) | 49.2 | NR | NR | NR | Mortality | 28 days | 7 | (32) |
| | | | | | No BB | 8404 | 72(13) | 49.8 | | | | | | | |
Meta-analysis
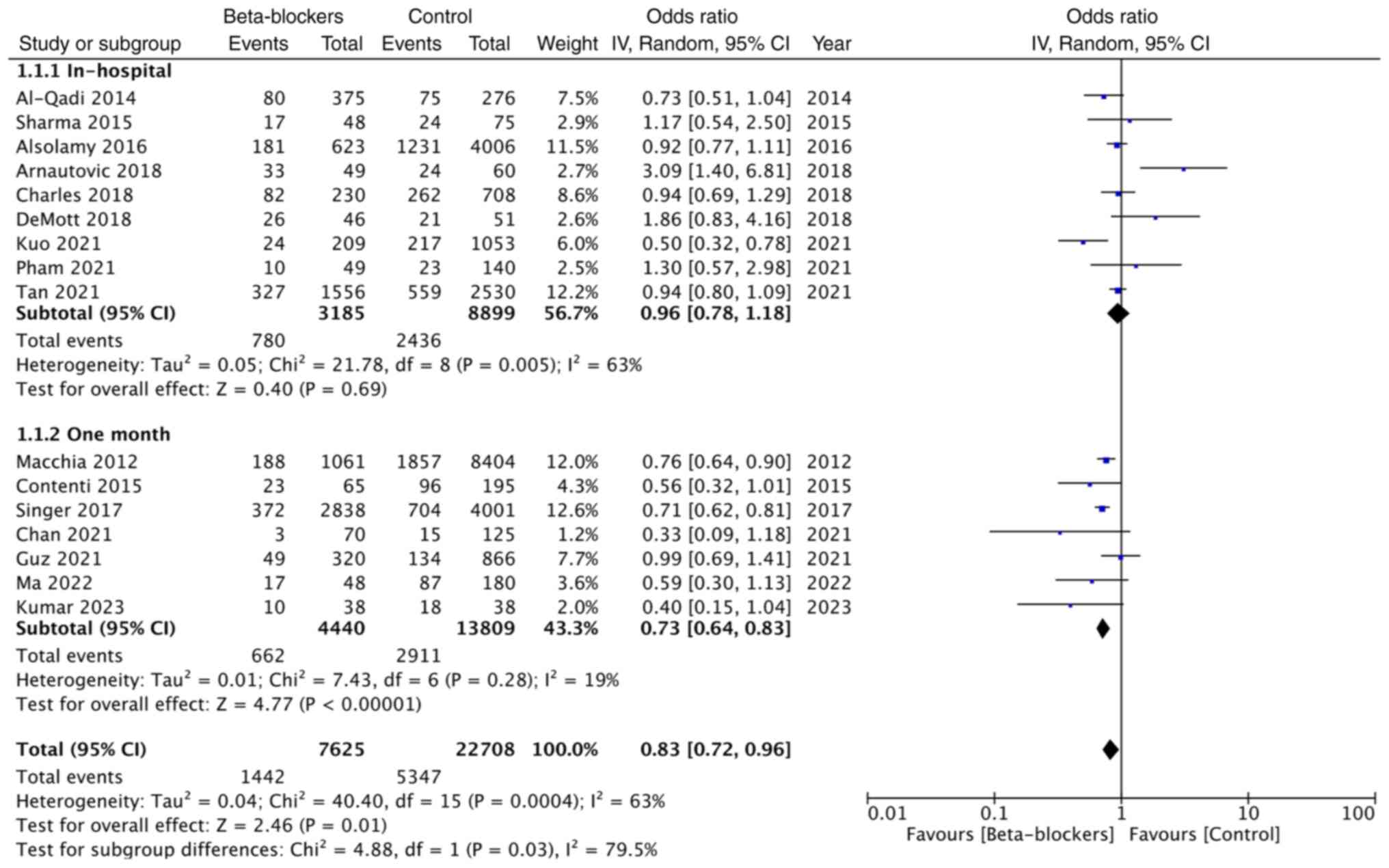
Pooled analysis of unadjusted mortality rates
included 3,185 patients on premorbid beta-blockers and 8,899
patients in the control group and showed that premorbid
beta-blocker use did not affect in-hospital mortality [OR: 0.96;
95% CI: (0.78, 1.18); and I2=63%].
However, a meta-analysis of one-month mortality data
of 4,440 patients on beta-blockers and 13,809 patients in the
control group demonstrated that use of premorbid use of
beta-blockers significantly reduced mortality (OR: 0.73; 95% CI:
0.64, 0.83; and I2=19%). Overall, the combined data from
16 studies demonstrated that premorbid use of beta-blockers did
offer a significant survival advantage in patients with sepsis (OR:
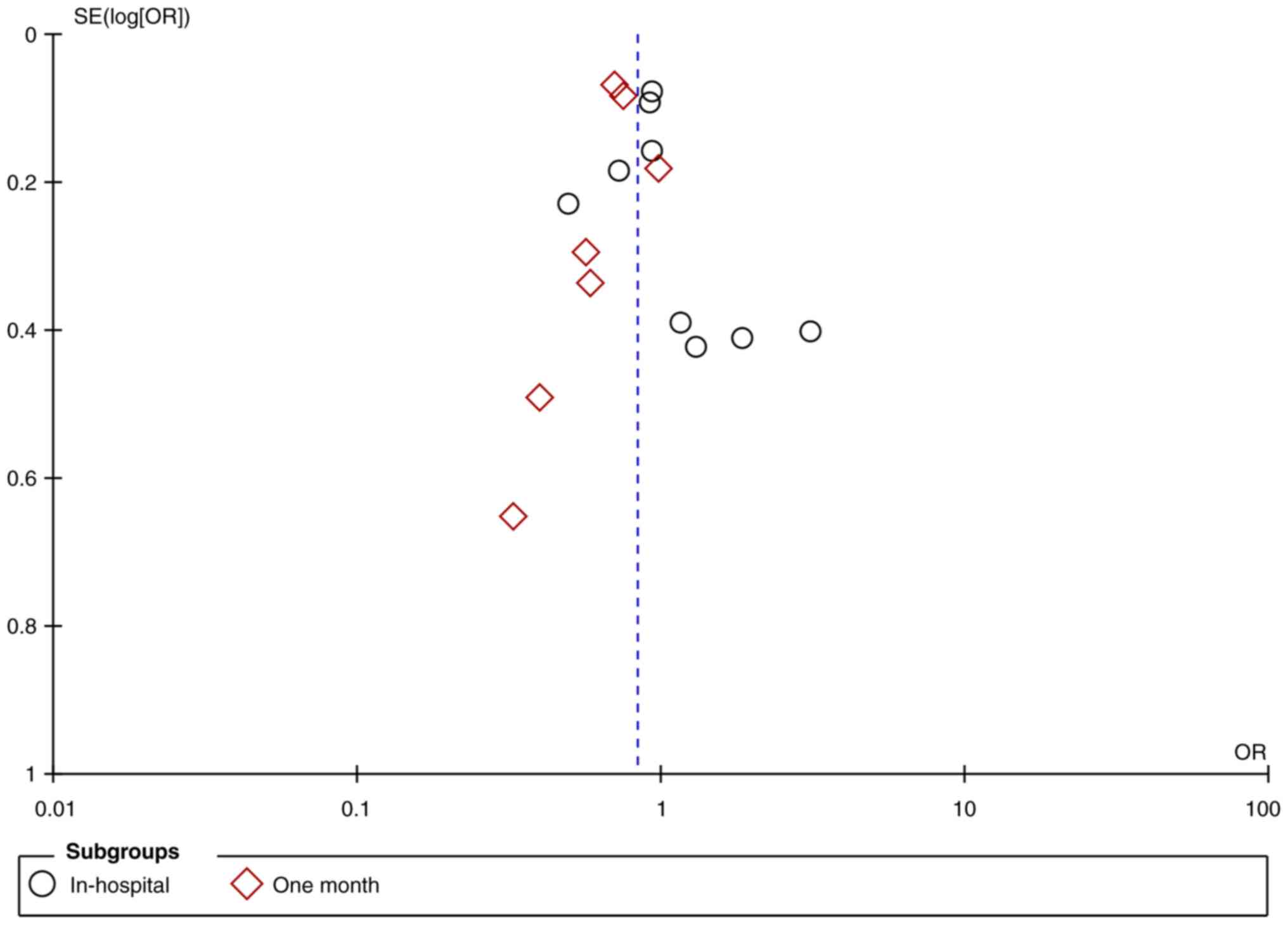
0.83; 95% CI: 0.72, 0.96; and I2=63%) (Fig. 2). The funnel plot showed no
publication bias (Fig. 3).
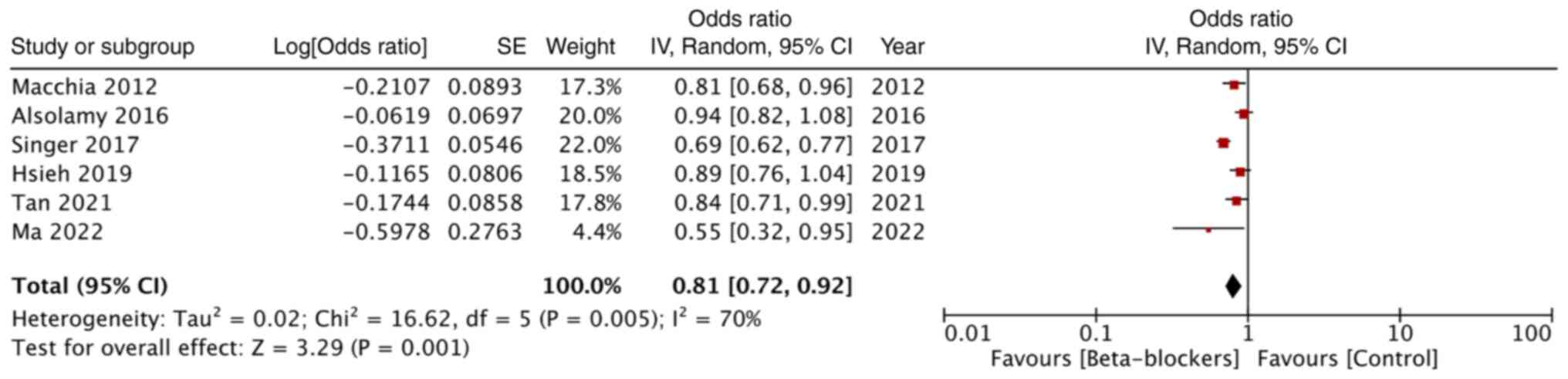
Adjusted mortality data was reported by only six
studies. Combined analysis revealed that premorbid beta-blockers
significantly reduced mortality rates in patients with sepsis (OR:
0.81; 95% CI: 0.72, 0.92; and I2=70%) (Fig. 4).
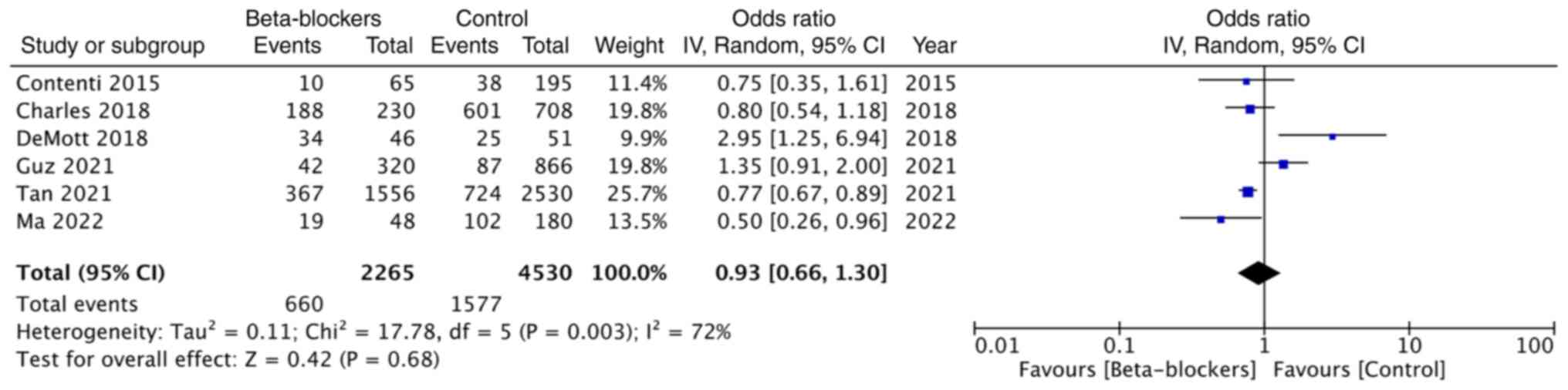
The data of the need for mechanical ventilation was
reported in six studies. Pooled analysis demonstrated no
significant impact of beta-blocker use on the need for mechanical
ventilation (OR: 0.93; 95% CI: 0.66, 1.30; and I2=72%)
(Fig. 5).
Sensitivity analysis
As presented in Table
II, the results of the sensitivity analysis for the
meta-analysis of unadjusted mortality rates demonstrated OR between
0.81 to 0.86. The upper limit of 95 CI% did reach the value of 1 on
the exclusion of two studies indicating no significant impact of
premorbid beta-blocker use on mortality after sepsis.
 | Table IIResults of sensitivity analysis for
crude mortality rates. |
Table II
Results of sensitivity analysis for
crude mortality rates.
| Excluded study,
year | Resultant odds
ratio | (Refs.) |
|---|
| Al-Qadi et
al, 2014 | 0.84 (0.72,
0.98) | (31) |
| Sharma et
al, 2015 | 0.83 (0.71,
0.96) | (17) |
| Alsolamy et
al, 2016 | 0.82 (0.70,
0.97) | (30) |
| Arnautovic et
al, 2018 | 0.81 (0.71,
0.92) | (16) |
| Charles et
al, 2018 | 0.82 (0.71,
0.96) | (29) |
| DeMott et
al, 2018 | 0.82 (0.71,
0.94) | (22) |
| Kuo et al,
2021 | 0.86 (0.74,
0.99) | (18) |
| Pham et al,
2021 | 0.82 (0.71,
0.95) | (25) |
| Tan et al,
2021 | 0.82 (0.70,
0.97) | (26) |
| Macchia et
al, 2012 | 0.84 (0.72,
1.00) | (32) |
| Contenti et
al, 2015 | 0.85 (0.73,
0.98) | (33) |
| Singer et
al, 2017 | 0.85 (0.73,
1.00) | (34) |
| Chan et al,
2021 | 0.84 (0.73,
0.97) | (35) |
| Guz et al,
2021 | 0.82 (0.70,
0.96) | (24) |
| Ma et al,
2022 | 0.84 (0.73,
0.98) | (27) |
| Kumar et al,
2023 | 0.85 (0.73,
0.98) | (28) |
As demonstrated in Table III, the results of the
sensitivity analysis for the meta-analysis of adjusted mortality
rates remained statistically significant on sequential exclusion of
all studies with the OR ranging from 0.78 to 0.87.
 | Table IIIResults of sensitivity analysis for
adjusted mortality rates. |
Table III
Results of sensitivity analysis for
adjusted mortality rates.
| Excluded study,
year | Resultant odds
ratio | (Refs.) |
|---|
| Macchia et
al, 2012 | 0.81 (0.69,
0.94) | (32) |
| Alsolamy et
al, 2016 | 0.78 (0.69,
0.89) | (30) |
| Singer et
al, 2017 | 0.87 (0.79,
0.95) | (34) |
| Hsieh et al,
2019 | 0.79 (0.68,
0.92) | (23) |
| Tan et al,
2021 | 0.80 (0.69,
0.94) | (26) |
| Ma et al,
2022 | 0.83 (0.73,
0.94) | (27) |
Discussion
This updated systematic review and meta-analysis
examined the impact of premorbid use of beta-blockers on the
outcomes of sepsis. Importantly, due to the limited data, only two
outcomes, mortality and a need for mechanical ventilation, were
included in the analysis. Analysis of 16 studies reporting
unadjusted mortality rates demonstrated that premorbid use of
beta-blockers had a protective role on patient survival after
sepsis. However, mortality rates were reduced only at one-month
follow-up with no impact on in-hospital mortality. Premorbid use of
beta-blockers was found to reduce one-month mortality by 27% and
overall mortality rates by 17%. The validity of the results is
strengthened by the absence of publication bias, large sample size
and no evidence of any outliner study. A detailed sensitivity
analysis demonstrated minimal changes in the effect size on the
exclusion of one study at a time.
The difference in in-hospital and one-month outcomes
in the present meta-analysis is interesting. Forest plot analysis
of the unadjusted mortality rates detected significant variation in
the results of studies reporting in-hospital mortality with high
heterogeneity in the meta-analysis. On the other hand, data for
one-month mortality was more consistently in favor of
beta-blockers, with the OR values of all the included studies being
below 1. The interstudy heterogeneity of the meta-analysis was also
low, with I2=19%. It can be hypothesized that the
difference in results could be explained by the quality of the
studies, as the meta-analysis on in-hospital mortality included
four studies (17,29-31)
that were published as conference abstracts. Another reason could
be the unaccountable baseline differences among studies in terms of
patient population, sepsis severity, treatment protocols and so on,
which could have skewed the results.
Unadjusted mortality rates are often confounded and
may not be a correct measurement of the outcome (36). In the context of sepsis, several
variables including age, sex, comorbidities, SOFA, Acute Physiology
and Chronic Health Evaluation II score, baseline vital signs,
lactate levels, creatinine levels, complications such as renal
failure and intervention strategies (vasopressor use, mechanical
ventilation, continuous renal replacement therapy) can all impact
the prognosis (37-39).
Beta-blockers are often prescribed to patients who are hypertensive
or have chronic heart failure. Also, age of patients receiving
beta-blockers was higher in all included studies, compared with the
control group. Given such differences, adjusted mortality rates
would represent an improved measurement of survival outcomes.
In the present review, a meta-analysis of a limited
number of studies reporting adjusted data demonstrated a protective
role of premorbid beta-blockers on sepsis-associated mortality.
These results remained consistent after the sensitivity analysis,
without any change in the significance of the results. Lastly, only
few studies reported secondary outcome data, and the meta-analysis
did not reveal any effect of beta-blocker use on the need for
mechanical ventilation.
While the results are consistent with previous
reviews, the current analysis has significantly higher number of
included studies. Tan et al (19) reviewed evidence from nine studies
and conducted a meta-analysis with just three studies to
demonstrate the protective effect of premorbid beta-blockers.
Hasegawa et al (11)
reported similar results, although, just ten studies were included
in the review. The present review has added seven more studies with
an overall sample size of 64,586 patients to present the most
comprehensive evidence on the potential impact of premorbid
beta-blockers on the outcomes of sepsis.
The role of beta-blockers in the management of
patients with sepsis has received significant attention in the past
decade with very controversial results. A previous study found that
short-acting beta-blockers such as esmolol and landiolol are able
to efficiently control tachycardia in patients with sepsis without
any relative decrease in the mean arterial pressure, and improve
patient survival (40). However,
recently published STRESS-L RCT (41) has revealed that in patients with
septic shock and tachycardia that were managed by norepinephrine
for >24 h, the use of beta-blocker landiolol did not affect SOFA
scores or mortality rates. The trial had to be stopped prematurely
due to the possible adverse effects of beta-blockers. By contrast,
a retrospective study has shown that that in patients who receive
chronic beta-blockers, continuation of beta-blockers therapy was
significantly associated with reduced in-hospital, 28 and 90-day
mortality compared with drug cessation (42).
The effect of beta-blockers in sepsis is indeed as
complex as the pathophysiology of the disease itself. Sympathetic
response is an important initial phase of sepsis that leads to
increased myocardial contractility, heart rate and vasoconstriction
as a way of counteracting the effect of inflammatory response to
infection (43). Current
guidelines recommend the use of norepinephrine to treat vasoplegia
and capillary leakage due to its vascular α1-agonist effect
(9). However high catecholamine
levels are associated with adverse effects such as tachycardia,
dysautonomia and altered cardiac hemodynamics (10). Furthermore, they can increase
cardiac dysfunction by inducing cardiomyopathy and cardiomyocyte
necrosis (13). A RCT comparing
norepinephrine and dobutamine with epinephrine alone, revealed that
these regimens resulted in similar survival of septic shock
patients. These results indicated a lack of benefit of
beta-adrenergic simulation in septic shock (44). The concept of
‘decatecholaminization’ is based on the blockage of beta-receptors
which are predominantly present in the heart, while allowing
adrenergic stimulation of vascular alpha receptors that would lead
to vasoconstriction (12,13). Premorbid use of beta-blockers can
therefore reduce the adrenergic response of the heart, leading to a
reduction in heart rate, improved diastolic time and higher
coronary perfusion. They can also reduce myocardial oxygen
consumption and lower the risk of myocardial ischemia (19). The use of beta-blockers would
reduce tachycardia, improve stroke volume and ultimately reduce
mortality (11). Beta-blockade can
also blunt the hypercatabolic adrenergic response, often observed
in sepsis, and can be associated with proteolysis, lipolysis and
hyperglycemia. Beta-blockers have been shown to reverse
muscle-protein catabolism and reduce catabolic states (45). Premorbid use of beta-blockers is
also associated with higher mean arterial pressure and lower
lactate levels at admission in patients with sepsis which could
also lower mortality rates (11).
The strengths of the present systematic review lie
in the detailed and updated literature search. The present analysis
included a total of 17 studies examining the role of premorbid
beta-blockers on outcomes of sepsis. The current study presented
the most current and comprehensive evidence on the subject, thereby
allowing clinicians to take informed decisions. Sensitivity
analysis and separate meta-analysis for unadjusted and adjusted
data further contributed to comprehensive evaluation of the
evidence.
There are limitations to the present study. Firstly,
most studies were retrospective. In addition, most studies did not
report adjusted data. Hence, selection and confounding bias are
important drawbacks of the current evidence. Secondly, studies did
not report if beta-blockers were continued or withheld during the
hospitalization period. Therefore, the current review was unable to
comment on the role of continued therapy on sepsis outcomes.
Thirdly, most studies did not report separate data on non-selective
and cardio-selective blockers. The type of beta-blocker was also
not reported in most studies. Furthermore, no information was
available on the duration and dosage of beta-blocker use in the
study group. Further investigations are therefore needed to provide
answers to these questions. Lastly, one cannot negate the
heterogeneity in the patient population and sepsis severity among
the studies. This could have primarily contributed to the high
heterogeneity in the primary meta-analysis.
In conclusion, premorbid use of beta-blockers may
contribute to improved survival in patients with sepsis. However,
there was no impact on the need for mechanical ventilation. Given
the observational nature of the data and the predominance of
unadjusted data, the results should be interpreted with caution.
Further prospective studies with large sample sizes and considering
confounding factors should be conducted to provide improved
evidence.
Supplementary Material
Details of search queries.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HC conceived and designed the study. FF, YS and HZ
collected the data and performed the literature search. HC
contributed to the writing of the manuscript. FF and YS confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yousuf F, Malik A, Saba A and Sheikh S:
Risk factors and compliance of surviving sepsis campaign: A
retrospective cohort study at tertiary care hospital. Pak J Med
Sci. 38:90–94. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rudd KE, Johnson SC, Agesa KM, Shackelford
KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer
S, et al: Global, regional, and national sepsis incidence and
mortality, 1990-2017: Analysis for the global burden of disease
study. Lancet. 395:200–211. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lei S, Li X, Zhao H, Xie Y and Li J:
Prevalence of sepsis among adults in China: A systematic review and
meta-analysis. Front public Health. 10(977094)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shahsavarinia K, Moharramzadeh P, Arvanagi
RJ and Mahmoodpoor A: qSOFA score for prediction of sepsis outcome
in emergency department. Pak J Med Sci. 36:668–672. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gorecki G, Cochior D, Moldovan C and Rusu
E: Molecular mechanisms in septic shock (Review). Exp Ther Med.
22(1161)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yoon SH, Choi B, Eun S, Bae GE, Koo CM and
Kim MK: Using the lactate-to-albumin ratio to predict mortality in
patients with sepsis or septic shock: A systematic review and
meta-analysis. Eur Rev Med Pharmacol Sci. 26:1743–1752.
2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Feng J, Wang L, Feng Y, Yu G, Zhou D and
Wang J: Serum levels of angiopoietin 2 mRNA in the mortality
outcome prediction of septic shock. Exp Ther Med.
23(362)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
de Montmollin E, Aboab J, Mansart A and
Annane D: Bench-to-bedside review: Beta-adrenergic modulation in
sepsis. Crit Care. 13(230)2009.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Hartmann C, Radermacher P, Wepler M and
Nußbaum B: Non-hemodynamic effects of catecholamines. Shock.
48:390–400. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hasegawa D, Sato R, Prasitlumkum N and
Nishida K: Effect of premorbid beta-blockers on mortality in
patients with sepsis: A systematic review and meta-analysis. J
Intensive Care Med. 37:908–916. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Singer M: Catecholamine treatment for
shock-equally good or bad? Lancet. 370:636–637. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Suzuki T, Suzuki Y, Okuda J, Kurazumi T,
Suhara T, Ueda T, Nagata H and Morisaki H: Sepsis-induced cardiac
dysfunction and β-adrenergic blockade therapy for sepsis. J
Intensive Care. 5(22)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Morelli A, Ertmer C, Westphal M, Rehberg
S, Kampmeier T, Ligges S, Orecchioni A, D'Egidio A, D'Ippoliti F,
Raffone C, et al: Effect of heart rate control with esmolol on
hemodynamic and clinical outcomes in patients with septic shock: A
randomized clinical trial. JAMA. 310:1683–1691. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hasegawa D, Sato R, Prasitlumkum N,
Nishida K, Takahashi K, Yatabe T and Nishida O: Effect of
ultrashort-acting β-blockers on mortality in patients with sepsis
with persistent tachycardia despite initial resuscitation: A
systematic review and meta-analysis of randomized controlled
trials. Chest. 159:2289–2300. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Arnautovic J, Mazhar A, Souther B,
Mikhijan G, Boura J and Huda N: Cardiovascular factors associated
with septic shock mortality risks. Spartan Med Res J.
3(6516)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sharma A, Vashisht R, Bauer S and Hanane
T: 1351: Effect of preadmission beta-blocker use on outcomes of
patients admitted with septic shock. Crit Care Med.
44(413)2016.
|
|
18
|
Kuo MJ, Chou RH, Lu YW, Guo JY, Tsai YL,
Wu CH, Huang PH and Lin SJ: Premorbid β1-selective (but not
non-selective) β-blocker exposure reduces intensive care unit
mortality among septic patients. J Intensive Care.
9(40)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tan K, Harazim M, Tang B, Mclean A and
Nalos M: The association between premorbid beta blocker exposure
and mortality in sepsis-a systematic review. Crit Care.
23(298)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. Int J Surg. 88(105906)2021.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Wells G, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The newcastle-ottawa scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses.
|
|
22
|
DeMott JM, Patel G and Lat I: Effects of
chronic antihypertensives on vasopressor dosing in septic shock.
Ann Pharmacother. 52:40–47. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hsieh MS, How CK, Hsieh VCR and Chen PC:
Preadmission antihypertensive drug use and sepsis outcome: Impact
of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin
receptor blockers (ARBs). Shock. 53:407–415. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guz D, Buchritz S, Guz A, Ikan A, Babich
T, Daitch V, Gafter-Gvili A, Leibovici L and Avni T: β-Blockers,
tachycardia, and survival following sepsis: An observational cohort
study. Clin Infect Dis. 73:e921–e926. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pham D, Ward H, Yong B, Raj JM, Awad M,
Harvey M, Doherty S and Cave G: Is lactate lower in septic patients
who are prescribed beta blockers? Retrospective cohort study of an
intensive care population. Emerg Med Australas. 33:82–87.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tan K, Harazim M, Simpson A, Tan YC,
Gunawan G, Robledo KP, Whitehead C, Tang B, Mclean A and Nalos M:
Association between premorbid beta-blocker exposure and sepsis
outcomes-the beta-blockers in european and Australian/American
septic patients (BEAST) study. Crit Care Med. 49:1493–1503.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ma Y, Ma J and Yang J: Association between
pre-existing long-term β-blocker therapy and the outcomes of
sepsis-associated coagulopathy: A retrospective study. Medicina
(Kaunas). 58(1843)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kumar S, Malviya D, Tripathi M, Rai S,
Nath SS, Tripathi SS and Mishra S: Exploring the impact of prior
beta-blocker and calcium channel blocker usage on clinical outcomes
in critically Ill patients with sepsis: An observational study.
Cureus. 15(e46169)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Charles D, Jean-Francois L, Matthieu J,
Charpentier J, Cariou A and Chiche JD: Hemodynamic parameters of
septic shock patients treated with prior BB therapy. In:
Proceedings of Réanimation. Ann Intensive Care, p8, 2018.
|
|
30
|
Alsolamy S, Ghamdi G, Alswaidan L, Alharbi
S and Alenezi F: Association between previous prescription of
βblockers and mortality rate among septic patients: A retrospective
observational study. In: 36th International Symposium on Intensive
Care and Emergency Medicine. Crit Care, p20, 2016.
|
|
31
|
Al-Qadi M, O'Horo J, Thakur L, Kaur S,
Berrios R and Caples S: Long- term use of beta blockers is
protective in severe sepsis and septic shock. Am J Resp Crit Care
Med. 189(A6655)2014.
|
|
32
|
Macchia A, Romero M, Comignani PD, Mariani
J, D'Ettorre A, Prini N, Santopinto M and Tognoni G: Previous
prescription of β-blockers is associated with reduced mortality
among patients hospitalized in intensive care units for sepsis.
Crit Care Med. 40:2768–2772. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Contenti J, Occelli C, Corraze H, Lemoël F
and Levraut J: Long-term β-blocker therapy decreases blood lactate
concentration in severely septic patients. Crit Care Med.
43:2616–2622. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Singer KE, Collins CE, Flahive JM, Wyman
AS, Ayturk MD and Santry HP: Outpatient beta-blockers and survival
from sepsis: Results from a national cohort of Medicare
beneficiaries. Am J Surg. 214:577–582. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chan JZW, Tan JH, Lather KS, Ng AJY, Ong
Z, Zou X, Chua MT and Kuan WS: Beta-blockers' effect on levels of
Lactate in patients with suspected sepsis-The BeLLa study. Am J
Emerg Med. 38:2574–2579. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nakayama T, Yoshiike N and Yokoyama T:
Clinicians and epidemiologists view crude death rates differently.
BMJ. 318(395)1999.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fleischmann C, Thomas-Rueddel DO, Hartmann
M, Hartog CS, Welte T, Heublein S, Dennler U and Reinhart K:
Hospital incidence and mortality rates of sepsis. Dtsch Arztebl
Int. 113:159–166. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Garg R, Tellapragada C, Shaw T, Eshwara
VK, Shanbhag V, Rao S, Virk HS, Varma M and Mukhopadhyay C:
Epidemiology of sepsis and risk factors for mortality in intensive
care unit: A hospital based prospective study in South India.
Infect Dis (Lond). 54:325–334. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ren Y, Zhang L, Xu F, Han D, Zheng S,
Zhang F, Li L, Wang Z, Lyu J and Yin H: Risk factor analysis and
nomogram for predicting in-hospital mortality in ICU patients with
sepsis and lung infection. BMC Pulm Med. 22(17)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lee YR, Seth MS, Soney D and Dai H:
Benefits of beta-blockade in sepsis and septic shock: A systematic
review. Clin Drug Investig. 39:429–440. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Whitehouse T, Hossain A, Perkins GD,
Gordon AC, Bion J, Young D, McAuley D, Singer M, Lord J, Gates S,
et al: Landiolol and organ failure in patients with septic shock:
The STRESS-L randomized clinical trial. JAMA. 330:1641–1652.
2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fuchs C, Wauschkuhn S, Scheer C, Vollmer
M, Meissner K, Kuhn SO, Hahnenkamp K, Morelli A, Gründling M and
Rehberg S: Continuing chronic beta-blockade in the acute phase of
severe sepsis and septic shock is associated with decreased
mortality rates up to 90 days. Br J Anaesth. 119:616–625.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dünser MW and Hasibeder WR: Sympathetic
overstimulation during critical illness: Adverse effects of
adrenergic stress. J Intensive Care Med. 24:293–316.
2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Annane D, Vignon P, Renault A, Bollaert
PE, Charpentier C, Martin C, Troché G, Ricard JD, Nitenberg G,
Papazian L, et al: Norepinephrine plus dobutamine versus
epinephrine alone for management of septic shock: A randomised
trial. Lancet. 370:676–684. 2007.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Herndon DN, Hart DW, Wolf SE, Chinkes DL
and Wolfe RR: Reversal of catabolism by beta-blockade after severe
burns. N Engl J Med. 345:1223–1229. 2001.PubMed/NCBI View Article : Google Scholar
|