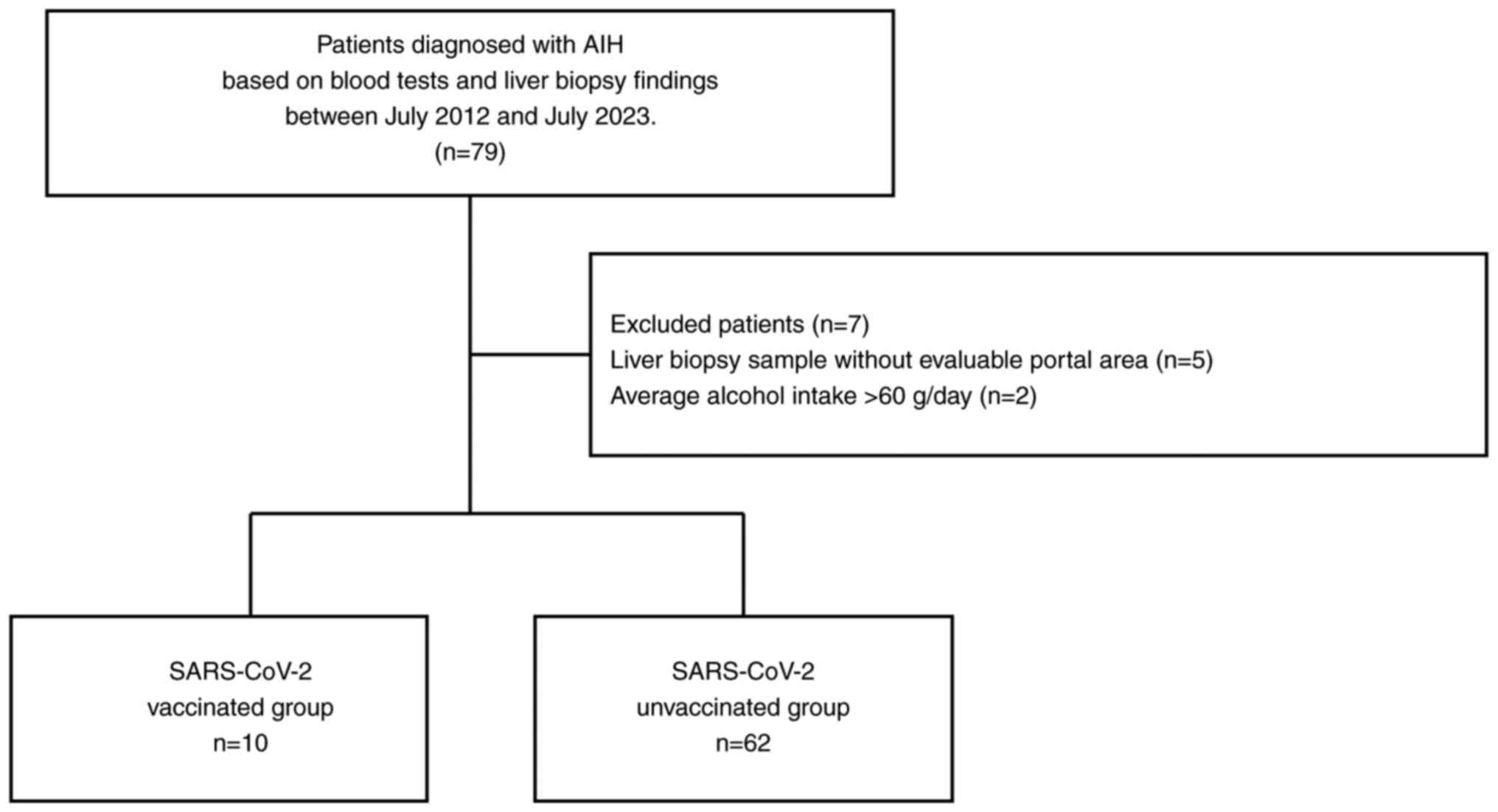
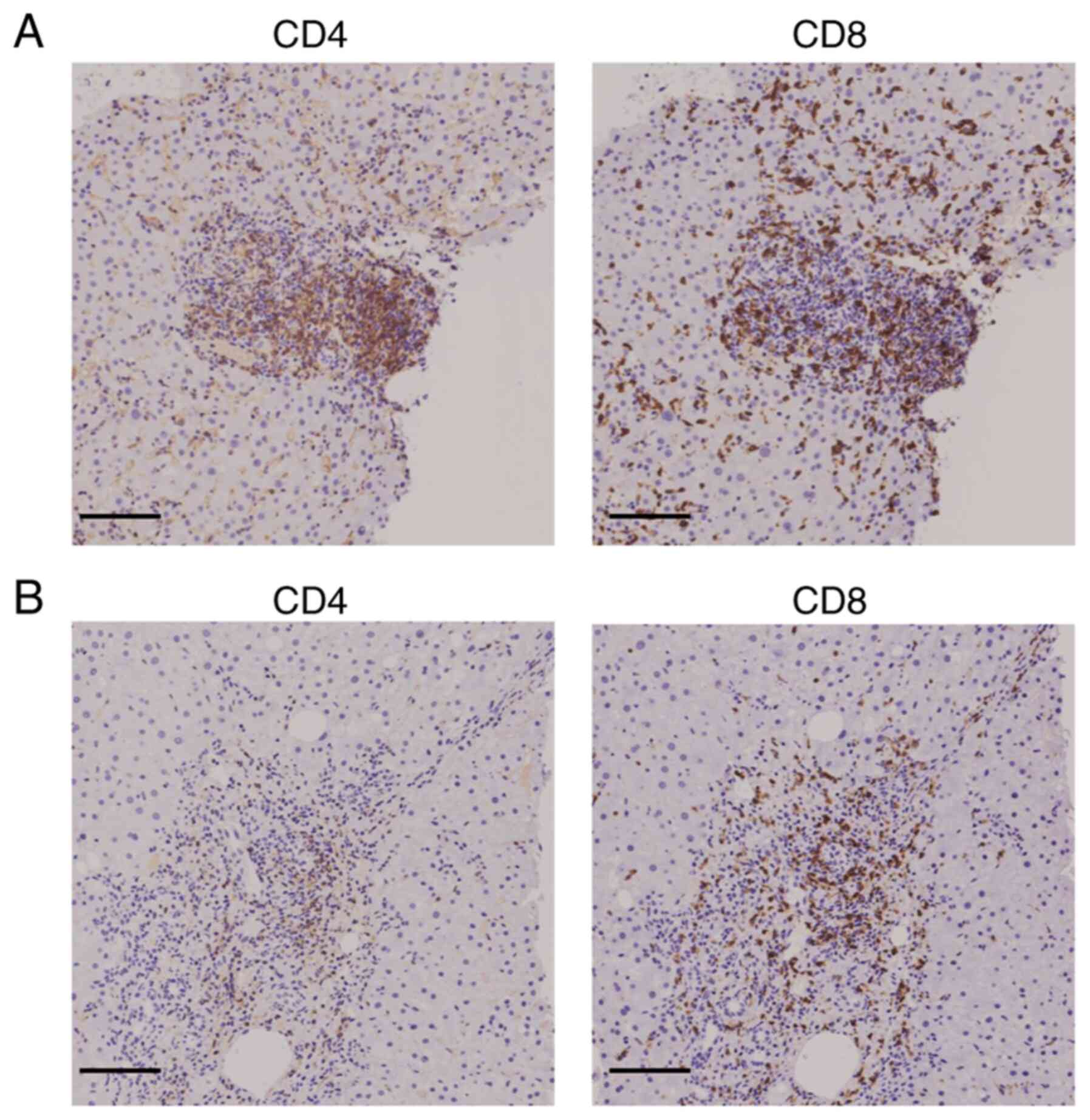
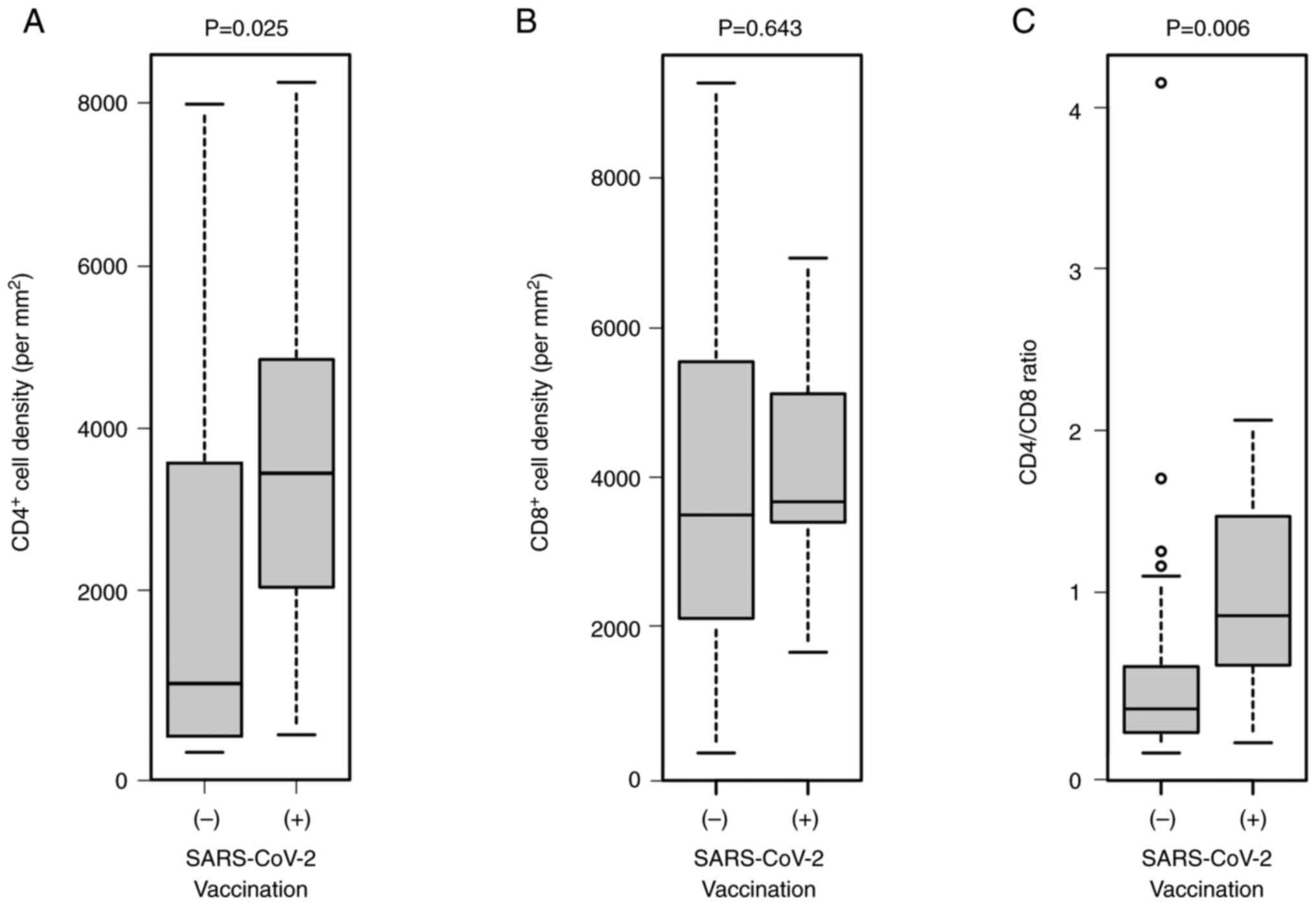
|
1
|
World Health Organization (WHO): COVID-19
epidemiological. WHO, Geneva, 2023. https://www.who.int/publications/m/item/covid-19-epidemiological-update-22-december-2023.
|
|
2
|
Ariño H, Heartshorne R, Michael BD,
Nicholson TR, Vincent A, Pollak TA and Vogrig A: Neuroimmune
disorders in COVID-19. J Neurol. 269:2827–2839. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tutal E, Ozaras R and Leblebicioglu H:
Systematic review of COVID-19 and autoimmune thyroiditis. Travel
Med Infect Dis. 47(102314)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gracia-Ramos AE, Martin-Nares E and
Hernández-Molina G: New onset of autoimmune diseases following
COVID-19 diagnosis. Cells. 10(3592)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Polatoğlu I, Oncu-Oner T, Dalman I and
Ozdogan S: COVID-19 in early 2023: Structure, replication
mechanism, variants of SARS-CoV-2, diagnostic tests, and vaccine
& drug development studies. MedComm. 4(e228)2023.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Guo M, Liu X, Chen X and Li Q: Insights
into new-onset autoimmune diseases after COVID-19 vaccination.
Autoimmun Rev. 22(103340)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lee EJ, Cines DB, Gernsheimer T, Kessler
C, Michel M, Tarantino MD, Semple JW, Arnold DM, Godeau B, Lambert
MP and Bussel JB: Thrombocytopenia following Pfizer and Moderna
SARS-CoV-2 vaccination. Am J Hematol. 96:534–537. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tarawneh O and Tarawneh H: Immune
thrombocytopenia in a 22-year-old post COVID-19 vaccine. Am J
Hematol. 96:E133–E134. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Watad A, De Marco G, Mahajna H, Druyan A,
Eltity M, Hijazi N, Haddad A, Elias M, Zisman D, Naffaa ME, et al:
Immune-mediated disease flares or new-onset disease in 27 subjects
following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel).
9(435)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mieli-Vergani G and Vergani D: Autoimmune
hepatitis. Nat Rev Gastroenterol Hepatol. 8:320–329.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Czaja AJ: Understanding the pathogenesis
of autoimmune hepatitis. Am J Gastroenterol. 96:1224–1231.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Björnsson E, Talwalkar J, Treeprasertsuk
S, Kamath PS, Takahashi N, Sanderson S, Neuhauser M and Lindor K:
Drug-induced autoimmune hepatitis: Clinical characteristics and
prognosis. Hepatology. 51:2040–2048. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Della Corte C, Carlucci A, Francalanci P,
Alisi A and Nobili V: Autoimmune hepatitis type 2 following
anti-papillomavirus vaccination in a 11-year-old girl. Vaccine.
29:4654–4656. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Perumalswami P, Peng L and Odin JA:
Vaccination as a triggering event for autoimmune hepatitis. Semin
Liver Dis. 29:331–334. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Muratori P, Serio I, Lalanne C and Lenzi
M: Development of autoimmune hepatitis after influenza vaccination;
trigger or killer? Clin Res Hepatol Gastroenterol. 43:e95–e96.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sasaki T, Suzuki Y, Ishida K, Kakisaka K,
Abe H, Sugai T and Takikawa Y: Autoimmune hepatitis following
influenza virus vaccination: Two case reports. Medicine
(Baltimore). 97(e11621)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Berry PA and Smith-Laing G: Hepatitis A
vaccine associated with autoimmune hepatitis. World J
Gastroenterol. 13:2238–2239. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
van Gemeren MA, van Wijngaarden P, Doukas
M and de Man RA: Vaccine-related autoimmune hepatitis: The same
disease as idiopathic autoimmune hepatitis? Two clinical reports
and review. Scand J Gastroenterol. 52:18–22. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Csepregi A, Treiber G, Röcken C and
Malfertheiner P: Acute exacerbation of autoimmune hepatitis induced
by Twinrix. World J Gastroenterol. 11:4114–4116. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lohse AW, Sebode M, Bhathal PS, Clouston
AD, Dienes HP, Jain D, Gouw ASH, Guindi M, Kakar S, Kleiner DE, et
al: Consensus recommendations for histological criteria of
autoimmune hepatitis from the International AIH Pathology Group:
Results of a workshop on AIH histology hosted by the European
Reference Network on Hepatological Diseases and the European
Society of Pathology: Results of a workshop on AIH histology hosted
by the European Reference Network on Hepatological Diseases and the
European Society of Pathology. Liver Int. 42:1058–1069.
2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hennes EM, Zeniya M, Czaja AJ, Parés A,
Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer
H, et al: Simplified criteria for the diagnosis of autoimmune
hepatitis. Hepatology. 48:169–176. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
LAlvarez F, Berg PA, Bianchi FB, Bianchi
L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ,
Desmet VJ, et al: International Autoimmune Hepatitis Group Report:
Review of criteria for diagnosis of autoimmune hepatitis. J
Hepatol. 31:929–938. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Czaja AJ: Performance parameters of the
diagnostic scoring systems for autoimmune hepatitis. Hepatology.
48:1540–1548. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Boettler T, Csernalabics B, Salié H,
Luxenburger H, Wischer L, Salimi Alizei E, Zoldan K, Krimmel L,
Bronsert P, Schwabenland M, et al: SARS-CoV-2 vaccination can
elicit a CD8 T-cell dominant hepatitis. J Hepatol. 77:653–659.
2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Grifoni A, Weiskopf D, Ramirez SI, Mateus
J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L,
Jadi RS, et al: Targets of T cell responses to SARS-CoV-2
coronavirus in humans with COVID-19 disease and unexposed
individuals. Cell. 181:1489–1501.e15. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sekine T, Perez-Potti A,
Rivera-Ballesteros O, Strålin K, Gorin JB, Olsson A,
Llewellyn-Lacey S, Kamal H, Bogdanovic G, Muschiol S, et al: Robust
T cell immunity in convalescent individuals with asymptomatic or
mild COVID-19. Cell. 183:158–168.e14. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sirbe C, Simu G, Szabo I, Grama A and Pop
TL: Pathogenesis of autoimmune hepatitis-cellular and molecular
mechanisms. Int J Mol Sci. 22(13578)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Béland K, Marceau G, Labardy A,
Bourbonnais S and Alvarez F: Depletion of B cells induces remission
of autoimmune hepatitis in mice through reduced antigen
presentation and help to T cells. Hepatology. 62:1511–1523.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
John K, Hardtke-Wolenski M, Jaeckel E,
Manns MP, Schulze-Osthoff K and Bantel H: Increased apoptosis of
regulatory T cells in patients with active autoimmune hepatitis.
Cell Death Dis. 8(3219)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wack S, Patton T and Ferris LK: COVID-19
vaccine safety and efficacy in patients with immune-mediated
inflammatory disease: Review of available evidence. J Am Acad
Dermatol. 85:1274–1284. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pacheco Y, Acosta-Ampudia Y, Monsalve DM,
Chang C, Gershwin ME and Anaya JM: Bystander activation and
autoimmunity. J Autoimmun. 103(102301)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Salemi S and D'Amelio R: Could
autoimmunity be induced by vaccination? Int Rev Immunol.
29:247–269. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ichiki Y, Aoki CA, Bowlus CL, Shimoda S,
Ishibashi H and Gershwin ME: T cell immunity in autoimmune
hepatitis. Autoimmun Rev. 4:315–321. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tanaka A, Iwabuchi S, Takatori M, Ohno A,
Yamada H, Hashimoto N, Ikeda Y, Kato T, Nishioka K, Iino S and
Yamamoto K: Clonotypic analysis of T cells in patients with
autoimmune and viral hepatitis. Hepatology. 25:1070–1076.
1997.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vojdani A and Kharrazian D: Potential
antigenic cross-reactivity between SARS-CoV-2 and human tissue with
a possible link to an increase in autoimmune diseases. Clin
Immunol. 217(108480)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Uzun S, Zinner CP, Beenen AC, Alborelli I,
Bartoszek EM, Yeung J, Calgua B, Reinscheid M, Bronsert P, Stalder
AK, et al: Morphologic and molecular analysis of liver injury after
SARS-CoV-2 vaccination reveals distinct characteristics. J Hepatol.
79:666–676. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Codoni G, Kirchner T, Engel B, Villamil
AM, Efe C, Stättermayer AF, Weltzsch JP, Sebode M, Bernsmeier C,
Lleo A, et al: Histological and serological features of acute liver
injury after SARS-CoV-2 vaccination. JHEP Rep.
5(100605)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Efe C, Kulkarni AV, Terziroli
Beretta-Piccoli B, Magro B, Stättermayer A, Cengiz M, Clayton-Chubb
D, Lammert C, Bernsmeier C, Gül Ö, et al: Liver injury after
SARS-CoV-2 vaccination: Features of immune-mediated hepatitis, role
of corticosteroid therapy and outcome. Hepatology. 76:1576–1586.
2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Manns MP, Czaja AJ, Gorham JD, Krawitt EL,
Mieli-Vergani G, Vergani D and Vierling JM: American Association
for the Study of Liver Diseases. Diagnosis and management of
autoimmune hepatitis. Hepatology. 51:2193–2213. 2010.PubMed/NCBI View Article : Google Scholar
|