Introduction
Coronavirus disease 2019 (COVID-19), caused by
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has
impacted millions of individuals across the globe, with profound
social and economic consequences. Confirmed COVID-19 cases have
surpassed 772 million worldwide, with nearly seven million
mortalities reported to date (1).
In addition to causing COVID-19, SARS-CoV-2 may interfere with
immune regulation, potentially initiating autoimmune responses.
Indeed, several studies have reported the emergence of autoimmune
diseases following SARS-CoV-2 infection (2-4).
Vaccines against SARS-CoV-2 have been developed and widely
distributed to reduce the severity of COVID-19 and the spread of
SARS-CoV-2. Since December 2020, following the first approval of
SARS-CoV-2 vaccine by the United States Food and Drug
Administration and European Medicines Agency, 50 vaccines including
Pfizer/BioNTech, Moderna, Johnson and Johnson, AstraZeneca/Oxford,
Sinovac, Sinopharm, Covaxin, Covovax and Nuvaxovid have been
approved worldwide and SARS-CoV-2 vaccination is recommended for
everyone aged 6 months and older for the prevention of
SARS-CoV-2(5). The majority of
these vaccines are delivered via intramuscular injection and have
been instrumental in reducing the severity of COVID-19 and
preventing fatalities. However, there is an increasing body of
evidence indicating that vaccination against SARS-CoV-2 could lead
to the onset of autoimmune conditions, such as autoimmune
glomerulonephritis and various autoimmune rheumatic diseases
(6-9).
The onset of autoimmune hepatitis (AIH) is
considered to be influenced by genetics, (particularly specific
human leukocyte antigens), while epigenetic, immunological and
environmental factors also contribute to its development. Certain
viral infections and medications have also been implicated in the
onset of AIH (10-12).
Recent reports have noted instances of AIH being potentially
triggered by vaccines, suggesting that the SARS-CoV-2 mRNA vaccine
might also be implicated in AIH development (13-19).
However, no study, to the best of the authors' knowledge, has
compared the clinical and pathological characteristics of AIH in
patients who were or were not vaccinated against SARS-CoV-2. The
present study examined the clinical and pathological attributes of
AIH patients who had or had not received the SARS-CoV-2 mRNA
vaccine.
Materials and methods
Patients
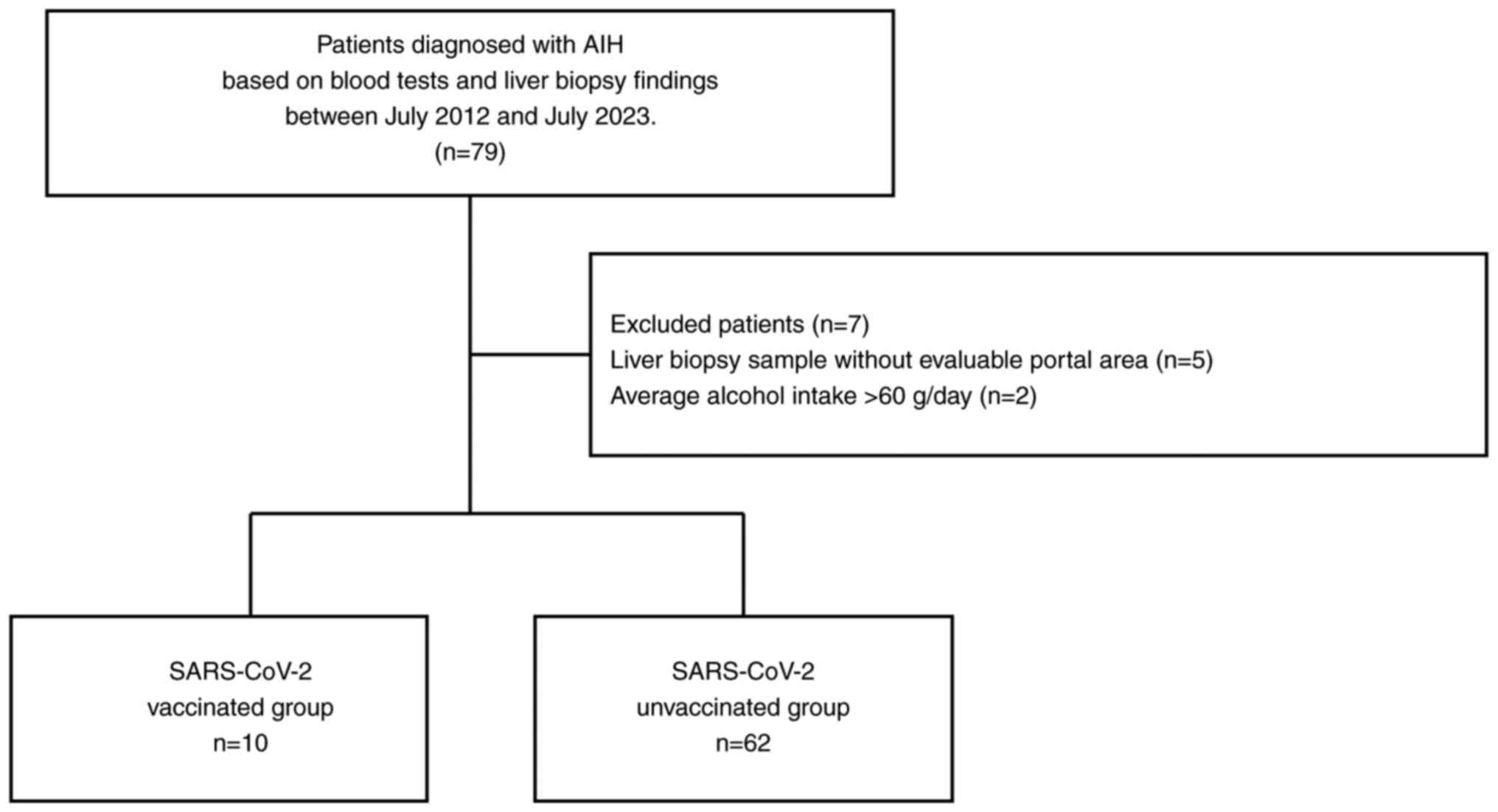
The present single-center, retrospective study
included 79 patients diagnosed with AIH, based on blood tests and
liver biopsy findings, between July 2012 and July 2023(20) at Aso Iizuka Hosipital. The
following exclusion criteria were applied: patients with i)
hepatitis B virus or hepatitis C virus, ii) those with alcohol use
disorder, who had an average daily intake of more than 60 g of
alcohol/day, iii) a history of SARS-CoV-2 infection and iv) those
with inadequate pathology specimens. Following the exclusion of
seven patients, 72 patients were included in the final analysis
(Fig. 1).
The present study was conducted in accordance with
the guidelines of the Declaration of Helsinki and was approved by
the Ethics Committee of Aso Iizuka Hospital (Fukuoka, Japan;
approval no. 23101). The opt-out method was used to obtain consent
for this study.
Immunohistochemistry (IHC)
Liver biopsy specimens were fixed in 10% formalin
for 10-48 h at room temperature. Subsequently, the tissue sections
were dehydrated in 80, 90, 95 and 100% gradient ethanol for 2-4 h.
Serial sections (5 µm) were cut from the paraffin-embedded blocks
and stained with hematoxylin and eosin (hematoxylin for 3 min and
eosin for 45 sec at room temperature). IHC was performed by
Morphotechnology Co. Ltd. Paraffin slides were deparaffinized using
two changes of xylene for 10 min each and hydrated using graded
alcohol and distilled water (two changes of 100% ethanol, two
changes of 95% ethanol and two changes of distilled water) for 10
min each at room temperature. Heat-induced epitope retrieval with
citrate buffer was performed for 20 min at 95˚C. Slides were then
cooled and rinsed with distilled water. Slides were then rinsed
with 0.3% hydrogen peroxide, followed by a rinse with Tris-buffered
saline. Specimens were then incubated for 60 min at room
temperature with the primary antibody CD4 (clone 4B12; cat. no.
NCL-L-CD4-368; 1:200, Leica Biosystems) and CD8 (clone C8/144B;
cat. no. M7103; 1:150, Dako; Agilent Technologies, Inc.). Sections
were incubated with peroxidase-labeled anti-goat or anti-rabbit
antibodies (Histofine Simple Stain MAX PO (MULTI); cat. no. 724152;
Nichirei Biosciences, Inc.) for 30 min at room temperature.
Afterwards, the secondary reagent, diaminobenzidine (Histofine
Simple Stain DAB solution; cat. no. 725191; Nichirei Biosciences,
Inc.) was applied for 5 min and the slides were rinsed with
distilled water. Counterstaining was performed with hematoxylin for
1.5 min at room temperature and slides were washed in tap water at
room temperature. Slides were then blued in ammonia water, rinsed
in tap water, dehydrated in graded alcohol (95 and 100% ethanol),
cleared in xylene (two changes) for 10 min each at room temperature
and coverslipped for light microscopic examination. The sections
were visualized under a Keyence BZ-X700 microscope (Keyence
Corporation). Positive cells in the selected microscopic fields
(magnification, x20) of the portal region of the liver were
quantified using analysis software (BZ-X analyzer, Keyence
Corporation). A total of three hepatologists evaluated IHC.
Statistical analysis
JMP Pro Version 11 statistical software (SAS
Institute Inc.) was used for all the statistical analyses. Data
were presented as the median (interquartile range) or n (%), as
appropriate. Significant differences between groups were examined
using the χ2 test. The χ2 test or the
Fisher's exact test were used for analyses involving categorical
variables. The accuracy of the statistical analyses were verified
by two experienced statisticians. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
The characteristics of the 72 patients diagnosed
with AIH are shown in Table I.
Scoring parameters according to the Simplified Diagnostic Criteria
or Revised Original Diagnostic Criteria for AIH were used to define
the reliability of the diagnosis (i.e., 53 simplified and 19
revised diagnoses) made from the evaluation of liver injury
(21). This resulted in 56
patients being diagnosed with definite AIH and 16 patients being
diagnosed with probable AIH (21-23).
A total of 10 patients developed AIH after receiving the SARS-CoV-2
mRNA vaccination (Pfizer/BioNTech or Moderna; the SARS-CoV-2
vaccinated group), while 62 patients were not vaccinated against
SARS-CoV-2 prior to AIH onset (the SARS-CoV-2 unvaccinated group).
A total of four patients received single dose of vaccine, four
patients received two doses of vaccine and three patients received
three doses of vaccine. AIH in SARS-CoV-2-vaccinated patients was
diagnosed at 123.5 (44.3-165.0) days after vaccine administration.
There were no significant differences in the age, sex, or the
aspartate aminotransferase, alanine aminotransferase (ALT),
alkaline phosphatase level, total bilirubin, immunoglobulin G,
anti-nuclear antibody, anti-smooth muscle antibody and
anti-mitochondrial antibody levels between the SARS-CoV-2
vaccinated and unvaccinated patients. History of recent or current
use of known or suspected hepatotoxic drugs, average alcohol
intake, or the presence of other autoimmune diseases also did not
differ markedly between the two groups. All patients were treated
with steroid therapy (mostly prednisone or prednisolone) without
azathioprine. There were no differences in the treatment success
rate between the groups.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | All | SARS-CoV-2
vaccinated group | SARS-CoV-2
unvaccinated group | P-value |
|---|
| Number | 72 | 10 | 62 | |
| Age, years | 64.0
(57.3-71.0) | 66.0
(47.5-71.0) | 63.5
(57.8-71.0) | 0.666 |
| Sex, n
male/female | 13/59 | 2/8 | 11/51 | 0.865 |
| AST, U/l | 424.5
(222.0-767.0) | 246.5
(202.5-953.0) | 484.0
(226.5-741.0) | 0.784 |
| ALT, U/l | 532.5
(247.5-886.0) | 397.0
(264.5-772.5) | 578.0
(241.8-892.5) | 0.968 |
| ALP, U/l | 165.9
(128.3-217.5) | 149.0
(105.8-183.3) | 165.9
(134.1-231.2) | 0.173 |
| Total bilirubin,
mg/dl | 1.4 (1.1-7.5) | 1.3 (0.8-5.3) | 1.6 (1.1-7.8) | 0.485 |
| IgG, mg/dl | 2,016.5
(1,656.3-2830.8) | 1,711.0
(1,232.5-2,355.5) | 2,087.5
(1,705.5-2,862.5) | 0.5283 |
| ANA, n | | | | 0.436 |
|
>1:80 | 52 | 6 | 46 | |
|
>1:40 | 11 | 3 | 8 | |
|
<1:40 | 9 | 1 | 8 | |
| ASMA, n | | | | 0.819 |
|
>1:80 | 15 | 2 | 13 | |
|
>1:40 | 3 | 0 | 3 | |
|
<1:40 | 32 | 4 | 28 | |
|
NA | 22 | 4 | 18 | |
| AMA, n | | | | 0.529 |
|
>1:40 | 1 | 0 | 1 | |
|
<1:40 | 52 | 6 | 46 | |
|
NA | 19 | 4 | 15 | |
| Average alcohol
intake, n | | | | 0.841 |
|
<25
g/day | 66 | 9 | 57 | |
|
<60
g/day | 6 | 1 | 5 | |
| Drug history,
na | | | | 0.734 |
|
None | 44 | 7 | 37 | |
|
Statin | 15 | 1 | 14 | |
|
Chinese
herbal medicine | 4 | 1 | 3 | |
|
Others | 9 | 1 | 8 | |
|
Other
autoimmune disease(s), n | 12 | 0 | 12 | 0.190 |
| Diagnosis, n | | | | 0.169 |
|
Definite | 56 | 6 | 50 | |
|
Probable | 16 | 4 | 12 | |
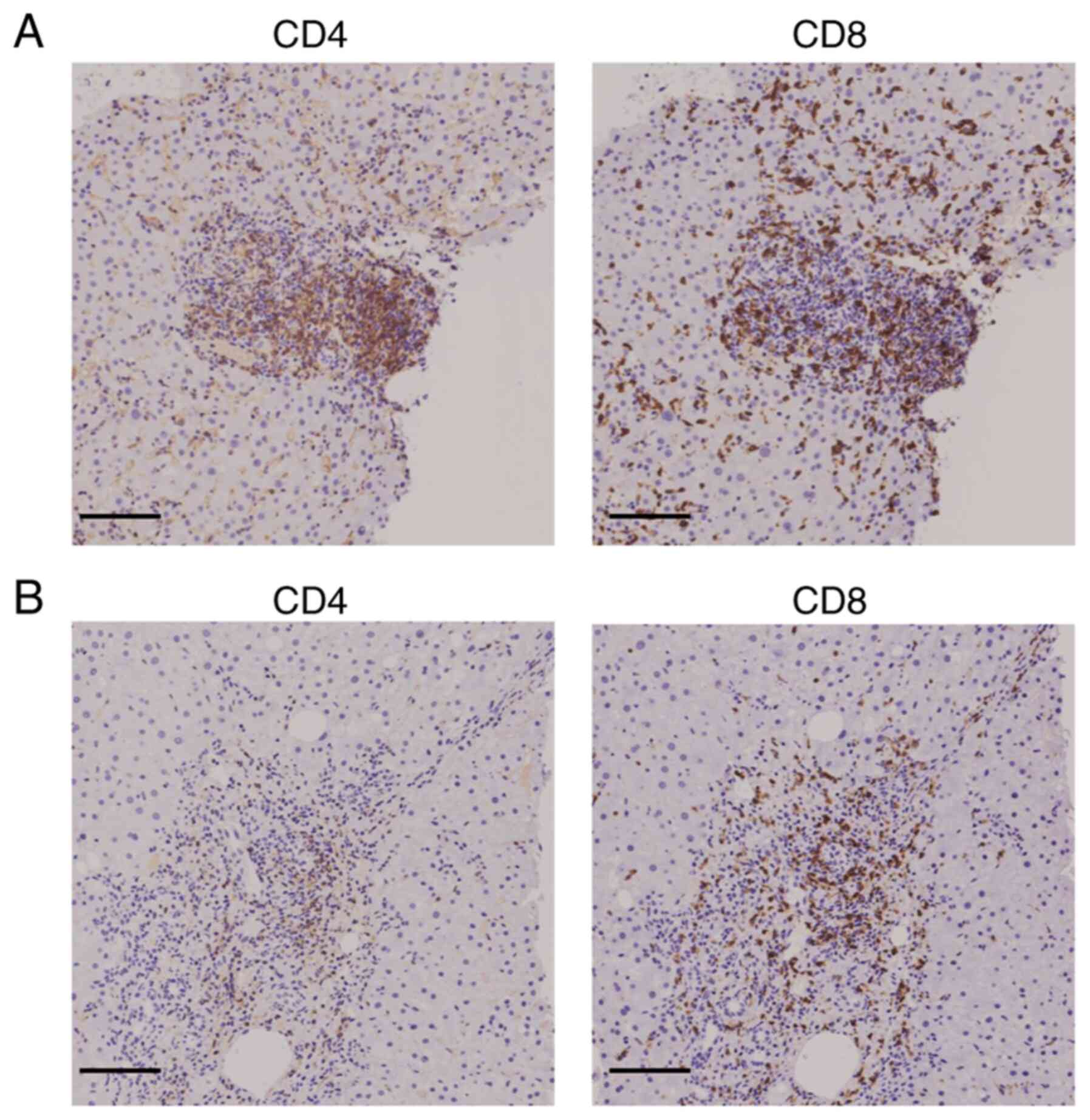
IHC analysis of CD4+ and
CD8+ T cells in liver tissue
The present study next examined the infiltration of
T cells into the portal region of the liver in the two groups of
patients. The IHC results showed that the extent of CD4+
and CD8+ T cell infiltration was comparable in the
SARS-CoV-2 vaccinated group, while the extent of CD4+ T
cell infiltration was lower than that of CD8+ T cell
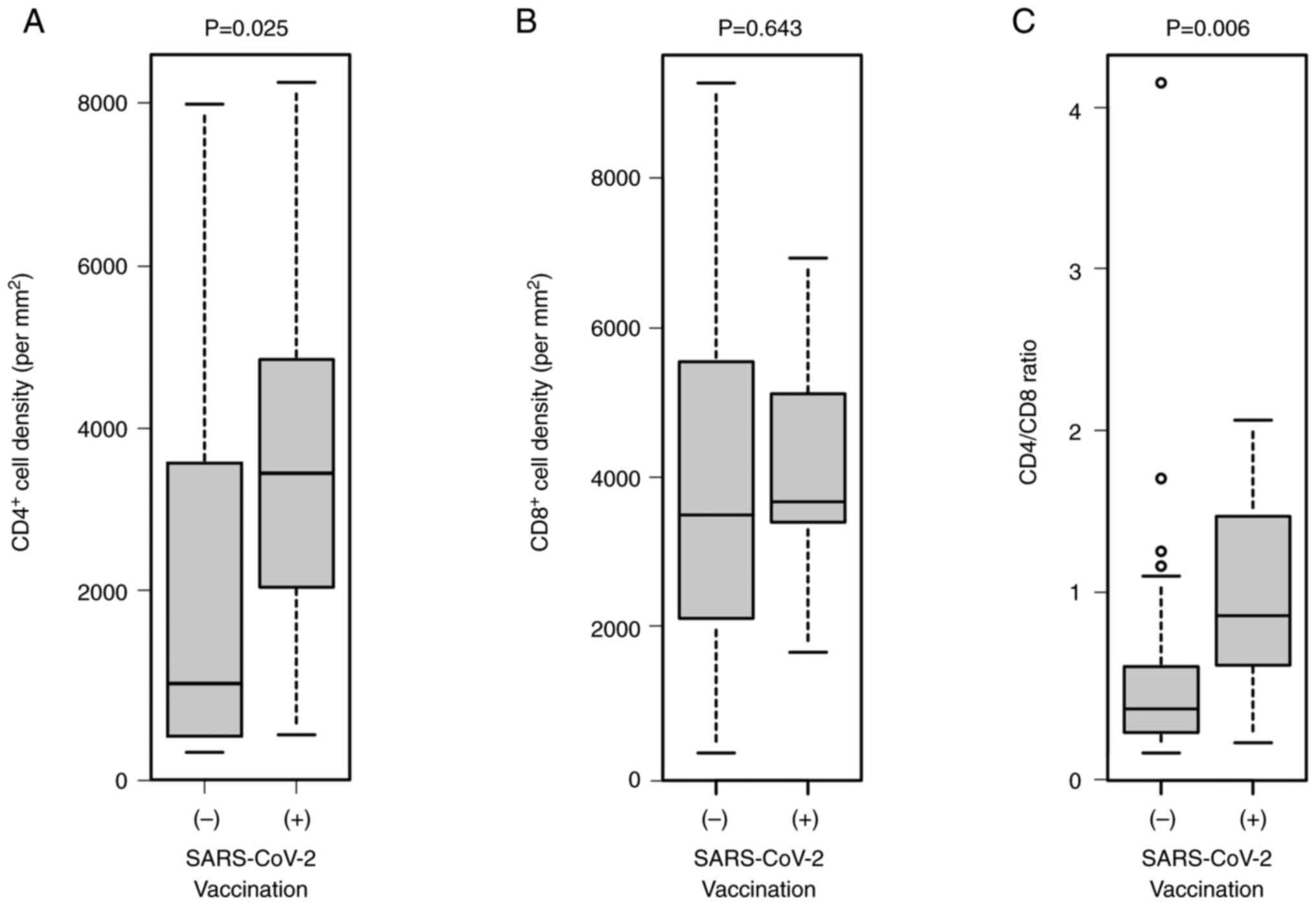
infiltration in the SARS-CoV-2 unvaccinated group (Fig. 2). Moreover, the density of
CD4+ T cells in the portal region of the liver was
significantly higher in the SARS-CoV-2 vaccinated group than the
unvaccinated group (P=0.025), while there was no significant
difference in the abundance of CD8+ T cells between the
groups (P=0.643; Fig. 3). Thus,
the CD4+/CD8+ T cell ratio was significantly
higher in the SARS-CoV-2 vaccinated group than in the unvaccinated
group (P=0.006). Moreover, serum ALT level was associated with
CD8+ T cell but not CD4+ T cell numbers in
liver tissue (P=0.0478) (data not shown).
Discussion
In the present study, the patients with AIH who had
been vaccinated against SARS-CoV-2 exhibited a greater infiltration
of CD4+ T cells into the portal region of the liver than
those who were unvaccinated. There was no difference in the extent
of CD8+ T cell infiltration between the two groups.
Previous research indicates that both SARS-CoV-2 vaccination and
infection can trigger strong CD4+ and CD8+ T
cell responses (24-26).
Notably, a previous study showed that the frequencies of both T
cell subsets within the liver tissue and peripheral blood increased
following SARS-CoV-2 vaccination in a patient who experienced two
acute hepatitis episodes (24).
SARS-CoV-2 infection typically induces stronger CD4+ T
cell than CD8+ T cell responses (25,26).
AIH development is thought to result from a
combination of genetic susceptibility and environmental triggers
(27). Molecular mimicry between
viral- and self-antigens may cause immune dysregulation and involve
a complex network of cells such as CD4+ T cells,
regulatory T cells, CD8+ T cells and B-cell-derived
autoantibodies (28,29). However, the precise process leading
to increased CD4+ T cell infiltration in the liver
following SARS-CoV-2 vaccination remains to be elucidated.
In Japan, the Moderna (mRNA-1273) and
Pfizer/BioNTech (BNT162b2) mRNA vaccines are most commonly
administered. These vaccines work by delivering the mRNA which
encodes the SARS-CoV-2 spike protein into host cells, where it is
translated into the spike protein. The SARS-CoV-2 spike protein is
then identified by the immune system, initiating strong
CD8+ and CD4+ T cell responses (30). Bystander activation, which refers
to the antigen-independent activation of T or B cells, aids in
pathogen elimination; however, it can also contribute to AIH
development (31,32). Serum ALT level was associated with
CD8+ T cell but not CD4+ T cell numbers in
liver tissue in this study. Until recently CD4+ T cells
were considered to be critical for development of AIH; previous
studies reported identical CD8+ T cells were universally
present throughout the liver of AIH and CD8+ T cells
played a significant role in the immune pathogenesis of AIH
(33,34).
Vojdani and Kharrazian (35) demonstrated cross-reactivity between
SARS-CoV-2 antibodies and human tissue antigens in the 21 out of 50
patients with autoimmune disease examined, suggesting that
molecular mimicry could potentially lead to autoimmune damage and
AIH in predisposed individuals. The development of AIH following
vaccination for influenza, hepatitis A, measles-mumps-rubella,
typhoid, polio and diphtheria/tetanus, has been documented,
indicating that vaccine-induced AIH is not exclusive to SARS-CoV-2
vaccines (14-18).
Uzun et al (36) explored the morphologic and
molecular features of SARS-CoV-2 vaccine-induced liver injury
(VILI), highlighting the challenges in distinguishing VILI from
AIH, as these conditions share clinical, biochemical, morphological
and serological characteristics. However, not all VILI cases meet
the AIH diagnostic criteria (23,36-39).
While VILI is typically marked by CD8+ T cell dominance,
AIH features a stronger presence of CD4+ T cells and
B/plasma cells (36). Moreover,
VILI can be identified in patients as early as at 2-28 days
post-vaccination, whereas AIH is typically diagnosed at a later
stage. In the present study, AIH in SARS-CoV-2-vaccinated patients
was dominated by CD4+ T cells and was diagnosed at
30-532 days after vaccine administration. These findings confirmed
that our SARS-CoV-2-vaccinated patients had AIH rather than
VILI.
The present study had several limitations, including
the small sample size, single-center scope and the absence of the
type of vaccine and gene expression analysis of liver tissues.
However, it showed that SARS-CoV-2-vaccinated patients with AIH had
more extensive CD4+ T cell liver infiltration compared
with those who were not vaccinated. In addition, no significant
difference was observed in the amount of CD8+ T cell
infiltration between the two groups. Our understanding of AIH
pathology has been altered by the COVID-19 pandemic. Further
studies are needed to differentiate between AIH and VILI.
Acknowledgements
The authors would like to thank Ms. Yukie Ishibashi
(Department of Hepatology, Iizuka Hospital, Iizuka, Japan) for
assistance with manuscript preparation.
Funding
Funding: The present study was conducted with the assistance of
an Aso Iizuka Hospital (Fukuoka, Japan) Clinical Research Grant
(grant no. AIH-CRG2024-3).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AK, SN, MY and KT designed the study. AK, SN, YK, KT
and MY assisted with data analysis. AK wrote the initial draft of
the manuscript. AK and SN performed and analyzed pathological
examinations, including immunostaining. AK and KT contributed to
the analysis and interpretation of the data. MY, AM and KM assisted
with the preparation and critical review of the manuscript. All
authors agreed to be accountable for all aspects of the work
presented within. AK and KT confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study protocols were performed in
accordance with the principles and ethical guidelines of the 1975
Declaration of Helsinki. The present study received approval from
the Aso Iizuka Hospital Ethics Committee (Fukuoka, Japan; approval
no. 23101). An opt-out method was used to obtain consent for this
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO): COVID-19
epidemiological. WHO, Geneva, 2023. https://www.who.int/publications/m/item/covid-19-epidemiological-update-22-december-2023.
|
|
2
|
Ariño H, Heartshorne R, Michael BD,
Nicholson TR, Vincent A, Pollak TA and Vogrig A: Neuroimmune
disorders in COVID-19. J Neurol. 269:2827–2839. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tutal E, Ozaras R and Leblebicioglu H:
Systematic review of COVID-19 and autoimmune thyroiditis. Travel
Med Infect Dis. 47(102314)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gracia-Ramos AE, Martin-Nares E and
Hernández-Molina G: New onset of autoimmune diseases following
COVID-19 diagnosis. Cells. 10(3592)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Polatoğlu I, Oncu-Oner T, Dalman I and
Ozdogan S: COVID-19 in early 2023: Structure, replication
mechanism, variants of SARS-CoV-2, diagnostic tests, and vaccine
& drug development studies. MedComm. 4(e228)2023.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Guo M, Liu X, Chen X and Li Q: Insights
into new-onset autoimmune diseases after COVID-19 vaccination.
Autoimmun Rev. 22(103340)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lee EJ, Cines DB, Gernsheimer T, Kessler
C, Michel M, Tarantino MD, Semple JW, Arnold DM, Godeau B, Lambert
MP and Bussel JB: Thrombocytopenia following Pfizer and Moderna
SARS-CoV-2 vaccination. Am J Hematol. 96:534–537. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tarawneh O and Tarawneh H: Immune
thrombocytopenia in a 22-year-old post COVID-19 vaccine. Am J
Hematol. 96:E133–E134. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Watad A, De Marco G, Mahajna H, Druyan A,
Eltity M, Hijazi N, Haddad A, Elias M, Zisman D, Naffaa ME, et al:
Immune-mediated disease flares or new-onset disease in 27 subjects
following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel).
9(435)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mieli-Vergani G and Vergani D: Autoimmune
hepatitis. Nat Rev Gastroenterol Hepatol. 8:320–329.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Czaja AJ: Understanding the pathogenesis
of autoimmune hepatitis. Am J Gastroenterol. 96:1224–1231.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Björnsson E, Talwalkar J, Treeprasertsuk
S, Kamath PS, Takahashi N, Sanderson S, Neuhauser M and Lindor K:
Drug-induced autoimmune hepatitis: Clinical characteristics and
prognosis. Hepatology. 51:2040–2048. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Della Corte C, Carlucci A, Francalanci P,
Alisi A and Nobili V: Autoimmune hepatitis type 2 following
anti-papillomavirus vaccination in a 11-year-old girl. Vaccine.
29:4654–4656. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Perumalswami P, Peng L and Odin JA:
Vaccination as a triggering event for autoimmune hepatitis. Semin
Liver Dis. 29:331–334. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Muratori P, Serio I, Lalanne C and Lenzi
M: Development of autoimmune hepatitis after influenza vaccination;
trigger or killer? Clin Res Hepatol Gastroenterol. 43:e95–e96.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sasaki T, Suzuki Y, Ishida K, Kakisaka K,
Abe H, Sugai T and Takikawa Y: Autoimmune hepatitis following
influenza virus vaccination: Two case reports. Medicine
(Baltimore). 97(e11621)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Berry PA and Smith-Laing G: Hepatitis A
vaccine associated with autoimmune hepatitis. World J
Gastroenterol. 13:2238–2239. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
van Gemeren MA, van Wijngaarden P, Doukas
M and de Man RA: Vaccine-related autoimmune hepatitis: The same
disease as idiopathic autoimmune hepatitis? Two clinical reports
and review. Scand J Gastroenterol. 52:18–22. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Csepregi A, Treiber G, Röcken C and
Malfertheiner P: Acute exacerbation of autoimmune hepatitis induced
by Twinrix. World J Gastroenterol. 11:4114–4116. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lohse AW, Sebode M, Bhathal PS, Clouston
AD, Dienes HP, Jain D, Gouw ASH, Guindi M, Kakar S, Kleiner DE, et
al: Consensus recommendations for histological criteria of
autoimmune hepatitis from the International AIH Pathology Group:
Results of a workshop on AIH histology hosted by the European
Reference Network on Hepatological Diseases and the European
Society of Pathology: Results of a workshop on AIH histology hosted
by the European Reference Network on Hepatological Diseases and the
European Society of Pathology. Liver Int. 42:1058–1069.
2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hennes EM, Zeniya M, Czaja AJ, Parés A,
Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer
H, et al: Simplified criteria for the diagnosis of autoimmune
hepatitis. Hepatology. 48:169–176. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
LAlvarez F, Berg PA, Bianchi FB, Bianchi
L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ,
Desmet VJ, et al: International Autoimmune Hepatitis Group Report:
Review of criteria for diagnosis of autoimmune hepatitis. J
Hepatol. 31:929–938. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Czaja AJ: Performance parameters of the
diagnostic scoring systems for autoimmune hepatitis. Hepatology.
48:1540–1548. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Boettler T, Csernalabics B, Salié H,
Luxenburger H, Wischer L, Salimi Alizei E, Zoldan K, Krimmel L,
Bronsert P, Schwabenland M, et al: SARS-CoV-2 vaccination can
elicit a CD8 T-cell dominant hepatitis. J Hepatol. 77:653–659.
2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Grifoni A, Weiskopf D, Ramirez SI, Mateus
J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L,
Jadi RS, et al: Targets of T cell responses to SARS-CoV-2
coronavirus in humans with COVID-19 disease and unexposed
individuals. Cell. 181:1489–1501.e15. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sekine T, Perez-Potti A,
Rivera-Ballesteros O, Strålin K, Gorin JB, Olsson A,
Llewellyn-Lacey S, Kamal H, Bogdanovic G, Muschiol S, et al: Robust
T cell immunity in convalescent individuals with asymptomatic or
mild COVID-19. Cell. 183:158–168.e14. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sirbe C, Simu G, Szabo I, Grama A and Pop
TL: Pathogenesis of autoimmune hepatitis-cellular and molecular
mechanisms. Int J Mol Sci. 22(13578)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Béland K, Marceau G, Labardy A,
Bourbonnais S and Alvarez F: Depletion of B cells induces remission
of autoimmune hepatitis in mice through reduced antigen
presentation and help to T cells. Hepatology. 62:1511–1523.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
John K, Hardtke-Wolenski M, Jaeckel E,
Manns MP, Schulze-Osthoff K and Bantel H: Increased apoptosis of
regulatory T cells in patients with active autoimmune hepatitis.
Cell Death Dis. 8(3219)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wack S, Patton T and Ferris LK: COVID-19
vaccine safety and efficacy in patients with immune-mediated
inflammatory disease: Review of available evidence. J Am Acad
Dermatol. 85:1274–1284. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pacheco Y, Acosta-Ampudia Y, Monsalve DM,
Chang C, Gershwin ME and Anaya JM: Bystander activation and
autoimmunity. J Autoimmun. 103(102301)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Salemi S and D'Amelio R: Could
autoimmunity be induced by vaccination? Int Rev Immunol.
29:247–269. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ichiki Y, Aoki CA, Bowlus CL, Shimoda S,
Ishibashi H and Gershwin ME: T cell immunity in autoimmune
hepatitis. Autoimmun Rev. 4:315–321. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tanaka A, Iwabuchi S, Takatori M, Ohno A,
Yamada H, Hashimoto N, Ikeda Y, Kato T, Nishioka K, Iino S and
Yamamoto K: Clonotypic analysis of T cells in patients with
autoimmune and viral hepatitis. Hepatology. 25:1070–1076.
1997.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vojdani A and Kharrazian D: Potential
antigenic cross-reactivity between SARS-CoV-2 and human tissue with
a possible link to an increase in autoimmune diseases. Clin
Immunol. 217(108480)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Uzun S, Zinner CP, Beenen AC, Alborelli I,
Bartoszek EM, Yeung J, Calgua B, Reinscheid M, Bronsert P, Stalder
AK, et al: Morphologic and molecular analysis of liver injury after
SARS-CoV-2 vaccination reveals distinct characteristics. J Hepatol.
79:666–676. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Codoni G, Kirchner T, Engel B, Villamil
AM, Efe C, Stättermayer AF, Weltzsch JP, Sebode M, Bernsmeier C,
Lleo A, et al: Histological and serological features of acute liver
injury after SARS-CoV-2 vaccination. JHEP Rep.
5(100605)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Efe C, Kulkarni AV, Terziroli
Beretta-Piccoli B, Magro B, Stättermayer A, Cengiz M, Clayton-Chubb
D, Lammert C, Bernsmeier C, Gül Ö, et al: Liver injury after
SARS-CoV-2 vaccination: Features of immune-mediated hepatitis, role
of corticosteroid therapy and outcome. Hepatology. 76:1576–1586.
2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Manns MP, Czaja AJ, Gorham JD, Krawitt EL,
Mieli-Vergani G, Vergani D and Vierling JM: American Association
for the Study of Liver Diseases. Diagnosis and management of
autoimmune hepatitis. Hepatology. 51:2193–2213. 2010.PubMed/NCBI View Article : Google Scholar
|