1. Introduction
Intracerebral hemorrhage and
subarachnoid hemorrhage
Intracerebral hemorrhage (ICH) is a deleterious form
of stroke that results from cerebral vascular rupture and
hemorrhage. It commonly occurs in the deep basal ganglia region of
the brain and is associated with a high incidence and mortality
rate (1). This subtype of
hemorrhagic stroke comprises 10-20% of all documented stroke cases
worldwide, based on statistical data from 1970 to 2008(2). A systematic review and meta-analysis
published in 2010 showed that the overall incidence of ICH was 24.6
per 100,000 person-years; among them, incidence of ICH per 100,000
person-years was 51.8 in East and Southeast Asian individuals,
which was much higher compared with that in white individuals in
Oceania, Europe and northern Manhattan (24.2), black individuals in
Africa, Caribbean and northern Manhattan (22.9) and Hispanic
individuals in Brazil, northern Manhattan, Caribbean and northern
Chile (19.6); moreover, the incidence rate of ICH was higher in the
elderly, with an incidence per 100,000 person-years of ICH in
individuals >55 years old (36.5-196.0), which was much higher
compared with that in individuals aged ≤54 (1.9-19.1) (3).
ICH is a devastating condition with a reported
mortality of ~40% within the first month (3). Despite the temporary lack of
comprehensive epidemiological data on ICH of the last five years,
it is undeniable that, having being recognized as one of the most
challenging conditions for the prognosis of patients with stroke
(4), ICH is likely to remain a
serious public health problem that cannot be ignored in the near
future, even after the progress in medicine in the past few years.
There are two types of damage caused by ICH: Primary injury and
secondary injury (5). In ICH, the
primary injury occurs during the initial stage. It is characterized
by physical damage to the brain cell structure caused by the
hematoma (6). The increased
intracranial pressure from the hematoma mass leads to compression
of the brain region, causing mechanical damage (7). Following this, a series of reactions
occur, including neuroinflammation and oxidative stress (OS),
leading to further damage such as blood-brain barrier (BBB)
dysfunction and cerebral edema. This is referred to as a secondary
injury (4). They both seriously
harm the health of patients with ICH.
Subarachnoid hemorrhage (SAH) has also attracted
considerable interest in the study of hemorrhagic stroke in recent
years. It is a type of neurological emergency that arises from the
rupture of diseased blood vessels found either at the brain's
surface or at the base of the brain. The blood flows directly into
the subarachnoid space (8),
resulting in the third most prevalent subtype of stroke (9) mainly attributed to aneurysm rupture
(10). It may have severe
consequences for patients, such as cognitive decline and secondary
cerebral ischemia (11). It occurs
at a relatively young age, despite comprising only 5% of all
strokes (12). Unlike ICH, SAH
starts very quickly, and symptoms can peak in a few minutes, with a
thunderclap headache which can peak within just 1 minute after SAH
(13). The complex
pathophysiological cascade that occurs after SAH is primarily
triggered by increased intracranial pressure (ICP) and blood
components (14). These factors
can lead to unpredictable diffuse brain damage, which is
challenging to detect until it reaches an irreversible stage
(15).
Two areas in the pathophysiology of SAH have
attracted significant attention in scientific research: Early brain
injury (EBI) and cerebral vasospasm (CVS) (16). Among them, EBI after SAH refers to
secondary brain injury within 72 h after SAH, including
microcirculation dysfunction, neuroinflammation damage, BBB
disruption, cerebral edema, neuronal death and oxidative cascade
(17). The EBI after SAH is
associated with several pathogenic mechanisms, including
inflammation, OS, and ferroptosis (18,19).
Moreover, CVS is a significant contributor to the high mortality
and morbidity associated with SAH (20). CVS usually occurs on day 3 after
SAH, peaks on days 6 and 8, lasts 2 to 3 weeks (17) and has lasting and serious
consequences after SAH. After a review of the relevant literature,
it was observed that the EBI and CVS after SAH have similar
mediating pathways, including OS and inflammation. This suggests
that flavonoid compounds have the potential to protect against a
number of significant injury modes in SAH.
Flavonoids
Flavonoids, which have 2-phenylchromone as their
fundamental structural unit, are among the most abundant secondary
metabolites found in plants. They can be found in herbs and
different dietary sources, including fruits, vegetables, teas and
grains, in glycosides or a number of free forms (21). Flavonoid compounds consist of a
shared C6-C3-C6 backbone where two aromatic rings are linked by
3-carbon bridges, typically forming a phenyltropane configuration
(22). Several studies have shown
that natural flavonoid compounds have neuroprotective effects in
brain diseases (23,24), suggesting that understanding the
functions and mechanisms of flavonoids in preventing
neuroinflammation may hold immense significance in the advancement
of nutritional guidelines and therapeutic approaches for brain
diseases.
2. Protective effects of flavonoid compounds
against ICH
Flavonoids protect against brain
damage after ICH by inhibiting inflammation and OS
The potential of flavonoids to protect against ICH
through their superior anti-inflammatory and antioxidant properties
has been the main focus of recent studies.
Puerarin is a natural isoflavone extracted from the
roots of pueraria species. A previous study has documented the
protective effects of puerarin against various diseases such as
Alzheimer's disease and cerebral ischemic disease (23). The PI3K/Akt signaling pathway is
involved in regulating the NF-κB pathway. The activation of
PI3K/Akt signaling has been shown to effectively decrease the
levels of phosphorylated NF-κB p65 and to inhibit the production of
inflammatory cytokines such as TNF-α and IL-1β. Zeng et al
(24) showed that puerarin can
activate PI3K/Akt signaling, thereby inhibiting inflammatory
reactions caused by the NF-κB pathway and ultimately attenuating
the EBI after ICH. Specifically, puerarin activates the PI3K/Akt
pathway and reduces NF-κB activation, significantly increasing the
expression level of Bcl-2 and inhibiting the expression of Bax and
Caspase-3, thereby inhibiting ICH-induced activation of apoptosis
signaling (24). At the same time,
the inflammatory factors that are promoted by NF-κB are
downregulated, reducing the inflammatory damage after ICH. In
addition, puerarin also reduces the levels of 8-OHdG and 3-NT after
ICH, inhibiting the production of reactive oxygen species (ROS) and
thus alleviating OS damage after ICH (24).
In addition, a previous study has revealed that
luteolin, which is classified as a flavonoid compound and has
anti-inflammatory properties, has been found to stimulate the Nrf2
pathway and facilitate the nuclear translocation of Nrf2 after ICH
(25). The study has shown that
luteolin can prevent ICH by stimulating the p62/Keap1/Nrf2 pathway
and substantially increasing the expression levels of its
downstream antioxidant proteins heme oxygenase 1 (HO-1) and
NQO1(26). NQO1 regulates ROS
production to inhibit oxidative stress (5), while increased expression of HO-1 can
inhibit the activation of NF-κB, thereby inhibiting NF-κB-mediated
inflammatory responses (27).
Further, luteolin inhibits the activation of the TLR4/TRAF6/NF-κB
signaling pathway by binding to TRAF6. This mechanism of action
reduces neuroinflammation, thereby providing a protective effect
against ICH by attenuating the overproduction of inflammatory
cytokines, the inflammatory cascade, apoptosis and structural and
functional disruption of BBB may result (28).
Baicalein is a bioactive flavonoid with
anti-inflammatory and antitumor activities (29). Based on a report, ICH rats treated
with baicalein showed significant reductions in lesion volume and
brain water content. On the one hand, its action was manifested as
decreasing the expression levels of pro-inflammatory cytokines
(IL-1β, IL-4, IL-6 and TNF-α), and inhibiting apoptosis. On the
other hand, it also increased the activities of superoxide
dismutase (SOD) and glutathione peroxidase (GSH-Px), while
decreasing the malondialdehyde (MDA) level in the brain tissues of
rats (30). MDA is an important
marker in oxidative stress, and the enhanced activity of SOD and
GSH-Px can strongly inhibit the production of MDA by lipid
peroxidation to improve oxidative damage after ICH (31). The results of the research
indicated that baicalein may have a therapeutic impact on brain
injury by reducing apoptosis, OS and neuroinflammation. This
suggests that baicalein could be a promising treatment option for
ICH and related brain injury (30). Another study on ICH supports that
baicalein may reduce brain damage by inhibiting ROS and the NLRP3
inflammasome, thus inhibiting NLRP3-mediated inflammatory
responses. This research further highlights the potential of
baicalein to protect against ICH (32).
Moreover, didymin, a dietary citrus flavonoid, was
shown to upregulate RAF kinase inhibitor protein (RKIP) expression
in a preclinical model of ICH (33). The research has shown that RKIP can
directly bind ASC, thereby disrupting the formation of the NLRP3
inflammasome after ICH (33). In
this way, didymin blocks cell pyroptosis and the inflammatory
response in the Caspase-1/GSDMD pathway, protecting against injury
after ICH (33).
Quercetin, a special subclass of flavonoids, which
is a bioactive natural compound established on the structure of
flavonoids (34). Based on a
recent study, the efficacy of quercetin in a non-clinical model of
ICH has been demonstrated. The study reviewed that, the lesion
volume and brain water content are significantly reduced in ICH
rats after quercetin treatment. These outcomes provided evidence
that quercetin has the potential to suppress inflammation and cell
death, and is capable of aiding in the restoration of neural
function by reducing levels of inflammatory mediators and
inhibiting apoptosis mediated by cleaved Caspase-3(35).
Baicalin is an active ingredient of the traditional
Chinese drug baicalensis that shows biological activity, including
anti-inflammatory properties (36,37).
A study found that baicalin displays a dose-dependent suppression
of NF-κB expression in the surrounding tissues of the hematoma
caused by ICH, and thereby reduces the secretion of IL-1β and IL-6.
Additionally, baicalin inhibits the expression of matrix
metalloproteinases (MMP)-9, and blocks the degradation of the
extracellular matrix (ECM) by MMP-9, thereby helping maintain the
integrity of the BBB. It was also suggests that baicalin may have a
protective effect against ICH by regulating the expression of
protease-activating receptor-1 (PAR-1) to inhibit the cellular
apoptosis pathway mediated by PAR-1(38).
A number of other potential flavonoid compounds have
demonstrated a protective effect against the OS and inflammation
mediated by ICH; for example, a study has highlighted that
isoliquiritigenin can inhibit ROS and the activation of the
NF-κB-mediated NLRP3 inflammasome through the stimulation of the
Nrf2 antioxidant pathway, which in turn alleviates EBI after
experimental ICH (39).
Breviscapine, a medicinal plant, reportedly possesses a substantial
inhibitory effect on the expression of NF-κB pathway-related
factors after ICH (40). Another
study suggests that pinocembrin lowers the expression of TLR4,
myeloid differentiation primary response 88 (MyD88) and
TIR-domain-containing adapter-inducing interferon-β and
downregulates NF-κB signaling, alleviating brain injury after ICH
by inhibiting the inflammatory response (41). Except for pinocembrin, eupatilin
has also been confirmed to reduce the inflammatory response
triggered by ICH through the TLR4/MyD88 pathway (42). In addition to the aforementioned
studies, fisetin, naringin, calycosin and procyanidins have also
been demonstrated to protect against the neuroinflammation and/or
OS resulting from ICH via various mechanisms, such as inhibiting
NF-κB, inhibiting lipid peroxidation and activating the Nrf2
pathway (43-46).
Flavonoids protect ICH by facilitating
TGF-β1
The aforementioned materials demonstrate that
flavonoids have a wide range of potential as antioxidants and/or
anti-inflammatory agents for the prevention and treatment of ICH.
However, aside from neuroinflammation and OS, a number of
researchers have also identified alternative pathways through which
flavonoids can protect ICH. For example, hesperidin, a biologically
active flavonoid that can be found in citrus fruits (47), has been highlighted to promote the
expression of TGF-β1, while TGF-β1 can promote the production and
reconstitution of ECM and protects the BBB by inhibiting the
expression of MMP9 and MMP2, to attenuate the damage caused by
adverse symptomatic ICH caused by ischemic stroke (48). At present, there are few studies on
the protective effect of flavonoid compounds on ICH, which have
highlighted the role of TGF-β1; however, the present study provides
a reference for future research directions.
Based on the aforementioned studies it is evident
that flavonoids have the potential to provide significant
protection against ICH through various mechanisms, such as
anti-inflammatory, anti-oxidative and anti-pyroptotic actions
(Fig. 1; Table I). These findings suggest that
flavonoids may be promising candidates for the development of
preventive and therapeutic drugs.
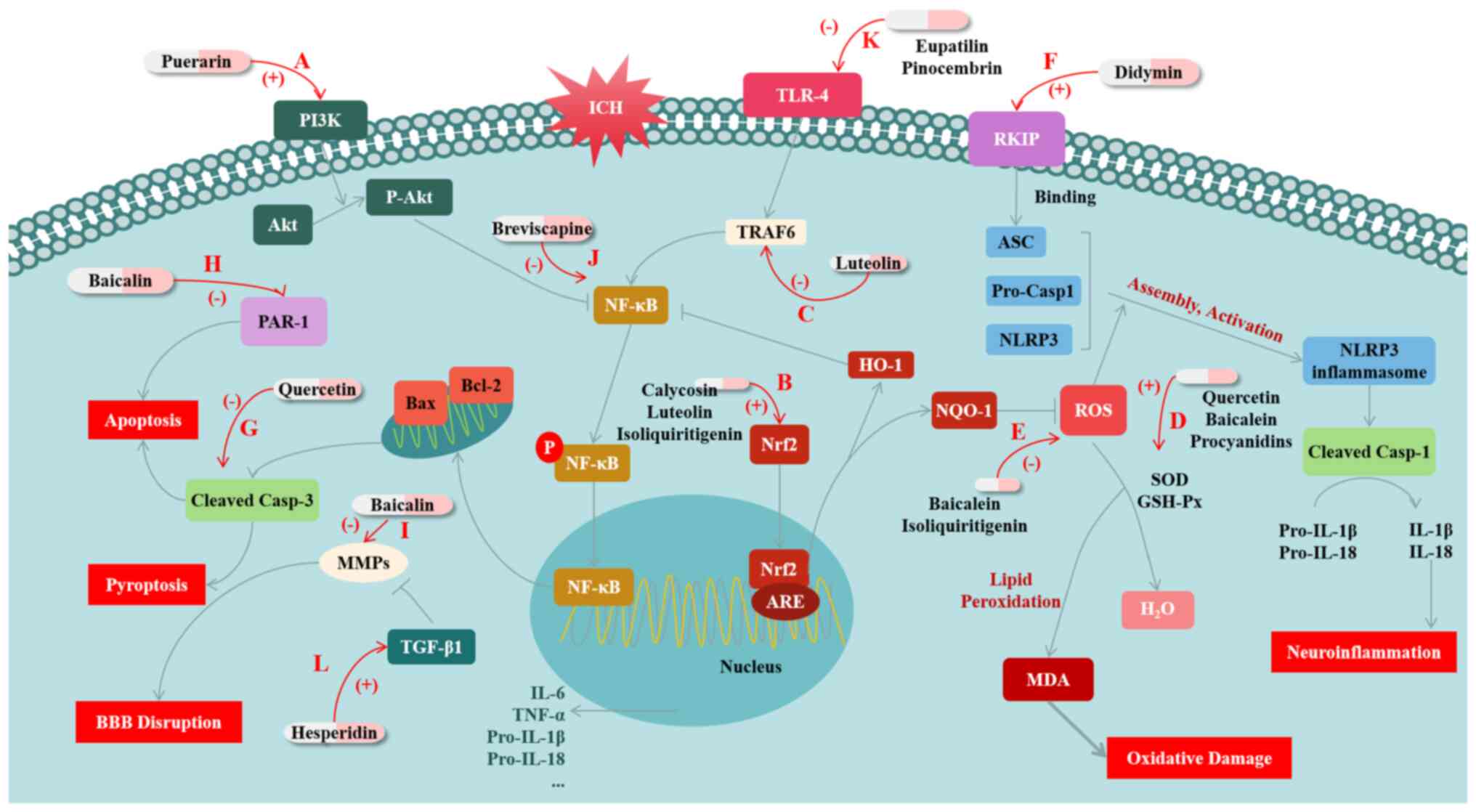 | Figure 1Mechanisms of flavonoid compounds
exerting protective effects against brain injury after ICH. (A)
Puerarin activates the PI3K/Akt pathway and reduces NF-κB
activation, increases the expression level of Bcl-2 and inhibits
the expression of Bax and Caspase-3, thereby inhibiting ICH-induced
apoptosis. (B) Calycosin, luteolin and isoliquiritigenin protects
ICH by modulating Nrf2. They both activate Nrf2 and promote its
nuclear translocation, thereby inhibiting OS. In addition,
calycosin and isoliquiritigenin further inhibit NF-κB and/or
NLRP3-mediated inflammation through promoting Nrf2. (C) Luteolin
inhibits the activation of the TLR4/TRAF6/NF-κB signaling pathway
by binding to TRAF6, thus reducing inflammation. (D) Quercetin,
baicalein and procyanidins increase the activities of SOD and
GSH-Px, while decreasing the MDA level, and thus improves oxidative
damage after ICH. (E) Baicalein and isoliquiritigenin inhibit
NLRP3-mediated inflammation by inhibiting ROS production. (F)
Didymin upregulates the expression of RKIP; RKIP combines with Asc,
thus inhibiting the assembly of NLRP3 inflammasomes, to inhibit
NLRP3-mediated inflammatory response. (G) Quercetin inhibits
apoptosis mediated by cleaved Caspase-3. (H) Baicalin downregulates
the expression of PAR-1 to inhibit cellular apoptosis. (I) Baicalin
inhibits the expression of MMPs to ameliorate the disruption of
BBB. (J) Breviscapine attenuates the inflammatory response by
inhibiting the activation of NF-κB. (K) Eupatilin and pinocembrin
lower the expression of TLR4 and downregulate NF-κB signaling to
inhibit the inflammatory response. (L) Hesperidin promotes the
expression of TGF-β1 to inhibit expression of MMPs, thus protecting
the BBB from disruption. ICH, intracerebral hemorrhage; OS,
oxidative stress; SOD, superoxide dismutase; GSH-Px, glutathione
peroxidase; MDA, malondialdehyde; ROS, reactive oxygen species;
RKIP, RAF kinase inhibitor protein; PAR-1, protease-activating
receptor-1; MMP, matrix metalloproteinases; BBB, blood-brain
barrier. |
 | Table IFlavonoids with protective effects on
ICH and their mechanisms of action. |
Table I
Flavonoids with protective effects on
ICH and their mechanisms of action.
| Compound | Protective
mechanism | Indexes | Pathways/targets of
action |
|---|
| Puerarin | Inhibiting OS and
inflammation | PI3K↑, Akt↑,
NF-κB↓, Bcl/Bax↑, 3-NT↓, 8-OHdG↓, ROS↓ | PI3K/Akt/NF-κB
pathway |
| Luteolin | Inhibiting OS and
inflammation | Nrf2↑, HO-1↑,
NQO1↑, TLR-4↓, TRAF6↓, NF-κB↓ | p62/Keap1/Nrf2;
TLR4/ TRAF6/NF-κB pathway |
| Baicalein | Inhibiting OS and
inflammation | SOD↑, GSH-Px↑,
MDA↓, ROS↓, NLRP3↓ | ROS/NLRP3 pathway
and lipid peroxidation pathway |
| Didymin | Inhibiting
pyroptosis and inflammation | RKIP↑, NLRP3↓,
GSDMD↓ |
Asc/NLRP3/Caspase-1/ GSDMD pathway |
| Quercetin | Inhibiting cell
apoptosis and inflammation | Cleaved
Caspase-3↓ | Caspase-3-mediated
apoptosis pathway |
| Baicalin | Inhibiting cell
apoptosis | PAR-1↓ | PAR-1-mediated
apoptosis pathway |
|
Isoliquiritigenin | Inhibiting OS and
inflammation | Nrf2↑, ROS↓,
NF-κB↓, NLRP3↓ | Nrf2/ROS, NF-κB,
NLRP3 pathway |
| Breviscapine | Inhibiting
inflammation | NF-κB↓, IL-6↓,
TNF-α↓ | NF-κB pathway |
| Pinocembrin | Inhibiting
inflammation | TLR4↓, TRIF↓,
MyD88↓, NF-κB↓ | TLR4/NF-κB
pathway |
| Eupatilin | Inhibiting
inflammation | TLR4↓, MyD88↓ | TLR4/NF-κB
pathway |
| Fisetin | Inhibiting
inflammation | NF-κB↓ | NF-κB pathway |
| Calycosin | Inhibiting OS and
inflammation | Nrf2↑, NLRP3↓,
NF-κB↓ | NACHT, NLRP3,
NF-κB |
| Hesperidin | Assist in rt-PA
treatment | TGF-β1↑, MMP-2↓,
MMP-9↓ | TGF-β1 |
| Procyanidins | Inhibiting OS | LDH↓, SOD↑,
MDA↓ | Lipid peroxidation
pathway |
3. Protective effects of flavonoid compounds
against SAH
Flavonoids protect SAH by inhibiting
inflammation, OS and apoptosis
Neuroinflammation and OS are significant factors in
the EBI and CVS that occur after SAH (49-51).
A number of studies have demonstrated that flavonoids can exert
protective effects on SAH by suppressing inflammation and OS. For
example, the study by Kuo et al (52) has highlighted the positive effects
of early baicalein treatment on rats after SAH. This treatment has
been shown to decrease the mortality rate and brain water content
in experimental rats, as well as reduce neuronal degeneration by
inhibiting CVS (52). In addition,
baicalein increases astrocyte activity and retains glutamate
transporter-1, thus attenuating OS induced by the glutamate surge
after SAH; baicalein also provided resistance against OS by
maintaining SOD and catalase activity and reducing MDA levels after
SAH (52). Several studies have
also emphasized the functions of flavonoid compounds in the similar
aspects in SAH, such as luteolin, rutin, apigenin, baicalein,
quercetin and proanthocyanidin, which have been shown to have
inhibitory effects on inflammation and/or OS after SAH (53-60).
Their mechanisms of action include several pathways as described
above, such as modulating Nrf2 to inhibit oxidative stress and
NLRP3 inflammasome-mediated inflammation, reducing NF-κB activation
by inhibiting TLR4 or RAGE, and inhibiting of MDA by increasing SOD
and GSH-Px to alleviate oxidative damage (53-60).
Simultaneously, the damage caused by apoptosis after
SAH is equally remarkable. Apoptosis is a significant factor in the
development of EBI and is also involved in the formation of CVS in
patients with SAH. According to the study of Zhang et al
(61), puerarin ameliorates the
neurological impairment observed in mice, suppresses cerebral
edema, decreases BBB destruction and also decreases the apoptosis
of cellular neurons. In addition, Zhang et al (61) has shown that puerarin-treated SAH
mice have a significantly higher Blc-2/Bax ratio and a reduced
level of cleaved Caspase-3 compared with vehicle-treated SAH
animals; that is to say, puerarin inhibits apoptosis mediated by
cleaved Caspase-3 by inhibiting Bax expression and promoting Blc-2
expression. Meanwhile, puerarin also blocks SAH-induced ROS
production, protects the synthesis of Sirt3 after SAH and enhances
the function of SOD2 after SAH, thus attenuating oxidative damage
after SAH. Overall, the study suggests that puerarin has the
potential to reduce neurological dysfunction in mice with SAH by
targeting specific pathways involved in apoptosis, including
Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2(61).
Flavonoids protect against brain
damage after SAH by activating SIRT1
In addition to the aforementioned pathways, a number
of other flavonoids have been shown to protect against brain damage
after SAH through other routes.
Pinocembrin is a natural compound distributed in
propolis. In a study, Zeng et al (62) found that pinocembrin has a
significant impact on improving behavior and reducing brain tissue
damage after SAH, and that its mechanism of action might be related
to Sirtuin-1(62). Sirtuin-1, also
known as SIRT-1, is a histone deacetylase that can be found in
different parts of the cerebral cortex. Accumulating preclinical
evidence has indicated that SIRT1 is a promising molecular
candidate for treating SAH. By deacetylating a variety of
intracellular targets such as Pgc-1α and ac-NF-κB, SIRT1 provides
protection by reducing inflammatory injury, free radical damage and
cell death (62). Specifically,
inhibition of ac-NF-κB can inhibit its downstream inflammatory
pathway, while activation of Pgc-1α can promote the entry of Pgc-1α
into the nucleus to promote the expression of antioxidant enzymes,
such as SOD, to reduce intracellular ROS levels, thereby protecting
normal mitochondrial function and protecting cells from oxidative
damage (63). In the study by Zeng
et al (62), treatment with
pinocembrin significantly increases the levels of SIRT1 and Pgc-1α,
and suppresses the expression of ac-NF-κB; therefore, treatment
with pinocembrin plays a protective role against brain injury after
SAH (62). Although SIRT1 was
shown in this aforementioned study to exert a protective effect on
SAH by inhibiting inflammation and OS, as a potential target for
the treatment of SAH, SIRT1 is less studied in the direction of the
protective effects of flavonoids on SAH. Overall, the potential of
flavonoids to play a role in the treatment of SAH through SIRT1
still needs to be further explored.
Flavonoids protect against brain
injury after SAH by promoting the endothelial nitric oxide (NO)
pathway
Concerning the pathophysiology of CVS, the
endothelial NO pathway is also regarded as one of the primary
mechanisms. The production of NO by endothelial nitric oxide
synthase (eNOS) in the cerebrovascular endothelium can spread to
nearby smooth muscle cells, triggering the activation of soluble
guanylyl cyclase. This, in turn, results in the production of
cyclic guanosine monophosphate (cGMP). The stimulation of
intracellular calcium channels by cGMP facilitates the transport of
free Ca2+ to the intracellular zone compartment,
resulting in the relaxation of smooth muscle cells, and eventually
inhibits CVS (16). Li et
al (64) demonstrated that
scutellarin can reduce vasospasm and neurological deficits by
regulating the Erk5-KLF2-eNOS pathway, confirming the protective
effect of scutellarin through the endothelial NO pathway on CVS
after SAH (64).
Flavonoids protects against brain
injury after SAH by inhibiting ferroptosis
Ferroptosis is a type of programmed cell death
characterized by iron-dependent lipid peroxidation. Ferroptosis can
trigger some inflammatory mediators, such as TNF-α, IL-1β and IL-6,
which can contribute to inflammation by promoting the aggregation
and activation of inflammatory cells (65,66).
Ferroptosis can also enhance the accumulation of ROS within cells,
thereby intensifying the OS damage (67,68).
Recently, ferroptosis has been reported in the pathological course
of hemorrhagic stroke, including SAH (69). One study has indicated that
baicalin may be effective in reducing the damage caused by SAH by
preventing ferroptosis. The research highlighted that baicalin
attenuates SAH-induced elevation of Fe2+ levels and
production of OS markers MDA and ROS in rat brain tissue, and
eliminates SAH-induced reduction of GSH levels. In addition to
validating Baicalin's inhibition of lipid peroxidation, the study
also revealed that baicalin maintains the expression level of
glutathione peroxidase 4 (GPX4) protein, which can reduce
phospholipid hydroperoxide and inhibit lipoxygenase-mediated lipid
peroxidation, thereby exerting a protective effect against
ferroptosis. In addition, baicalin also inhibits the protein level
of beclin1 and the ratio of LC3-II/I in the brain tissues of SAH
rats, suggesting the suppressive effect of baicalin on autophagy in
SAH rats. This study demonstrates the ability of baicalin to
attenuate brain damage following SAH by inhibiting
autophagy-dependent ferroptosis (70).
In conclusion, flavonoids regulate and improve brain
damage after SAH in various ways. These are a class of drugs with
potential for the treatment of SAH (Fig. 2; Table II). However, further research is
still required to elucidate their mode of action.
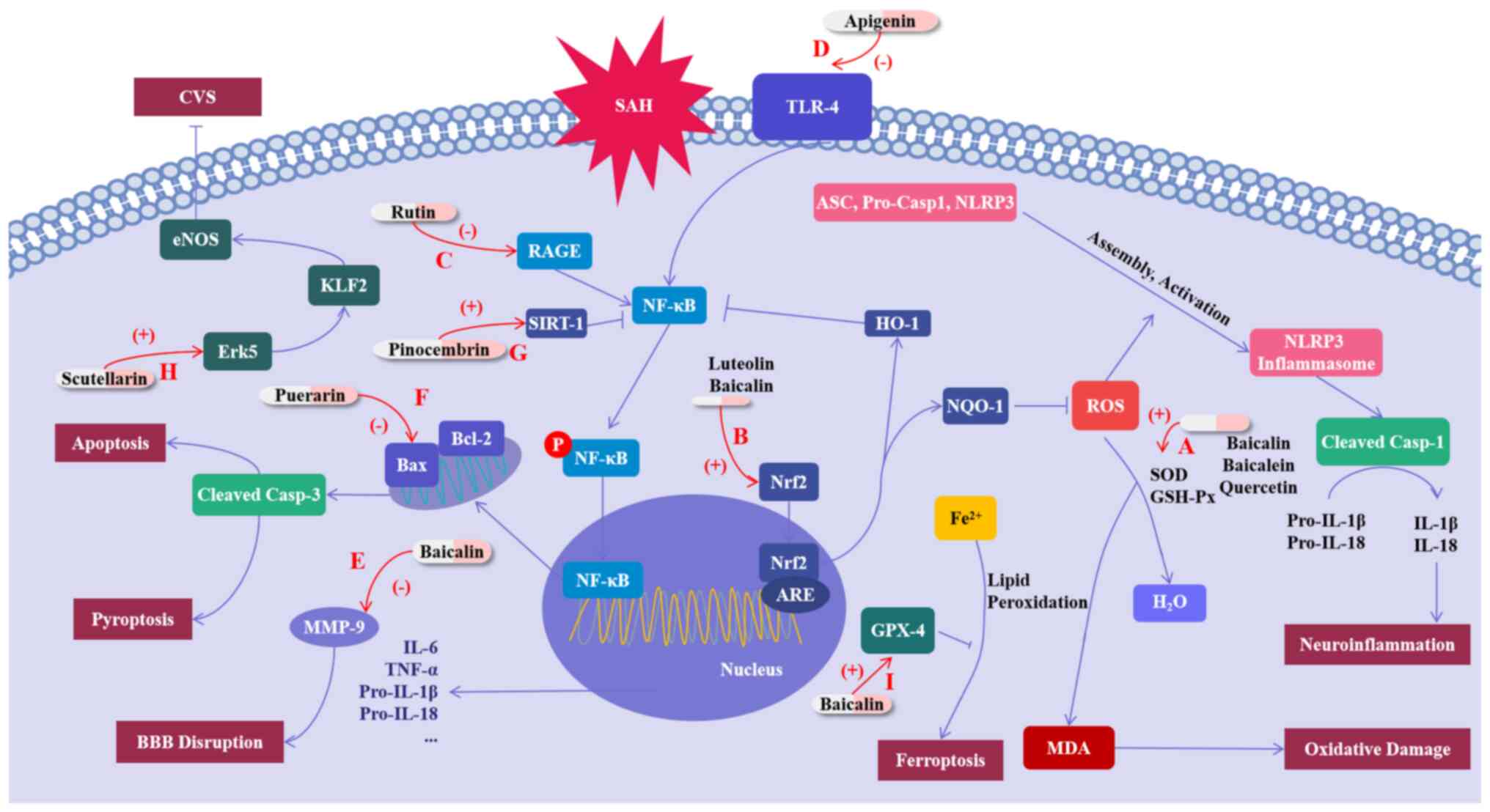 | Figure 2Mechanisms of flavonoid compounds
exerting protective effects on SAH. (A) Baicalin, baicalein and
quercetin increase the activities of SOD and GSH-Px, while
decreasing the MDA level, thus inhibiting oxidative damage. (B)
Luteolin and baicalin inhibit oxidative damage by activating Nrf2,
and luteolin also inhibits the NLRP3-mediated inflammatory response
through regulating Nrf2. (C) Rutin inhibits the inflammatory
response by inhibiting the RAGE-NF-κB pathway. (D) Apigenin
inhibits the TLR4-induced inflammatory response. (E) Baicalin
prevents t blood-brain barrier disruption by inhibiting the
expression of MMP-9. (F) Puerarin attenuates apoptosis by
regulating the Bcl-2/Bax/cleaved Caspase-3 pathway. (G) Pinocembrin
inhibits the inflammation mediated by NF-κB by promoting SIRT-1.
(H) Scutellarin promotes the Erk5-KLF2-eNOS pathway, thus reducing
CVS by promoting smooth muscle cell relaxation. (I) Baicalin
maintains the expression levels of GPX4 protein and inhibits the
ferroptosis after SAH. SAH, subarachnoid hemorrhage; OS, oxidative
stress; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase;
MDA, malondialdehyde; ROS, reactive oxygen species; RKIP, RAF
kinase inhibitor protein; CVS, cerebral vasospasm; MMP, matrix
metalloproteinases; BBB, blood-brain barrier. |
 | Table IIFlavonoids with protective effects on
SAH and their mechanisms of action. |
Table II
Flavonoids with protective effects on
SAH and their mechanisms of action.
| Compound | Protective
mechanism | Improved
damage | Indexes | Pathways/targets of
action |
|---|
| Baicalein | Inhibiting OS | CVS | GLT-1↑, SOD↑,
MDA↓ | Lipid peroxidation
pathway |
| Luteolin | Inhibiting OS and
inflammation | CVS | Nrf2↑, NLRP3↓ | Nrf2/NLRP3
pathway |
| Rutin | Inhibiting
inflammation | EBI | RAGE↓, NF-κB↓ | RAGE-NF-κB
pathway |
| Apigenin | Inhibiting
inflammation | EBI | TLR4↓, NF-κB↓ | TLR4-NF-κB
pathway |
| Baicalin | Inhibiting OS and
ferroptosis | EBI | SOD↑, MDA↓, Nrf2↑,
MMP9↓, Fe2+↓, ROS↓ | Nrf2 pathway, lipid
peroxidation pathway, and ferroptosis pathway |
| Quercetin | Inhibiting OS and
apoptosis | CVS | MDA↓, CuZn- SOD↑,
GSH-Px↑, MDA↓, Cleaved Caspase-3↓ | Lipid peroxidation
pathway, Caspase-3-mediated apoptosis pathway |
| Puerarin | Inhibiting OS and
apoptosis | EBI | Blc-2/Bax↑, cleaved
Caspase-3↓, ROS↓, SIRT3↑, SOD2↑ |
Bcl-2/Bax/caspase-3; Sirt3/SOD2
pathway |
| Pinocembrin | Inhibiting OS and
inflammation | EBI | SIRT1↑, Pgc-1α↑,
ac-NF-κB↓, SOD↑, GSH↑, MDA↓, IL-1β↓, IL-6↓ | SIRT1 pathway and
lipid peroxidation pathway |
| Scutellarin | Promoting
endothelial NO pathway | CVS | p-Erk5↑, KLF2↑,
eNOS↑ | Erk5/KLF2/eNOS
pathway |
4. Conclusion
ICH and SAH are harmful brain diseases that cause
severe damage to patients globally. Flavonoids, as potent
antioxidants and anti-inflammatory agents, have consistently
demonstrated their neuroprotective effects in preclinical studies
involving ICH and SAH. They have been shown to regulate
inflammation, pyroptosis and OS pathways, which in turn involve,
for example, the NF-κB-related inflammatory pathways, Nrf2-related
antioxidant pathways, lipid peroxidation pathways and
caspase-3-mediated pyroptosis. Although their anti-inflammatory,
antioxidant and other effects have been demonstrated, flavonoid
compounds still need more research to investigate their potential,
and they are still constrained by a number of obstructions on the
way to the clinic, for example, the mechanism research is not
in-depth enough, and the potential toxic and side effects of
flavonoids need to be discovered and avoided.
More pathways, targets and more
specific mechanisms remain to be discovered and exploited
Other than the researches mentioned in the previous
chapters, it is important to highlight that some targets have
attracted less attention in the available research. Targets such as
TGF-β1, SIRT1, ERK1/2 and RAGE, and pathways such as ferroptosis
may potentially be affected by a number of flavonoids. Especially,
the activation of the ERK1/2 signaling pathway is implicated in the
pathological process of vascular wall proliferation in CVS
(71). It has been revealed that
cell proliferation in the vessel wall is a crucial factor in the
development of CVS in SAH (71),
and TLR4, downstream of ERK1/2, can exacerbate the inflammatory
response by promoting the secretion of inflammatory factors through
c-Fos phosphorylation (72). This,
in turn, increases the damage caused by SAH. Moreover, numerous
studies have confirmed the inhibitory effects of various flavonoid
compounds on ERK1/2-related pathways (73,74).
These findings also provide considerable reference value for
studies targeting SAH. These targets and pathways may serve as
potential sites of damage for different flavonoids, and their
research directions are both attractive and immensely valuable.
While studying the effects of flavonoids on new
pathways and targets, the studies of pathways and targets that have
been demonstrated to be affected by flavonoids should not be
stopped. While numerous studies have explored the mechanisms by
which flavonoids exert protective effects on ICH and SAH in the
studies cited in the present paper, the ways in which flavonoids
interact with the specific biomarkers they affect are rarely
mentioned. Future research is still necessary to explore this point
in depth; for example, to study whether flavonoids play a role as
activators or inhibitors of certain enzymes, and to study the
binding mode and binding sites of flavonoids to various targets, so
as to lay a more reliable theoretical basis for their clinical
treatment of ICH and SAH.
Flavonoids may also have negative
effects
In a number of studies flavonoid compounds have
played a role that can exert protective effects against a variety
of diseases. Inevitably, however, there are exceptions to
everything and the effects of flavonoid compounds on hemorrhagic
stroke are also complex and two-sided. In a certain study, the
present study noted that rotenone, a flavonoid compound, has been
shown to accelerate ferroptosis after ICH (75). It appears that this type of
compound may not be beneficial for treating ICH and SAH. This may
be due to the special structure of rotenone, which makes it an
inhibitor of mitochondrial respiratory chain complex I, which can
enhance the production of mitochondrial ROS (76). The increased production of ROS
aggravates lipid peroxidation, which ultimately promotes
ferroptosis. This also indicates that some flavonoid compounds may
not have a positive impact on hemorrhagic strokes. Upon closer
investigation, the present study revealed that there are a number
of flavonoid compounds with some potential toxic side effects,
including but not limited to: i) Baicalin can induce acetylation of
Smad3 through the interaction of Smad3 with the transcriptional
coactivator p300 and reduce phosphorylation of AMPK, a metabolic
master kinase, leading to increased kidney damage, including
glomerular hypertrophy, collagen deposition, inflammatory cell
infiltration among the renal tubules and even kidney fibrosis
(77); and ii) under the
conditions of high dose and long-term use, luteolin induces
glutathione depletion and activates the metabolism of CYP450,
mediating the formation of o-benzoquinone metabolites, thereby
causing cytotoxicity including damage to multiple structures and
functions in cells, and even cell apoptosis in primary rat
hepatocytes (78).
The potential toxicity of flavonoid compounds has
become a non-negligible obstacle to their clinical development. Not
only that, but the decision on the dosage and the risks involved in
the long-term use of the drug should not be ignored. A too-high
dosage or long-term use of a drug may have strong toxic side
effects and even a risk of death. Nowadays, there is still a lack
of more specific and comprehensive clinical studies on the efficacy
and toxicity of flavonoids at different doses and the risks of
long-term use of flavonoid drugs. Therefore, in future studies,
flavonoid compounds with specific structures, toxicity, functions,
dose-dependent toxicity and/or side effects and long-term
medication risks should be identified and screened and selectively
used. This shows a new opportunity for further research, while also
raising new challenges. At present, some studies have made attempts
to investigate this; for example, Choi et al (79) revealed that long-term combined
administration of quercetin and daidzein can inhibit
quercetin-induced suppression of glutathione antioxidant defenses
(79). Such studies undoubtedly
provide evidence to further study the safety of flavonoids and
propose treatment options those are safer and more effective.
Issues of bioavailability and
biological activities of flavonoid compounds
Another challenge for flavonoids moving towards
clinical practice is that the bioavailability of flavonoid
compounds varies (80). However,
new delivery systems may help different flavonoid compounds play a
fuller role in the prevention and treatment of diseases such as ICH
and SAH. As research reports, the application of quercetin in
nanoemulsion has been found to significantly boost its antioxidant
effect (81). This study indicated
that the biological activity and pharmacological effects of
flavonoids may vary depending on the dosage form of their
administration. This finding offers a strong basis and motivation
for further research into the potential of these compounds.
Presently, there is a continuous influx of new
dosage forms being identified. Several potent flavonoids may be
formulated into various dosage forms so that their protective and
toxic levels in each formulation can be determined. This could be
another interesting point of research. On the other hand,
derivatives are also important in enhancing the efficiency of
flavonoid compounds. A previous study has shown that the C- and
O-glycosides of flavonoids generally show higher radical scavenging
activity compared with aglycones; for example, kaempferol
C3-O-glycoside (astragalin) shows higher activity compared with
kaempferol (82). This study
reveals that making flavonoid compounds into different derivatives
may reveal unexpected effects. At the same time, in future
research, researchers should also pay attention to the selection of
patients. Patients with different constitutions may also show
considerable differences in drug compliance, which is also a
direction worthy of further study.
Considering the aforementioned research progress,
flavonoid compounds have shown significant bioactivity and
promising clinical potential in ICH and SAH. Nevertheless, the
current studies still have some limitations. Firstly, in the
studies mentioned in the present review, the experimental animal
breeds, animal models, measurement methods and measurement
indicators are uneven, making a systematic comparison of their
specific efficacy difficult. Therefore, for each compound, it is
necessary to compare the protective effects of flavonoids on ICH
and SAH in different experimental animal breeds and by different
ICH or SAH model construction methods. This not only helps to
compare the efficacy of different flavonoids, but also supports the
existing research results.
In addition, to the best of our knowledge, there is
a lack of large-scale clinical trials and extensive exploration of
mechanistic studies. The ‘from the bench to the bedside’ process
still contains more unknowns waiting to be explored. Despite this,
in our opinion, foods containing flavonoids may play an unexpected
role in preventing diseases such as ICH, SAH and other interrelated
diseases, and would be readily available as a daily dietary
supplement by the concept of ‘homology of medicine and food’ in
traditional Chinese medicine. In daily life, individuals can
selectively increase their intake of foods that contain beneficial
flavonoid compounds, in order to prevent diseases such as ICH and
SAH. For example, a study showed that individuals with higher daily
intake of flavonoids, such as anthocyanin (predominantly from
blueberries and strawberries), had a lower incidence of
hypertension (83). Hypertension
is an important cause of ICH (84), therefore, increasing the intake of
these flavonoids will also probably reduce the risk of ICH
accordingly. Flavonoid compounds are widely found in a variety of
fruits and vegetables, for example, celery and parsley contain
apigenin (85), apples contain
rutin (86) and strawberries and
blueberries contain anthocyanin (83), and advocating for an appropriate
increase in the intake of flavonoids containing those beneficial
flavonoids may be of considerable benefit in preventing ICH and SAH
and reducing their incidence.
A number of previous reviews have summarized studies
on flavonoids. Jäger and Saaby (87) discussed the effects of flavonoids
on the central nervous system, including oral bioavailability, BBB
permeability and the interaction of flavonoids with several
biomarkers. The review by Parrella et al (88) summarized the therapeutic effects of
polyphenols, including flavonoids, shown in non-clinical and
clinical studies of stroke, while the article by Chen et al
(21) summarized the beneficial
effects of natural flavonoids on neuroinflammation. However, to the
best of our knowledge, there is no review that specifically
summarizes the protective effects of flavonoids on ICH and SAH;
therefore, the present review summarized the protective effects of
flavonoids on ICH and SAH and the mechanisms by which they exert
protective effects in detail, which provides a reference for the
treatment of ICH and SAH by flavonoids. The current review
discussed the challenges of flavonoids towards clinical practice,
the limitations of current researches on the protective effects of
flavonoids in ICH and SAH and the beneficial directions for future
research on flavonoids in ICH and SAH. In our opinion, flavonoids
are a class of compounds with potential, and it is hypothesized
that their therapeutic potential for ICH and SAH can be more and
more fully explored in future studies.
Acknowledgements
Not applicable.
Funding
Funding: This work was partially supported by the National
Natural Science Foundation of China (grant no. 81973547).
Availability of data and materials
Not applicable.
Authors' contributions
HD designed and drafted the manuscript. HD, XG, HL
and JG revised the manuscript. LZ conceived and designed the whole
project. All authors read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Magid-Bernstein J, Girard R, Polster S,
Srinath A, Romanos S, Awad IA and Sansing LH: Cerebral hemorrhage:
Pathophysiology, treatment, and future directions. Circ Res.
130:1204–1229. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Feigin VL, Lawes CM, Bennett DA,
Barker-Collo SL and Parag V: Worldwide stroke incidence and early
case fatality reported in 56 population-based studies: A systematic
review. Lancet Neurol. 8:355–369. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
van Asch CJ, Luitse MJ, Rinkel GJ, van der
Tweel I, Algra A and Klijn CJ: Incidence, case fatality, and
functional outcome of intracerebral haemorrhage over time,
according to age, sex, and ethnic origin: A systematic review and
meta-analysis. Lancet Neurol. 9:167–176. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen Y, Chen S, Chang J, Wei J, Feng M and
Wang R: Perihematomal edema after intracerebral hemorrhage: An
update on pathogenesis, risk factors, and therapeutic advances.
Front Immunol. 12(740632)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xiao L, Zheng H, Li J, Wang Q and Sun H:
Neuroinflammation mediated by NLRP3 inflammasome after
intracerebral hemorrhage and potential therapeutic targets. Mol
Neurobiol. 57:5130–5149. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Keep RF, Hua Y and Xi G: Intracerebral
haemorrhage: Mechanisms of injury and therapeutic targets. Lancet
Neurol. 11:720–731. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wan Y, Holste KG, Hua Y, Keep RF and Xi G:
Brain edema formation and therapy after intracerebral hemorrhage.
Neurobiol Dis. 176(105948)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Muehlschlegel S: Subarachnoid hemorrhage.
Continuum (Minneap Minn). 24:1623–1657. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Claassen J and Park S: Spontaneous
subarachnoid haemorrhage. Lancet. 400:846–862. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sveinsson ÓÁ, Ólafsson IH, Kjartansson Ó
and Valdimarsson EM: Spontaneous subarachnoid haemorrhage-review.
Laeknabladid. 97:355–362. 2011.PubMed/NCBI View Article : Google Scholar : (In Icelandic).
|
|
11
|
Lucke-Wold B, Logsdon A, Manoranjan B,
Turner RC, McConnell E, Vates GE, Huber JD, Rosen CL and Simard JM:
Aneurysmal subarachnoid hemorrhage and neuroinflammation: A
comprehensive review. Int J Mol Sci. 17(497)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
van Gijn J, Kerr RS and Rinkel GJ:
Subarachnoid haemorrhage. Lancet. 369:306–318. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dilli E: Thunderclap Headache. Curr Neurol
Neurosci Rep. 14(437)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hao G, Conzen-Dilger C, Schmidt TP, Harder
E, Schöps M, Clauser JC, Schubert GA and Lindauer U: Effect of
isolated intracranial hypertension on cerebral perfusion within the
phase of primary disturbances after subarachnoid hemorrhage in
rats. Front Cell Neurosci. 17(1115385)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lynch DG, Shah KA, Powell K, Wadolowski S,
Tambo W, Strohl JJ, Unadkat P, Eidelberg D, Huerta PT and Li C:
Neurobehavioral impairments predict specific cerebral damage in rat
model of subarachnoid hemorrhage. Transl Stroke Res: Jul 26, 2023
(Epub ahead of print).
|
|
16
|
Ciurea AV, Palade C, Voinescu D and Nica
DA: Subarachnoid hemorrhage and cerebral vasospasm-literature
review. J Med Life. 6:120–125. 2013.PubMed/NCBI
|
|
17
|
Lauzier DC, Jayaraman K, Yuan JY, Diwan D,
Vellimana AK, Osbun JW, Chatterjee AR, Athiraman U, Dhar R and
Zipfel GJ: Early brain injury after subarachnoid hemorrhage:
Incidence and mechanisms. Stroke. 54:1426–1440. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yuan B, Zhao XD, Shen JD, Chen SJ, Huang
HY, Zhou XM, Han YL, Zhou LJ, Lu XJ and Wu Q: Activation of SIRT1
alleviates ferroptosis in the early brain injury after subarachnoid
hemorrhage. Oxid Med Cell Longev. 2022(9069825)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang Z, Fang Y, Lenahan C and Chen S: The
role of immune inflammation in aneurysmal subarachnoid hemorrhage.
Exp Neurol. 336(113535)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Clower BR, Yamamoto Y, Cain L, Haines DE
and Smith RR: Endothelial injury following experimental
subarachnoid hemorrhage in rats: Effects on brain blood flow. Anat
Rec. 240:104–114. 1994.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen Y, Peng F, Xing Z, Chen J, Peng C and
Li D: Beneficial effects of natural flavonoids on
neuroinflammation. Front Immunol. 13(1006434)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Santos-Buelga C and Feliciano AS:
Flavonoids: From structure to health issues. Molecules.
22(477)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu T, Su K, Cai W, Ao H and Li M:
Therapeutic potential of puerarin against cerebral diseases: From
bench to bedside. Eur J Pharmacol. 953(175695)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zeng J, Zheng S, Chen Y, Qu Y, Xie J, Hong
E, Lv H, Ding R, Feng L and Xie Z: Puerarin attenuates
intracerebral hemorrhage-induced early brain injury possibly by
PI3K/Akt signal activation-mediated suppression of NF-κB pathway. J
Cell Mol Med. 25:7809–7824. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Franza L, Carusi V, Nucera E and Pandolfi
F: Luteolin, inflammation and cancer: Special emphasis on gut
microbiota. Biofactors. 47:181–189. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tan X, Yang Y, Xu J, Zhang P, Deng R, Mao
Y, He J, Chen Y, Zhang Y, Ding J, et al: Luteolin exerts
neuroprotection via modulation of the p62/Keap1/Nrf2 pathway in
intracerebral hemorrhage. Front Pharmacol. 10(1551)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sivandzade F, Prasad S, Bhalerao A and
Cucullo L: NRF2 and NF-κB interplay in cerebrovascular and
neurodegenerative disorders: Molecular mechanisms and possible
therapeutic approaches. Redox Biol. 21(101059)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang Y, Tan X, Xu J, Wang T, Liang T, Xu
X, Ma C, Xu Z, Wang W, Li H, et al: Luteolin alleviates
neuroinflammation via downregulating the TLR4/TRAF6/NF-κB pathway
after intracerebral hemorrhage. Biomed Pharmacother.
126(110044)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu M, Qi B, Xiaoxiang W, Xu J and Liu X:
Baicalein increases cisplatin sensitivity of A549 lung
adenocarcinoma cells via PI3K/Akt/NF-κB pathway. Biomed
Pharmacother. 90:677–685. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wei N, Wei Y, Li B and Pang L: Baicalein
promotes neuronal and behavioral recovery after intracerebral
hemorrhage via suppressing apoptosis, oxidative stress and
neuroinflammation. Neurochem Res. 42:1345–1353. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Masomi-Bornwasser J, Kurz E, Frenz C,
Schmitt J, Wesp DMA, König J, Lotz J, Ringel F, Kerz T, Krenzlin H
and Keric N: The influence of oxidative stress on neurological
outcomes in spontaneous intracerebral hemorrhage. Biomolecules.
11(1615)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen X, Zhou Y, Wang S and Wang W:
Mechanism of baicalein in brain injury after intracerebral
hemorrhage by inhibiting the ROS/NLRP3 inflammasome pathway.
Inflammation. 45:590–602. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gu L, Sun M, Li R, Zhang X, Tao Y, Yuan Y,
Luo X and Xie Z: Didymin suppresses microglia pyroptosis and
neuroinflammation through the Asc/caspase-1/GSDMD pathway following
experimental intracerebral hemorrhage. Front Immunol.
13(810582)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Di Petrillo A, Orrù G, Fais A and Fantini
MC: Quercetin and its derivates as antiviral potentials: A
comprehensive review. Phytother Res. 36:266–278. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang Y, Yi B, Ma J, Zhang L, Zhang H,
Yang Y and Dai Y: Quercetin promotes neuronal and behavioral
recovery by suppressing inflammatory response and apoptosis in a
rat model of intracerebral hemorrhage. Neurochem Res. 40:195–203.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fu Y, Xu B, Huang S, Luo X, Deng XL, Luo
S, Liu C, Wang Q, Chen JY and Zhou L: Baicalin prevents LPS-induced
activation of TLR4/NF-κB p65 pathway and inflammation in mice via
inhibiting the expression of CD14. Acta Pharmacol Sin. 42:88–96.
2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Guo LT, Wang SQ, Su J, Xu LX, Ji ZY, Zhang
RY, Zhao QW, Ma ZQ, Deng XY and Ma SP: Baicalin ameliorates
neuroinflammation-induced depressive-like behavior through
inhibition of toll-like receptor 4 expression via the
PI3K/AKT/FoxO1 pathway. J Neuroinflammation. 16(95)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhou QB, Jia Q, Zhang Y, Li LY, Chi ZF and
Liu P: Effects of baicalin on protease-activated receptor-1
expression and brain injury in a rat model of intracerebral
hemorrhage. Chin J Physiol. 55:219–226. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zeng J, Chen Y, Ding R, Feng L, Fu Z, Yang
S, Deng X, Xie Z and Zheng S: Isoliquiritigenin alleviates early
brain injury after experimental intracerebral hemorrhage via
suppressing ROS- and/or NF-κB-mediated NLRP3 inflammasome
activation by promoting Nrf2 antioxidant pathway. J
Neuroinflammation. 14(119)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen Z, Wang C, Liu Y, Liang X, Yang C,
Zhang X and Li X: Protective effects of medicinal plant
breviscapine on postcerebral hemorrhage in rats. J Integr Neurosci.
19:101–109. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lan X, Han X, Li Q, Li Q, Gao Y, Cheng T,
Wan J, Zhu W and Wang J: Pinocembrin protects hemorrhagic brain
primarily by inhibiting toll-like receptor 4 and reducing M1
phenotype microglia. Brain Behav Immun. 61:326–339. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fei X, Chen C, Kai S, Fu X, Man W, Ding B,
Wang C and Xu R: Eupatilin attenuates the inflammatory response
induced by intracerebral hemorrhage through the TLR4/MyD88 pathway.
Int Immunopharmacol. 76(105837)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen C, Yao L, Cui J and Liu B: Fisetin
protects against intracerebral hemorrhage-induced neuroinflammation
in aged mice. Cerebrovasc Dis. 45:154–161. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Singh N, Bansal Y, Bhandari R, Marwaha L,
Singh R, Chopra K and Kuhad A: Naringin reverses neurobehavioral
and biochemical alterations in intracerebroventricular
collagenase-induced intracerebral hemorrhage in rats. Pharmacology.
100:172–187. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chen C, Cui J, Ji X and Yao L:
Neuroprotective functions of calycosin against intracerebral
hemorrhage-induced oxidative stress and neuroinflammation. Future
Med Chem. 12:583–592. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gao Y and Dong Z: Protective effect of
procyanidins on experimental rats with intracerebral hemorrhage.
Zhongguo Zhong Yao Za Zhi. 34:3078–3081. 2009.PubMed/NCBI(In Chinese).
|
|
47
|
Pyrzynska K: Hesperidin: A review on
extraction methods, stability and biological activities. Nutrients.
14(2387)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Qin Z, Chen L, Liu M, Tan H and Zheng L:
Hesperidin reduces adverse symptomatic intracerebral hemorrhage by
promoting TGF-β1 for treating ischemic stroke using tissue
plasminogen activator. Neurol Sci. 41:139–147. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Dumont AS, Dumont RJ, Chow MM, Lin CL,
Calisaneller T, Ley KF, Kassell NF and Lee KS: Cerebral vasospasm
after subarachnoid hemorrhage: Putative role of inflammation.
Neurosurgery. 53:123–135. 2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Erdi F, Keskin F, Esen H, Kaya B,
Feyzioglu B, Kilinc I, Karatas Y, Cuce G and Kalkan E: Telmisartan
ameliorates oxidative stress and subarachnoid haemorrhage-induced
cerebral vasospasm. Neurol Res. 38:224–231. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Xu W, Li T, Gao L, Zheng J, Yan J, Zhang J
and Shao A: Apelin-13/APJ system attenuates early brain injury via
suppression of endoplasmic reticulum stress-associated TXNIP/NLRP3
inflammasome activation and oxidative stress in a AMPK-dependent
manner after subarachnoid hemorrhage in rats. J Neuroinflammation.
16(247)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kuo CP, Wen LL, Chen CM, Huh B, Cherng CH,
Wong CS, Liaw WJ, Yeh CC, Lin BF and Wu CT: Attenuation of
neurological injury with early baicalein treatment following
subarachnoid hemorrhage in rats. J Neurosurg. 119:1028–1037.
2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hao G, Dong Y, Huo R, Wen K, Zhang Y and
Liang G: Rutin Inhibits neuroinflammation and provides
neuroprotection in an experimental rat model of subarachnoid
hemorrhage, possibly through suppressing the RAGE-NF-κB
inflammatory signaling pathway. Neurochem Res. 41:1496–1504.
2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhang T, Su J, Guo B, Wang K, Li X and
Liang G: Apigenin protects blood-brain barrier and ameliorates
early brain injury by inhibiting TLR4-mediated inflammatory pathway
in subarachnoid hemorrhage rats. Int Immunopharmacol. 28:79–87.
2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Shi X, Fu Y, Zhang S, Ding H and Chen J:
Baicalin attenuates subarachnoid hemorrhagic brain injury by
modulating blood-brain barrier disruption, inflammation, and
oxidative damage in mice. Oxid Med Cell Longev.
2017(1401790)2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang H, Tu X, Song S, Liang R and Shi S:
Baicalin reduces early brain injury after subarachnoid hemorrhage
in rats. Chin J Integr Med. 26:510–518. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Gül Ş, Aydoğmuş E, Bahadir B, Büyükuysal
MÇ and Güven B: Neuroprotective effects of quercetin on cerebral
vasospasm following experimental subarachnoid haemorrhage in rats.
Turk J Med Sci. 50:1106–1110. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Dong YS, Wang JL, Feng DY, Qin HZ, Wen H,
Yin ZM, Gao GD and Li C: Protective effect of quercetin against
oxidative stress and brain edema in an experimental rat model of
subarachnoid hemorrhage. Int J Med Sci. 11:282–290. 2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Tekiner A, Yilmaz MB, Bolat E, Goker T,
Sargon MF and Arat A: The therapeutic value of proanthocyanidin in
experimental cerebral vasospasm following subarachnoid hemorrhage.
Turk Neurosurg. 24:885–890. 2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhang ZH, Liu JQ, Hu CD, Zhao XT, Qin FY,
Zhuang Z and Zhang XS: Luteolin confers cerebroprotection after
subarachnoid hemorrhage by suppression of NLPR3 inflammasome
activation through Nrf2-dependent pathway. Oxid Med Cell Longev.
2021(5838101)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Zhang Y, Yang X, Ge X and Zhang F:
Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved
caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid
hemorrhage mice. Biomed Pharmacother. 109:726–733. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zeng Y, Fang Z, Lai J, Wu Z, Lin W, Yao H,
Hu W, Chen J, Guo X and Chen X: Activation of sirtuin-1 by
pinocembrin treatment contributes to reduced early brain injury
after subarachnoid hemorrhage. Oxid Med Cell Longev.
2022(2242833)2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Xu W, Yan J, Ocak U, Lenahan C, Shao A,
Tang J, Zhang J and Zhang JH: Melanocortin 1 receptor attenuates
early brain injury following subarachnoid hemorrhage by controlling
mitochondrial metabolism via AMPK/SIRT1/PGC-1α pathway in rats.
Theranostics. 11:522–539. 2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Li Q, Chen Y, Zhang X, Zuo S, Ge H, Chen
Y, Liu X, Zhang JH, Ruan H and Feng H: Scutellarin attenuates
vasospasm through the Erk5-KLF2-eNOS pathway after subarachnoid
hemorrhage in rats. J Clin Neurosci. 34:264–270. 2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Sun Y, Chen P, Zhai B, Zhang M, Xiang Y,
Fang J, Xu S, Gao Y, Chen X, Sui X and Li G: The emerging role of
ferroptosis in inflammation. Biomed Pharmacother.
127(110108)2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wang F, He J, Xing R, Sha T and Sun B:
Molecular mechanisms of ferroptosis and their role in inflammation.
Int Rev Immunol. 42:71–81. 2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11(88)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G,
Liu Y, Zhao X, Qian L, Liu P and Xiong Y: Ferroptosis: A cell death
connecting oxidative stress, inflammation and cardiovascular
diseases. Cell Death Discov. 7(193)2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gao S, Zhou L, Lu J, Fang Y, Wu H, Xu W,
Pan Y, Wang J, Wang X, Zhang J and Shao A: Cepharanthine attenuates
early brain injury after subarachnoid hemorrhage in mice via
inhibiting 15-lipoxygenase-1-mediated microglia and endothelial
cell ferroptosis. Oxid Med Cell Longev.
2022(4295208)2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Zheng B, Zhou X, Pang L, Che Y and Qi X:
Baicalin suppresses autophagy-dependent ferroptosis in early brain
injury after subarachnoid hemorrhage. Bioengineered. 12:7794–7804.
2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Chen D, Chen JJ, Yin Q, Guan JH and Liu
YH: Role of ERK1/2 and vascular cell proliferation in cerebral
vasospasm after experimental subarachnoid hemorrhage. Acta
Neurochir (Wien). 151:1127–1134. 2009.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Curson JEB, Liu L, Luo L, Muusse TW, Lucas
RM, Gunther KS, Vajjhala PR, Abrol R, Jones A, Kapetanovic R, et
al: TLR4 phosphorylation at tyrosine 672 activates the ERK/c-FOS
signaling module for LPS-induced cytokine responses in macrophages.
Eur J Immunol. 53(e2250056)2023.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Lin CW, Chen PN, Chen MK, Yang WE, Tang
CH, Yang SF and Hsieh YS: Kaempferol reduces matrix
metalloproteinase-2 expression by down-regulating ERK1/2 and the
activator protein-1 signaling pathways in oral cancer cells. PLoS
One. 8(e80883)2013.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Zong J, Zhang DP, Zhou H, Bian ZY, Deng W,
Dai J, Yuan Y, Gan HW, Guo HP and Tang QZ: Baicalein protects
against cardiac hypertrophy through blocking MEK-ERK1/2 signaling.
J Cell Biochem. 114:1058–1065. 2013.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Cheng Y, Zhang Z, Tang H, Chen B, Cai Y,
Wei Y, Zhao W, Wu ZB and Shang H: Mitochondrial inhibitor rotenone
triggers and enhances neuronal ferroptosis following intracerebral
hemorrhage. ACS Chem Neurosci. 14:1071–1079. 2023.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Li N, Ragheb K, Lawler G, Sturgis J, Rajwa
B, Melendez JA and Robinson JP: Mitochondrial complex I inhibitor
rotenone induces apoptosis through enhancing mitochondrial reactive
oxygen species production. J Biol Chem. 278:8516–8525.
2003.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Li K, Liang Y, Cheng A, Wang Q, Li Y, Wei
H, Zhou C and Wan X: Antiviral properties of baicalin: A concise
review. Rev Bras Farmacogn. 31:408–419. 2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Yao C, Dai S, Wang C, Fu K, Wu R, Zhao X,
Yao Y and Li Y: Luteolin as a potential hepatoprotective drug:
Molecular mechanisms and treatment strategies. Biomed Pharmacother.
167(115464)2023.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Choi EJ, Lee BH, Lee K and Chee KM:
Long-term combined administration of quercetin and daidzein
inhibits quercetin-induced suppression of glutathione antioxidant
defenses. Food Chem Toxicol. 43:793–798. 2005.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Di Lorenzo C, Colombo F, Biella S,
Stockley C and Restani P: Polyphenols and human health: The role of
bioavailability. Nutrients. 13(273)2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Galho AR, Cordeiro MF, Ribeiro SA, Marques
MS, Antunes MF, Luz DC, Hädrich G, Muccillo-Baisch AL, Barros DM,
Lima JV, et al: Protective role of free and quercetin-loaded
nanoemulsion against damage induced by intracerebral haemorrhage in
rats. Nanotechnology. 27(175101)2016.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Waki T, Nakanishi I, Matsumoto K, Kitajima
J, Chikuma T and Kobayashi S: Key role of chemical hardness to
compare 2,2-diphenyl-1-picrylhydrazyl radical scavenging power of
flavone and flavonol O-glycoside and C-glycoside derivatives. Chem
Pharm Bull (Tokyo). 60:37–44. 2012.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Cassidy A, O'Reilly ÉJ, Kay C, Sampson L,
Franz M, Forman JP, Curhan G and Rimm EB: Habitual intake of
flavonoid subclasses and incident hypertension in adults. Am J Clin
Nutr. 93:338–347. 2011.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Elijovich L, Patel PV and Hemphill JC III:
Intracerebral hemorrhage. Semin Neurol. 28:657–667. 2008.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Sung B, Chung HY and Kim ND: Role of
apigenin in cancer prevention via the induction of apoptosis and
autophagy. J Cancer Prev. 21:216–226. 2016.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Ganeshpurkar A and Saluja AK: The
pharmacological potential of rutin. Saudi Pharm J. 25:149–164.
2017.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Jäger A and Saaby L: Flavonoids and the
CNS. Molecules. 16:1471–1485. 2011.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Parrella E, Gussago C, Porrini V, Benarese
M and Pizzi M: From preclinical stroke models to humans:
Polyphenols in the prevention and treatment of stroke. Nutrients.
13(85)2020.PubMed/NCBI View Article : Google Scholar
|
















