Introduction
Primary papillary adenoma of the lung is a rare
tumor first described by Spencer et al (1) in 1980. To date, only 44 cases of
pulmonary papillary adenoma have been reported, predominantly
involving peripheral lung tissue. Owing to its rarity, current
understanding of papillary adenoma of the lung is limited, which
makes it easily misdiagnosed as other types of primary benign or
malignant lung tumors in clinical practice, especially lung
adenocarcinoma with a papillary growth pattern. Typically, clinical
symptoms of primary lung papillary adenoma are non-specific, and
can manifest as cough, shortness of breath, asthma and chest pain;
patients are often asymptomatic and are diagnosed accidentally by
physical examination. Most of the patients are male and range in
age from 2 months to 78 years-old. Most reported cases have
predominantly involved peripheral lung tissue, and also in the
hilar region, mainly appearing as a solitary pulmonary nodule on
chest computed tomography (CT) images. Under imaging examination,
pulmonary papillary adenomas were mostly solitary round or
spherical nodules with smooth margins. The most common location was
the lower lobe of the left lung, followed by the upper and lower
lobes of the right lung. Pathologic examination revealed that the
diameter of the tumors ranged from 0.2 to 9 cm, most of them had no
capsule and were clearly demarcated from surrounding lung tissues
(1-26).
Most reported patients underwent only surgical treatment, with no
recurrence or metastasis generally observed during follow-up;
papillary adenomas of the lung are classified as benign tumors
(1-26).
Pulmonary papillary adenomas were extremely rare; pathologists do
not have sufficient knowledge of the histological morphology of the
tumor. In the present study, a rare case of papillary adenoma of
the lung was reported and the relevant literature was reviewed.
Case report
Ethical approval. The present study was
approved [approval no: LS (2021)009] by the Institutional Review
Board of The First Hospital of China Medical University (Shenyang,
China). Written informed consent was obtained from the patient for
the participation in the present study and for the publication of
the associated images. The study was conducted in accordance with
the principles of the Declaration of Helsinki (2013 version).
Clinical history
A 61-year-old man with no history of smoking
presented with a nodule in the lower lobe of the left lung during
an examination and was admitted to the First Hospital of China
Medical University (Shenyang, China) in December 2022 for further
treatment. The patient had no relevant medical, personal, or family
history. The male patient had no chest pain or any other relevant
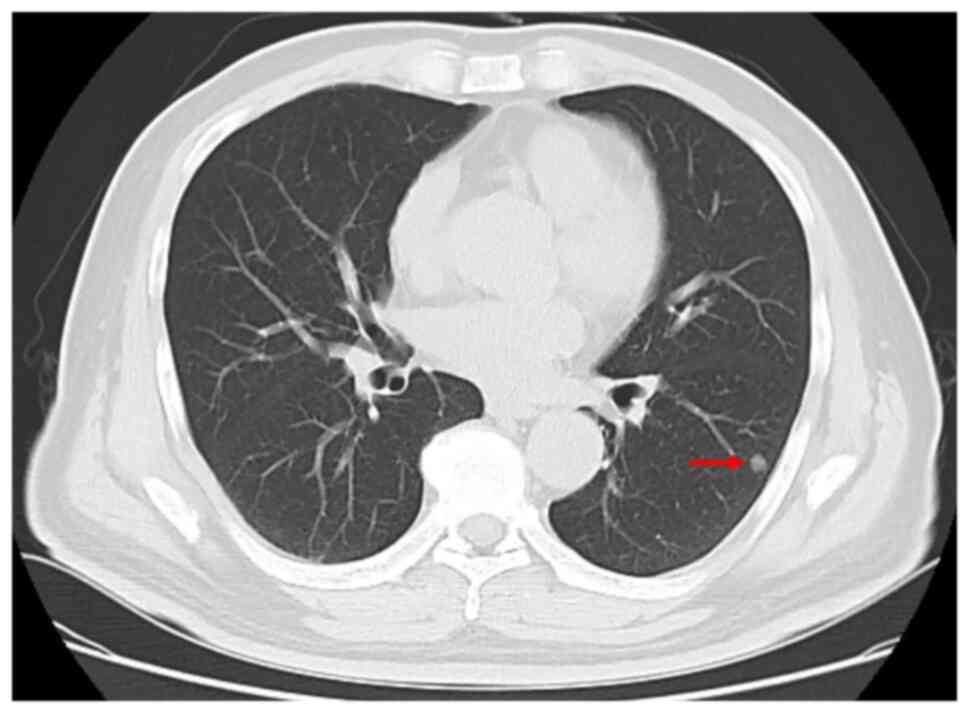
symptoms and was afebrile on presentation. A CT scan revealed a
well-defined solid mass nodule in the left lower lobe measuring ~1
cm in diameter. The tracheobronchial and subcarinal lymph nodes
were not enlarged (Fig. 1). The
preoperative diagnosis was a lung mass, and a wedge resection of
the lower lobe of the left lung was performed. Intraoperatively,
the nodule was located in the left lower lobe and was ~1 cm in
diameter. The surface of the tumor was rough and uneven. The edge
of the mass was located 2 cm from the incisal margin. The resected
lung specimen was sent for rapid intraoperative frozen tissue
pathological examination, with a suggested diagnosis provided as
follows: ‘Consider sclerosing pneumocytoma or papillary adenoma, to
be determined and excluded from malignancy by paraffin section and
immunohistochemical examination’. No lymph node sampling or
lymphadenectomy was performed. The male patient did not receive
postoperative radiotherapy or chemotherapy and had a favorable
postoperative recovery. Follow-up at 15 months (to March 2024)
revealed no evidence of recurrence or other metastatic occurrences.
The follow-up result aligns with previous studies. Based on the
available data, excluding two special cases and seven patients for
whom follow-up information was unavailable, the remaining patients
(36/45) did not experience any recurrence and exhibited positive
outcomes (1-26).
Immunohistochemical staining
The resected specimens were fixed with 10%
neutral-buffered formalin for 24 h at room temperature, embedded in
paraffin blocks, and cut into 4-µm thick serial sections. Sections
were stained for 5 min with hematoxylin and eosin (H&E; (cat.
no. G1120; Beijing Solarbio Science & Technology Co., Ltd.) at
room temperature for histological assessment under a light
microscope (Nikon Corporation). For immunohistochemical analysis,
after washing three times in 0.01 M phosphate buffered saline (pH
7.4) for 5 min each time at room temperature, the sections were
incubated with 3% hydrogen peroxide at room temperature for 10 min.
Antigen retrieval was performed with ethylene diamine tetra-acetic
acid at 100˚C for 2.5 min. The sections were then incubated with
undiluted primary antibodies at 37˚C for 60 min and ready-to-use
secondary antibody at 37˚C for 20 min. The following primary
antibodies were used: Broad-spectrum cytokeratin (CK, cat. no.
MAB-0671), CK7 (cat. no. MAB-0828), CK5/6 (cat. no. MAB-0744), P40
(cat. no. RMA-0815), thyroid transcription factor 1 (TTF-1, cat.
no. MAB-0677), Napsin-A (cat. no. MAB-0704) and Ki-67 (cat. no.
MAB-0672; all Fuzhou Maixin Biotech Co., Ltd.). Ready-to-use
biotinylated goat anti-mouse and rabbit secondary antibodies (cat.
no. KIT-9710; Fuzhou Maixin Biotech Co., Ltd.) . Elastic fiber
staining at room temperature according to the manufacturer's
protocol (cat. no. BA4083B; Zhuhai Beso Biotechnology Co.,
Ltd.).
Morphological and immunohistochemical
findings
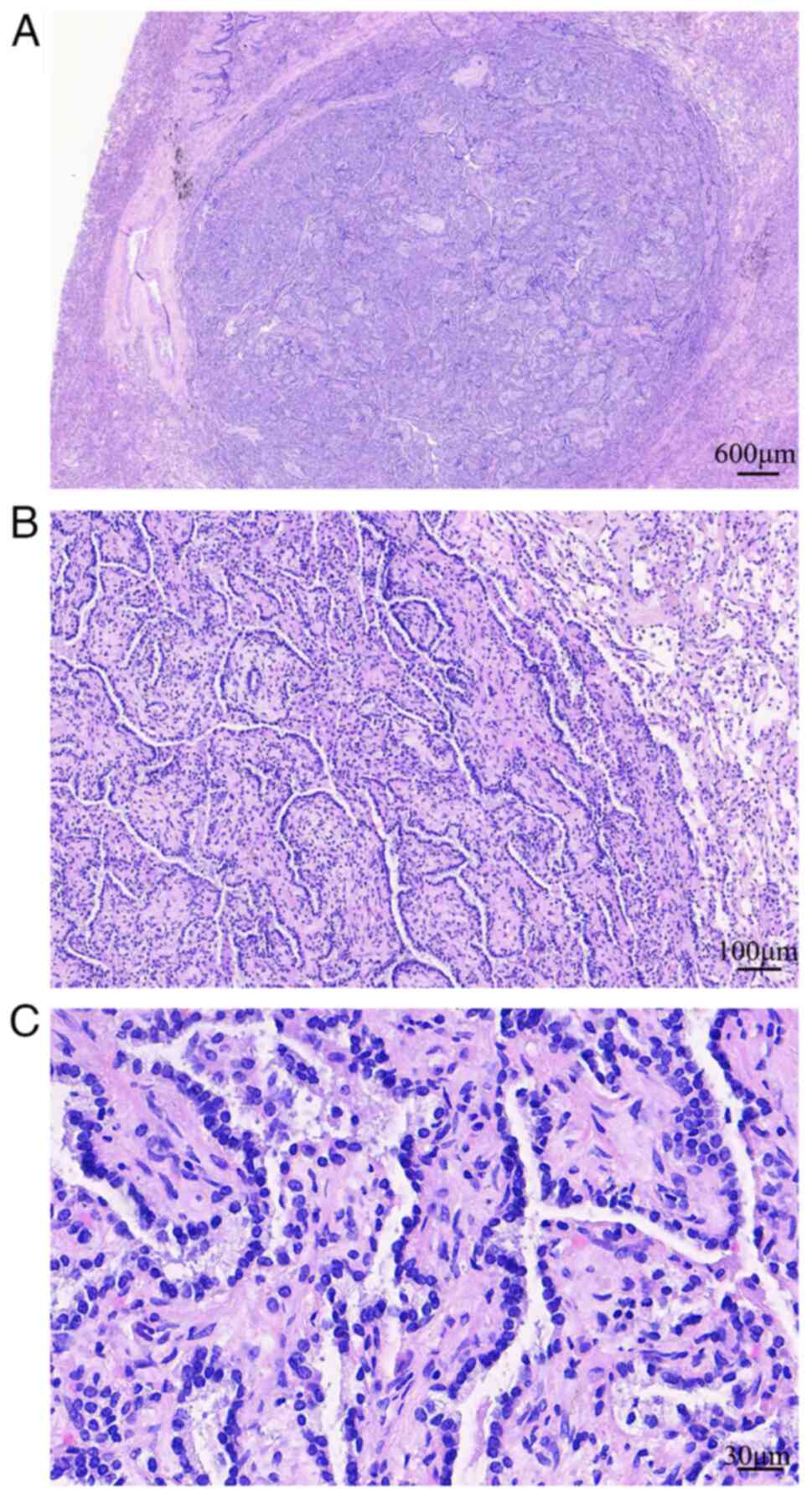
Morphologically, the well-circumscribed tumor
(maximum diameter, ~0.7 cm) had no surrounding fibrous capsule,
revealed expansive growth and was pressed against healthy lung
tissue. The tumor comprised branched papillae with a fibrovascular
core and no other structural components. The papillary structures
were covered with a single layer of cuboidal epithelial cells. The
tumor cells were relatively uniform in shape and well arranged with
round or oval nuclei (Fig. 2). No
nucleoli or mitotic figures were observed. Lymphocyte infiltration
was observed in the stroma of the tumor; however no significant
edematous change was noted. No healthy lung tissue infiltration or
vascular or neural invasion were observed in relation to the
tumor.
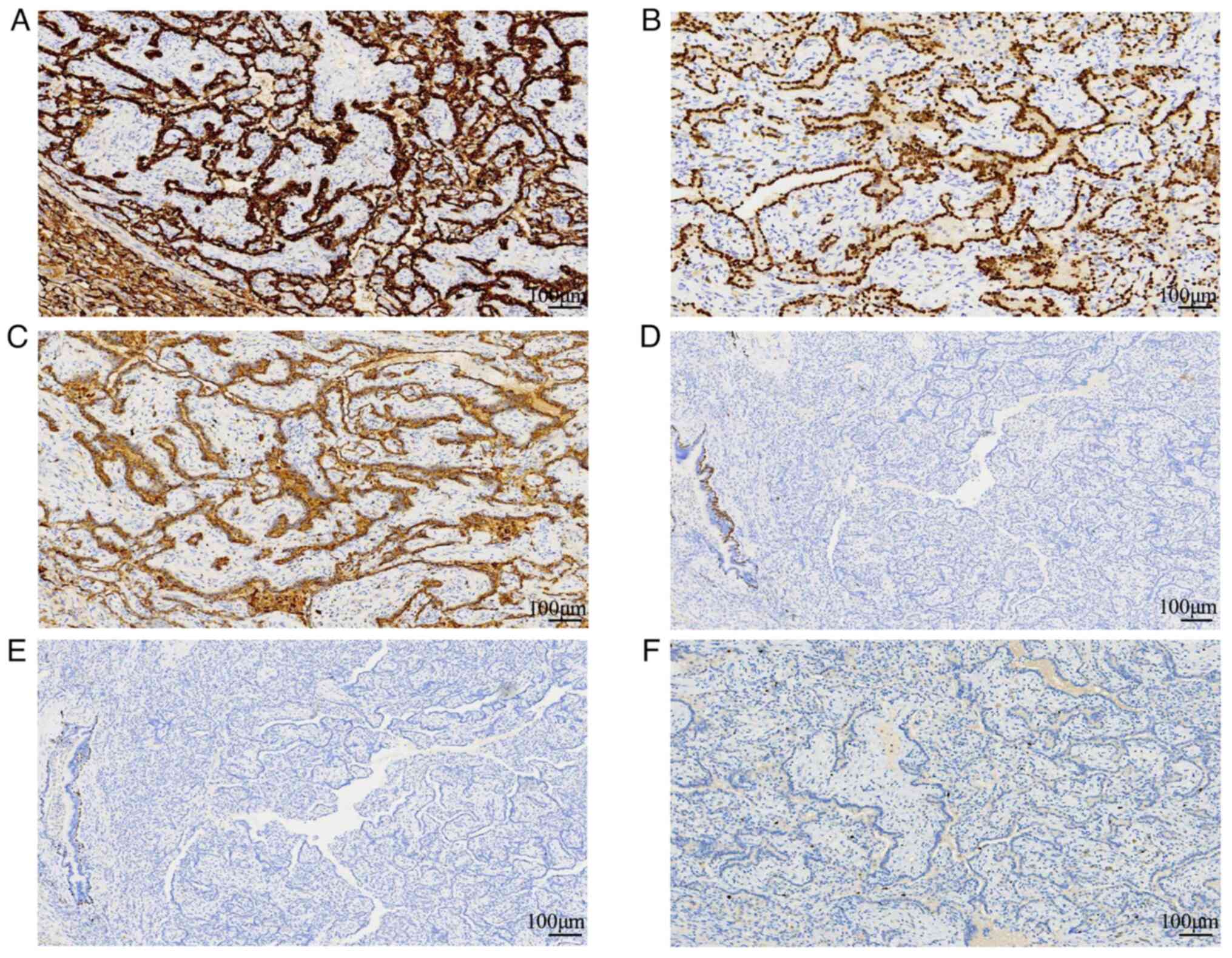
Immunohistochemically, the papillary structures of
the tumor cells were strongly and diffusely positive for CK, CK7,
Napsin A and TTF-1. The stroma of the papillary structures was
negative for CK, CK7 and TTF-1. The tumor was negative for CK5/6
and P40. The Ki-67 index was ~1% (Figs. 3 and S1). Positive staining for CK, CK7, and
Napsin-A in the papillary structures of the tumor cells, along with
a diffusely lower Ki67 index, aided in the diagnosis of pulmonary
papillary adenoma. Elastic fiber staining was performed and
discontinuous or fractured elastic fibers in papillary adenoma
tissue were observed (Fig. S1);
therefore, elastic fiber staining could not be used to
differentiate between papillary adenoma and papillary
adenocarcinoma.
Discussion
Based on the aforementioned clinical information,
morphological features and immunohistochemical results, the tumor
was diagnosed as a primary papillary adenoma of the lung. Papillary
adenoma is a rare tumor that occurs mainly in the peripheral lung.
In 1980, Spencer et al (1)
first reported two cases of primary papillary adenoma of the lung
(1). Only 45 patients with primary
papillary adenoma of the lung have been reported to date. The
clinicopathological features of the 45 reported cases (women, n=19;
men, n=26) (1-26)
are summarized in Table I, with
most patients being asymptomatic, and the tumor being an incidental
finding in 27 of the 45 cases. Patient ages varied widely between 2
months and 78 years (mean age, 50.5 years). The diameter of the
tumors ranged from 0.2 to 9 cm (mean diameter, 2.5 cm), with the
exception of 11 cases where tumor size was not reported. Tumor
locations were as follows: Right lung, 20 cases; left lung, 18
cases; main airway, one case; both lungs, one case; and location
not mentioned, four cases (1-26).
 | Table IClinicopathological characteristics of
patients with primary pulmonary papillary adenoma. |
Table I
Clinicopathological characteristics of
patients with primary pulmonary papillary adenoma.
| Case | Symptoms | Year | Sex | Age, years | Size, cm | Site | Therapy | Outcome | (Refs.) |
|---|
| 1 | 2-month history of
cough | 1980 | F | 26 | 4.0 | L | S | NM | (1) |
| 2 | None | 1980 | M | 7 | NM | L | S | NM | (1) |
| 3 | None | 1982 | F | 25 | 2.1 | R | S | 10 years FD | (2) |
| 4 | None | 1986 | M | 57 | 1.5 | R | S | 8 years FD | (3) |
| 5 | None | 1992 | M | 23 | 1.8 | R | S | 10 years FD | (4) |
| 6 | None | 1992 | M | 56 | 1.8 | R | S | 2 years FD | (4) |
| 7 | None | 1992 | F | 52 | 1.2 | R | S | 11 months FD | (5) |
| 8 | None | 1993 | M | 13 | 0.2 | R,L | S | 6 years FD | (6) |
| 9 | None | 1994 | F | <1 | 2 | R | S | 2 years FD | (7) |
| 10 | None | 1996 | M | 35 | 2 | L | S | 3 years FD | (8) |
| 11 | None | 2000 | M | 15 | 2.5 | L | S | 9 years FD | (9) |
| 12 | None | 2000 | M | 27 | 2.4 | R | S | 2 years FD | (9) |
| 13 | With osteosarcoma
lung metastases | 2002 | M | 9 | 0.4 | L | S,C | 6 years and 9
months FD | (10) |
| 14 | None | 2009 | M | 61 | 1.5 | L | S | 7 years FD | (11) |
| 15 | None | 2010 | M | 70 | 1.1 | L | S | 3 years FD | (12) |
| 16 | Shortness of
breath | 2011 | M | 75 | 1.5 | L | S | NM | (13) |
| 17 | None | 2011 | M | 75 | 1.8 | L | S | 38 months FD | (14) |
| 18 | History of
asthma | 2013 | M | 24 | 6.0 | L | S | 6 months FD | (15) |
| 19 | History of renal
cancer | 2014 | F | 68 | 2.5 | L | S | NM | (16) |
| 20 | None | 2015 | F | 17 | 3.1 | R | S | 12 months FD | (17) |
| 21 | None | 2016 | F | 78 | 3.8 | L | S | 26 months FD | (18) |
| 22 | None | 2017 | F | 64 | 1.7 | R | S | 6 months FD | (19) |
| 23 | None | 2017 | F | 41 | 2.0 | L | S | NM | (19) |
| 24 | None | 2019 | M | 59 | 1.5 | R | S | NM | (20) |
| 25 | Dizziness for 1
week | 2020 | M | 65 | 4.2 | R | NM | Brain
metastasis | (21) |
| 26 | History of
asthma | 2020 | M | 56 | 1.5 | L | S | Developed
adenocarcinoma after 2 years | (22) |
| 27 | Cough | 2020 | M | 59 | 9 | R | S | 6 years FD | (23) |
| 28 | None | 2020 | F | 62 | NM | L | No | Favorable | (24) |
| 29 | Cough | 2020 | M | 60 | 1.1 | L | S | Favorable | (24) |
| 30 | Chest pain | 2020 | F | 65 | ECH | R | S | Favorable | (24) |
| 31 | Cough | 2020 | M | 64 | ECH | R | No | Favorable | (24) |
| 32 | None | 2020 | F | 62 | 1 | R | S | Favorable | (24) |
| 33 | None | 2020 | F | 72 | 1 | R | S | Favorable | (24) |
| 34 | Cough | 2020 | M | 47 | EC | NM | No | Favorable | (24) |
| 35 | None | 2020 | F | 78 | 3.4 | R | No | Favorable | (24) |
| 36 | Cough | 2020 | F | 68 | BE exudation | NM | No | Favorable | (24) |
| 37 | Cough | 2020 | M | 48 | exudation | R | No | Favorable | (24) |
| 38 | Cough | 2020 | F | 63 | BE exudation | NM | No | Favorable | (24) |
| 39 | Cough | 2020 | F | 39 | EC | NM | No | Favorable | (24) |
| 40 | Cough | 2020 | F | 76 | EC | NM | No | Favorable | (24) |
| 41 | Chest pain | 2020 | M | 70 | 4 | L | S | Favorable | (24) |
| 42 | Cough | 2020 | M | 45 | BE | R | No | Favorable | (24) |
| 43 | None | 2021 | M | 69 | 4 | Left main
airway | S | 1 year FD | (25) |
| 44 | None | 2022 | F | 66 | 5.5 | R | S | NM | (26) |
| 45 present
case | None | 2023 | M | 61 | 1 | L | S | 15 months FD | |
Owing to its rarity, pathologists may have
insufficient knowledge of the histological morphology of this type
of tumor. At present, papillary adenomas are considered to
originate from the primitive multipotential respiratory epithelium,
which shows bidirectional differentiation into type II alveolar
epithelium and club cells (7,8,21).
Primary papillary adenomas of the lung may be confused with other
primary malignant or benign lung tumors such as adenocarcinoma with
papillary growth patterns, metastatic papillary thyroid carcinoma,
sclerosing pneumocytomas and bronchiolar adenomas. Papillary
adenomas should be distinguished from adenocarcinomas with
papillary growth patterns. Adenocarcinomas generally exhibit a high
degree of cellular proliferation, nuclear atypia and a complex
branching architecture. Lung adenocarcinoma may occasionally
present with relatively mild papillary structures, showing
infiltrative growth without a clear boundary. In addition, owing to
the heterogeneity of lung adenocarcinomas, the papillary structure
is usually not the only growth pattern and is accompanied by other
structures, such as lepidic or glandular patterns. EGFR and
KRAS gene mutations may play a role in the development of
pulmonary adenocarcinoma (17).
The diagnosis of metastatic papillary thyroid carcinoma relies
primarily on nuclear morphology and immunohistochemical staining.
Papillary thyroid carcinoma is characterized by the presence of
cells with ground glass nuclei and colloid within thyroid
follicles. Thyroglobulin and PAX8 are antibodies specifically
expressed in thyroid carcinoma, while Napsin A is a marker for lung
adenocarcinomas (27). Sclerosing
pneumocytomas originate from the primitive respiratory epithelium
(28). The histological morphology
of sclerosing pneumocytomas varies. Four growth patterns are
typically observed, namely, papillary, sclerotic, hemorrhagic and
solid. Sclerosing pneumocytomas are comprised of two cell types.
Tumor cells are arranged not only on the papillary surface and in
the stroma, with papillary structures comprising TTF-1-positive
stromal cells instead of fibrovascular cores (28). Bronchiolar adenomas (including
proximal and distal types) are derived from bronchiolar epithelium
and not from the alveolar epithelial cells. Therefore, the tumor
cells on the surface of the papillary structures display
differentiation of the ciliated columnar epithelium and mucus cells
with basal cells at the bottom layer. CK5/6, P40, or P63
immunostaining helps to identify the basal cells of bronchiolar
adenoma (29).
Owing to the rarity of papillary adenomas of the
lung, definitive histopathological prognostic factors have not been
elucidated. With the exception of 10 patients who did not receive
therapy, most reported patients underwent only surgical treatment
(excluding one with osteosarcoma lung metastases who received
chemotherapy). All patients had a favorable prognosis with no tumor
recurrence after surgery. Thus, papillary adenomas of the lung are
classified as benign tumors, implying that patient sex and tumor
characteristics, such as size and location, typically do not
associate with prognosis or recurrence. Moreover, apart from a low
Ki67 index, these adenomas lack specific biological markers. One
patient with pulmonary papillary adenoma developed acinar
adenocarcinoma and micropapillary adenocarcinoma components in the
same tumor after 2 years of follow-up (22). Another case report indicated that a
pulmonary papillary adenoma underwent malignant transformation. In
that case, a CT scan taken prior to biopsy had already identified a
lung tumor of diameter 4 cm and brain metastasis; therefore, the
diagnosis of pulmonary papillary adenoma in that case remains
contentious (21). Two reported
cases of papillary adenoma exhibited invasive growth behavior
(9). The prognostic factors and
long-term outcomes for papillary adenomas of the lung remain
unclear, and further longer-term follow-up studies are needed. A
small number of pulmonary papillary adenomas may have malignant or
transformation potential and should be treated early after
detection or through close follow-up. Complete surgical resection
of papillary lung adenomas is curative and feasible. After
diagnosis and treatment, excluding the aforementioned two cases and
seven patients with no follow-up information, the remaining
patients (36/45) had no recurrence and favorable prognosis. Based
on these findings, regular CT examinations can be employed for
preventive and follow-up monitoring.
In summary, a case of primary pulmonary papillary
adenoma was reported. Surgical resection is the primary treatment
option for such tumors. Careful examination of histological
features and immunohistochemistry is essential for accurate
diagnosis and prognostic evaluation. Additional studies on primary
pulmonary papillary adenoma are necessary to validate previously
reported findings.
Supplementary Material
(A) Tumor cells were positive for
cytokeratin 7 (magnification, x100). (B) Elastic fiber staining
demonstrated discontinuous or fractured elastic fibers in papillary
adenoma tissue (magnification, x100).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
HTX and MQY conceived the study and confirm the
authenticity of all the raw data. HTX conceptualized the present
study. LQC, SMG and ZJW developed the methodology. LQC, MQY and HTX
wrote the original manuscript, and reviewed and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The research protocol was approved [approval no. LS
(2021) 009] by the Institutional Review Board of The First Hospital
of China Medical University (Shenyang, China). The patient provided
written informed consent to participate in the present study.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the present case report and the
accompanying associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Spencer H, Dail DH and Arneaud J:
Non-invasive bronchial epithelial papillary tumors. Cancer.
45:1486–1497. 1980.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fantone JC, Geisinger KR and Appelman HD:
Papillary adenoma of the lung with lamellar and electron dense
granules. An ultrastructural study. Cancer. 50:2839–2844.
1982.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Noguchi M, Kodama T, Morinaga S, Shimosato
Y, Saito T and Tsuboi E: Multiple sclerosing hemangiomas of the
lung. Am J Surg Pathol. 10:134–139. 1986.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fukuda T, Ohnishi Y, Kanai I, Emura I,
Watanabe T, Kitazawa M and Okamura A: Papillary adenoma of the
lung. Histological and ultrastructural findings in two cases. Acta
Pathol Jpn. 42:56–61. 1992.PubMed/NCBI
|
|
5
|
Hegg CA, Flint A and Singh G: Papillary
adenoma of the lung. Am J Clin Pathol. 97:393–397. 1992.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kurotaki H, Kamata Y, Kimura M and Nagai
K: Multiple papillary adenomas of type II pneumocytes found in a
13-year-old boy with von Recklinghausen's disease. Virchows Arch A
Pathol Anat Histopathol. 423:319–322. 1993.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sánchez-Jiménez J, Ballester-Martínez A,
Lodo-Besse J, Huguet-Redecilla P, Martínez-González S and
Cobos-Barroso N: Papillary adenoma of type 2 pneumocytes. Pediatr
Pulmonol. 17:396–400. 1994.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mori M, Chiba R, Tezuka F, Kaji M, Kobubo
T, Nukiwa T and Takahashi T: Papillary adenoma of type II
pneumocytes might have malignant potential. Virchows Arch.
428:195–200. 1996.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dessy E, Braidotti P, Del Curto B, Falleni
M, Coggi G, Cruz GS, Carai A, Versace R and Pietra GG: Peripheral
papillary tumor of type-II pneumocytes: A rare neoplasm of
undetermined malignant potential. Virchows Arch. 436:289–295.
2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Neusuess A, Claviez A, Schroeter T, Harms
D and Suttorp M: Synchronous detection of a pulmonary papillary
adenoma and lung metastases in a patient with osteosarcoma in
relapse. Med Pediatr Oncol. 38:125–127. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Papla B: Papillary adenoma of the lung.
Pol J Pathol. 60:49–51. 2009.PubMed/NCBI
|
|
12
|
Kuwahara M, Nagafuchi M, Rikimaru T,
Iwasaki A and Shirakusa T: Pulmonary papillary adenoma. Gen Thorac
Cardiovasc Surg. 58:542–545. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Morresi-Hauf AT, Weber N, Gesierich W and
Büsing CM: Biopsy diagnosis of papillary adenoma of the lung by
endobronchial ultrasound-guided transbronchial needle aspiration
(EBUS-TBNA). Pneumologie. 65:406–411. 2011.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
14
|
Nakano T, Yokose T, Hasegawa C, Kameda Y,
Kato Y, Ito H, Tsuboi M, Nakayama H, Yamada K, Noda K and Iwazaki
M: Papillary adenoma of the lung with a peculiar raw macroscopic
feature. Pathol Int. 61:475–480. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cornejo KM, Shi M, Akalin A, Uy K, Cagle
PT and Fraire AE: Pulmonary papillary adenoma: A case report and
review of the literature. J Bronchology Interv Pulmonol. 20:52–57.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Choi IH, Han J, Moon JW, Choi YS and Lee
KJ: A rare case of pulmonary papillary adenoma in old aged woman: A
brief case report. Korean J Pathol. 48:66–68. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lin XY, Han Q, Wang EH and Zhang Y:
Pulmonary papillary adenoma presenting in central portion: A case
report. Diagn Pathol. 10(190)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Frey A, Alatassi H, Wiese TA, Fraig M and
Yang X: Cytomorphologic findings and differential diagnosis of
pulmonary papillary adenoma: A case report and literature review.
Diagn Cytopathol. 44:543–547. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Wang XL, Jiang GJ, Zhang XZ, Chu LM and
Cao Y: Pulmonary papillary adenoma: Report of two cases. J Coll
Physicians Surg Pak. 27:582–583. 2017.PubMed/NCBI
|
|
20
|
Shomura S, Suzuki H, Sawada Y, Kondo C and
Shimpo H: Surgery of papillary adenoma;report of a case. Kyobu
Geka. 72:720–723. 2019.PubMed/NCBI(In Japanese).
|
|
21
|
Hu X, Chen Y, Ru G and Yu L: Cytological
features of pulmonary papillary adenoma with malignant
transformation and literature review. Anal Cell Pathol (Amst).
17(8827056)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ma H, Wang Y, Chen P, Zhang Z and Xu J:
Pulmonary papillary adenoma with malignant transformation: Report
of one case and review of the literature. Int J Clin Exp Pathol.
13:792–798. 2020.PubMed/NCBI
|
|
23
|
Stojšić J, Popović M, Pezzuto F and
Marković J: Massive relief: Papillary adenoma of the lung in
asymptomatic former smoker patient. Diagnostics (Basel).
10(906)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhou P, Yu W, Wang L, Xia Q and Chen K:
Retrospective study of clinical and pathologic features of
pulmonary papillary adenoma: A rare tumor and 15 cases report.
Medicine (Baltimore). 99(e23066)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gorostiaga I, Martinez-Aracil A, Catón B
and Perez-Rodriguez A: Central papillary adenoma of the lung
diagnosed in a bronchoscopy-guided FNA: Cytological and
histological characterization of this rare entity. Rev Esp Patol.
54:206–210. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu P, Feng J, Yang M, Chen J, Fu L and Lu
J: Pulmonary papillary adenoma with malignant potential: A case
report and literature review. Diagn Pathol. 17(81)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xue L, Luan Z, Liu Y, Zou S, Jiang J, Wu
N, Lu N and Lin D: Pulmonary metastasis of a papillary thyroid
carcinoma and primary lung adenocarcinoma: Two coincident
carcinomas at the same location. Diagn Pathol. 8(26)2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zheng Q, Zhou J, Li G, Man S, Lin Z, Wang
T, Chen B and Lin F: Pulmonary sclerosing pneumocytoma: Clinical
features and prognosis. World J Surg Oncol. 20(140)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shirsat H, Zhou F, Chang JC, Rekhtman N,
Saqi A, Argyropoulos K, Azour L, Simms A, Melamed J, Hung YP, et
al: Bronchiolar Adenoma/Pulmonary ciliated muconodular papillary
tumor. Am J Clin Pathol. 155:832–844. 2021.PubMed/NCBI View Article : Google Scholar
|