Introduction
Chronic kidney disease (CKD) is defined by a
decrease of glomerular filtration rate (eGFR) to <60 ml/min per
1.73 m2, which results in the loss of function due to
kidney damage (1). In addition, it
is a comorbidity that affects the world population with a
prevalence of 13.4% (1990-2021) (2). For >30 years, the main causes of
mortalities, morbidity and CKD risk factors worldwide, from birth
to 95 years of age, have been studied. Mortality due to diabetes
and CKD in 1990 ranked 14th and 18th, in 2019 ranked 8 and 9th and
in 2021 ranked 10th and 11th, respectively (2,3).
This shows that both CKD and diabetes have recently and
considerably advanced as causes of mortalities in the world
population, with the highest incidence in Latin America and the
Caribbean. Diabetes and kidney diseases have a negative effect on
life expectancy, resulting in a change in life expectancy that
represents a global loss of 0.1 years in life expectancy (2).
In Mexico, 11% of the population (~13 million
individuals) have CKD (4,5). The main cause of CKD is type II
diabetes mellitus, accounting for 12.8 million patients represent
30-50% of the CKD affected population (6). CKD is also triggered by arterial
hypertension (27.2%), glomerulonephritis (8.2%), type 1 diabetes
mellitus (3.9%), chronic tubulointerstitial nephritis (3.6%),
cystitis (3.1%) of patients and, to a lesser extent, hereditary,
autoimmune and obesity diseases (1,7).
During the progression of the disease, an inflammatory response is
generated, which triggers fibrosis, a tissue regeneration
mechanism. It is accompanied by infiltration of cells of the immune
response in renal tissue, synthesis and activation of
pro-inflammatory cytokines and activation of fibroblasts and the
consequent deposition of the extracellular matrix. Therefore, all
these mechanisms facilitate the advancement of CKD, concluding with
chronic renal failure (7).
Currently, drugs are used to treat patients with
CKD, such as angiotensin-converting enzyme inhibitors to treat as a
first-line antihypertensive therapy symptoms of hypertension in
coronary diseases and cardiovascular conditions (8,9).
However, in patients who cannot tolerate ACEI therapy due to an
ACEI-induced cough or angioneurotic edema, angiotensin II receptor
blockers (ARB) therapy is appropriate and suggested as an
alternative to treat hypertension, congestive heart failure and
diabetic nephropathy (8,10), angiotensin II (ATII) is the
principal vasoactive peptide in the renin-angiotensin-aldosterone
system and acts on two receptors, angiotensin 1 and 2 receptors
(AT1 and AT2). ATII activation of AT1 receptors increase blood
pressure due to contraction of vascular smooth muscle, increase
systemic vascular resistance, increase sympathetic activity, sodium
(Na), and water retention due to increase Na+
reabsorption in the proximal convoluted tubule. Sodium reabsorption
in the proximal convoluted tubule is a direct result of ATII and
indirectly by increased aldosterone production in the adrenal
cortex, promoting distal Na reabsorption (11). Chronically high levels of ATII
cause smooth muscle and cardiac muscle cell growth and
proliferation, endothelial dysfunction, platelet aggregation,
enhanced inflammatory responses, and mediation of apoptosis. On the
other hand, the effects of ATII binding to AT2 receptors result in
vasodilatation due to increased production of nitrous oxide and
bradykinin (8). Furthermore,
activation of AT2 receptors leads to renal sodium excretion.
Agonism at AT2 receptors have anti-proliferative and cardiovascular
protective effects (10); β
adrenergic receptor blockers in patients with hypertension and CKD
(9,12); statins, which decrease cholesterol
in patients with coronary diseases and in early stages of CKD block
the formation of atherosclerosis and the onset of hypertension
(9); xanthine oxidase inhibitors,
which reduce levels of uric acid in serum (9); and antidiabetics [for example,
sulfonylureas (depolarization of Ca+2 channels in
pancreatic β cells, secretagogue effect)] (13); dipeptidyl-peptidase 4 receptor
inhibitors (resisting oxidation, inflammation, and fibrosis,
destroying the advanced glycation end product (AGE)-RAGE signaling
pathway and raising the levels of GLP-1, thereby improving
endothelial dysfunction and providing multi-level kidney
protection) (14); sodium-glucose
cotransporter 2 inhibitor (reduces hyperglycemia in patients with
type 2 diabetes by reducing the renal reabsorption of glucose,
thereby increasing urinary glucose excretion) (15); α-glucosidase inhibitor (reversibly
inhibits intestinal alpha-glucosidases, enzymes responsible for the
metabolism of complex carbohydrates into absorbable monosaccharide
units). This action results in a diminished and delayed rise in
blood glucose following a meal) (16); thiazolidinediones/glitazones
(peroxisome proliferator activated receptors (PPARs) agonists)
(affect nuclear receptors (PPAR) and subsequently enhance the
effects of insulin) (17,18), which decrease serum glucose
values.
Pioglitazone
(5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione;hydrochloride)
(19) belongs to the family of
thiazolidinediones/glitazones agonists of PPARγ and partial
activators of PPARα. It is widely used in the treatment of patients
with type II diabetes because it regulates genes involved in
adipogenesis, fatty acid oxidation and the increase in adiponectin
secretion by adipocytes (16,17).
Adiponectin activates muscle and hepatic AMPK, which stimulates
fatty acid oxidation, which indirectly improves insulin
sensitization related to obesity, increasing glucose uptake by
cells through GLUT4 translocation (20-23).
In recent decades PPARs have stood out as homeostatic modulators as
they act as transcription factors, which coordinate various renal
processes such as lipid and glucose metabolism, fatty acid
oxidation and inflammatory responses. Some findings have shown that
the deregulation of these receptors contributes to the progression
of some diseases such as diabetes and cancer (24).
PPAR family consists of PPARα, PPARβ/δ and PPARγ,
which regulate fatty acid oxidation, adipogenesis, lipogenesis,
glucose metabolism and insulin sensitivity (24). PPARs are widely distributed in the
kidney, particularly, PPARγ is found in the distal collecting ducts
and, in smaller amounts, they are also found in mesangial cells,
podocytes and endothelial cells (25). Likewise, PPARγ coordinates various
functions, such as adipocyte differentiation, lipid absorption and
storage, thermogenesis, lipogenesis, oxidative stress, glucose
absorption, and insulin signaling (24). PPARγ also participates in the
negative regulation of NF-κB, as studies have highlighted the
beneficial effect in the inflammatory and fibrotic process
(26,27). Pioglitazone, which acts by
stimulating PPARγ receptors, has been used in models of kidney
damage; Ko et al (28)
administered 10 mg/kg pioglitazone to diabetic nephropathy rats,
showed the downregulation of genes involved in fibrosis and
extracellular matrix deposition, such as TGF-β1, plasminogen
activator inhibitor-1 and type IV collagen, through the decrease of
NF-κB activity, MCP-1 and collagen synthesis. On the other hand,
Németh et al (26) proposed
that pioglitazone acts as a protective drug in renal fibrosis
induced by TGF-β by repressing the signaling pathways of EGR-1,
STAT3 and AP-1 in a model with knockout mice that expressed
increased levels of TGF-β. Similarly, Sun et al (29) demonstrated that pioglitazone
attenuates renal fibrosis by decreasing damage caused by ureteral
obstruction through the decrease in the expression of fibronectin,
α-SMA and collagen I.
The aim of the present study was to analyze the
anti-inflammatory and antifibrotic effects of pioglitazone in a rat
model of CKD in an adenine-induced. Adenine is a purine base and
high consumption or administration is immediately metabolized to
2,8-dihydroxyadenine (DHA) by xanthine oxidase (30,31).
2,8-DHA precipitates and forms crystals with low solubility
resulting in recurrent urolithiasis leads to secondary nephropathy
(32). The adenine model has been
widely used to generate kidney damage because its metabolic
alterations reproduce CKD characterized by crystalline deposits,
foreign body granulomas formation in the renal tubules and
interstitium, and increase fibrosis and inflammation, leading to
tubule-interstitial disease; these abnormalities are similar to the
symptoms of CKD in humans (32).
Most animal models do not mimic the complexity of the human
disease; however, the adenine model of CKD in rodents is an
exception (33).
Materials and methods
Animals and ethical approval
A total of 40 male Wistar rats between 150-250 g
(age, 6 weeks old), were obtained from the animal facility at the
Basic Sciences Center of the Autonomous University of
Aguascalientes (Aguascalientes, Mexico). The animals were kept in
light/dark cycles of 12 h, relative humidity and controlled
temperature of 25˚C. They were previously dewormed (fenbendazole 55
mg, toltrazuril 20 mg and praziquantel 10 mg) at a dose of 1 ml/kg,
intragastrical, for 3 days. Likewise, throughout the
experimentation, a diet based on Purina Rodent Chow Nutricubes
(cat. no H87 Nestlé, PurinaÒ, Mexico) and purified water were
provided with free access. All animal experiments were approved by
the Ethics Committee for the use of animals in teaching and
research at UAA (CEADI-UAA, UAA: Autonomous University of
Aguascalientes, AUT-B-C-1121-077-Tipo C; approval no.
CEADI-02-2023), following the Mexican Official Standard
NOM-062-ZOO-1999(34), and the
guidelines of the National Institutes of Health for the care and
use of Laboratory animals (35).
During the experiment, various signs related to humane endpoints
were monitored weekly, including stress, pain, decreased mobility,
withdrawal, weight loss, reduced food and water intake,
self-mutilation and behavioral changes such as aggressiveness.
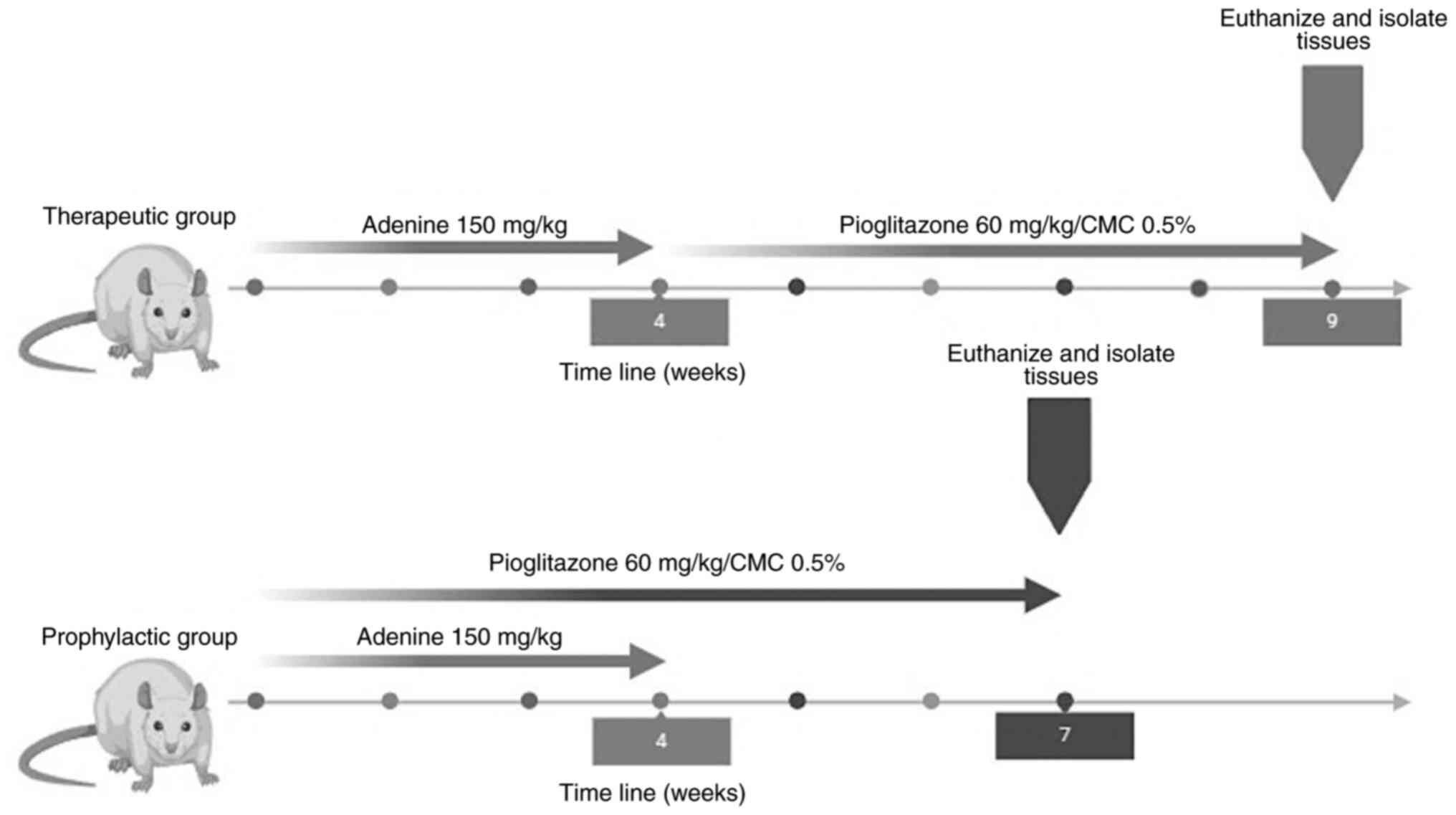
Experimental design
To evaluate the effects of pioglitazone in early and
late stages of CKD, two experimental groups were used.
i) The therapeutic group. The rats were
divided into three sub-groups (n=5): a) intact (healthy); b)
adenine (CKD); and c) adenine/pioglitazone (treatment). The
induction of CKD was performed by administering 150 mg/kg/day of
adenine (cat. no. A8626; Sigma-Aldrich; Merck KGaA) for 4 weeks,
the route of administration was oral and for this, a curved
stainless steel esophageal cannula (18x3"; Cadence Science) was
used. Once the induction was finished, the treatment was started,
which consisted of the administration of 60 mg/kg/day pioglitazone
(Pharmalife LTC) (36-38)
diluted in 0.5% carboxymethylcellulose for 5 weeks.
ii) The prophylactic group. The rats were
divided into five sub-groups (n=5): a) intact (healthy); b) adenine
(CKD); c) endogenous reversion (recovery without treatment); d)
adenine/pioglitazone (treatment); and e) pioglitazone. For the
prophylactic group, the induction of kidney damage (CKD) and the
administration of the treatment (pioglitazone) was performed
simultaneously, the induction time of CKD was 4 weeks, and the
treatment time was 7 weeks (Fig.
1).
At the end of the treatments, all rats were
euthanized with an overdose of sodium pentobarbital (≥100 mg/kg)
intraperitoneally, until rapid loss of consciousness, thus
minimizing stress and anxiety experienced by the animal, monitoring
respiratory and cardiac signs until their absence. During
euthanasia, renal tissue and blood samples were immediately
collected and processed, additionally, biomarkers of renal damage
and gene expression were analyzed. It is worth mentioning that the
group that was only administered pioglitazone was to evaluate the
toxicity of the drug. Euthanasia was carried out following accepted
animal euthanasia methods based on the guidelines of the American
Veterinary Medical Association (AVMA) (39). The blood collection was carried out
as a non-survival procedure.
Biomarkers of renal damage
By colorimetry in in the Dimension EXL-200 (Siemens
AG), the blood concentration of the metabolites urea, creatinine
and uric acid were determined. From the measurement of urea and
creatinine, the eGFR was calculated using the formula proposed by
Besseling et al (40).
Histopathological study
Hematoxylin-eosin (H&E) staining was performed
for evaluation of tissue damage and Masson's trichrome and Sirius
Red staining were performed for visualization of extracellular
matrix and collagen fibers. The kidneys were processed with
automatic tissue processers (Microm STP 120, Thermo Fisher
Scientific). Tissue sections were obtained at 5 µm thickness, using
a rotation microtome (Leica RM, 2125RT). The staining process
started with deparaffinization in a 60˚C laboratory oven for 1 h.
The tissues were placed in two xylol (100%), and alcohol (100 and
96%) solutions and transferred to distilled water. For hematoxylin
and eosin staining, rehydrated slides were immersed in hematoxylin
solution (cat. no HX9125853 Merck KGaA) at room temperature for 3
min, then rinsed briefly in tap water. Slides were then dipped in
acid alcohol for a few sec to remove excess hematoxylin and rinsed
with tap water. Counterstaining with eosin started with immersing
in eosin Y working solution (cat. no HX20198139 Merck KGaA) at room
temperature for 2.5 min and rinsing with tap water. Sirius Red
staining, the rehydrated slides were immersed in Weigert's
hematoxylin for 8 min and washed with tap water and PBS-1X. The
slides were placed at room temperature for 1 h in Picro-Sirius Red
Stain (0.5% Sirius red cat. no 2610-10-8 Sigma-Aldrich; Merck
KGaA), and excess dye was removed. Masson's trichrome staining, the
rehydrated slides were immersed at room temperature overnight in
Bouin´s fixative solution (formalin 10%). The slides were rinsed
briefly in tap water and distilled water. Subsequently, the slides
were incubated at room temperature; in Weigert's hematoxylin for 10
min (Sol. A 1% hematoxylin in 96% alcohol, Sol. B: 1.16 g iron
chloride + 1 ml 25% of hydrochloric acid in 99 ml distilled water.
In relation 1:1, sol A/sol B), Biebrich solution (1% Biebrich
scarlet in 1% acid fuchsin and 1 ml acetic acid) for 15 min,
phosphomolybdic 5% and phosphotungstic 5% in distilled water for 15
min and counterstain in aniline blue solution (5% blue aniline, 2
ml acetic acid glacial, 100 ml distilled water) for 10 min. The
slides were rinsed with 1% acetic acid in distilled water.
Finally, all the slides were performed by
transferring through a series of ethanol solutions (96 and 100%).
The slides were cleared in xylene and covered by Tissue-Tek (cat.
no HX90832861, Merck KGaA). Image analysis was performed through
the Axioscope 40/40 FL fluorescence microscope (Zeiss AG) and
processed with the Image ProPlus Software 4.5.1 (Media
Cybernetics).
Molecular biomarkers
Reverse transcription-quantitative PCR (RT-qPCR).
Total RNA extraction was performed using the Direct-zol™ RNA
MiniPrep kit (cat. no. R2050; Zymo Research Corp.) following the
manufacturer's specifications. The RNA was quantified using the
Biodrop (cat. no. 80-3006-51; Isogen Life Science B.V.) equipment
and subsequently the RNA was stored at -80˚C. For the cDNA
obtainment, reverse transcription was performed with 1 µg of RNA
using GoScript™ Reverse Transcription System (cat. no. A5000;
Promega Corp.). Subsequently, qPCR was performed using the Maxima
SYBR Green/ROX qPCR Master Mix (2X) (cat. no. K0221; Thermo Fisher
Scientific, Inc.), using StepOne™ Real-Time PCR Systems (Applied
Biosystems) to evaluate the relative expression of the genes:
Collagen 1, α-SMA and TGF-β. Thermocycling conditions were as
follows: 95˚C for 3 min for initial denaturation, 40 cycles of 95˚C
for 30 sec of denaturation, and 62˚C for 45 sec for annealing. The
oligonucleotide primers are shown in Table I. The relative expression levels
were normalized with respect to those of β-actin, and the
differences were determined by the 2-∆∆Cq method
(41).
 | Table IPrimer sequences for reverse
transcription-quantitative PCR. |
Table I
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer | Sequence
(5'-3') | pb | Amplicon (pb) |
|---|
| Col-1 | Fw |
AGGCATAAAGGGTCATCGTG | 20 | 157 |
| | Rv |
ACCGTTGAGTCCATCTTTGC | 20 | |
| ACTA-2 | Fw |
GCCAGTCGCCATCAGGAAC | 19 | 74 |
| | Rv |
CACACCAGAGCTGTGCTGTCTT | 22 | |
| TGF-β | Fw |
GACTCTCCACCTGCAAGACCA | 21 | 244 |
| | Rv |
CGGGTGACTTCTTTGGCGTA | 20 | |
| β-actin | Fw |
GTCGTACCACTGGCATTGTG | 20 | 175 |
| | Rv |
GCTGTGGTGAAGCTGTA | 20 | |
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8.0.2 software (Dotmatics). Differences mean ± standard error
of the mean between groups were assessed using non-parametric
multi-group analysis of variance with Kruskal-Wallis test with a
post hoc Dunn's test for multiple groups or Mann-Whitney U test for
two groups P<0.05 was considered to indicate a statistically
significant difference, n=3.
Results
Pioglitazone restores renal function
but does not promote regeneration of tubular architecture in a
therapeutic group
A macroscopic indicator of renal damage process is
the measurement of the kidney index. This index is calculated by
assessing the total weight of the animal with the weight of both
kidneys. Its purpose is to assess whether there are changes in
renal mass. Our results indicate that the kidney index in the
therapeutic group does not show significant differences with
respect to the intact, adenine and adenine/pioglitazone sub-groups
(Fig. 2A). On the other hand, the
kidney function tests evaluated by the serum measurement of
creatinine, urea, eGFR and uric acid (Fig. 2B-E), showed that creatinine is
lower in the adenine/pioglitazone sub-group (0.57 mg/dl) compared
with the adenine sub-group (1.05 mg/dl; P=0.414), and creatinine in
adenine/pioglitazone sub-group is similar to the intact sub-group
(0.40 mg/dl; P=0.939) (Fig. 2B).
By contrast, the amount of urea in the adenine/pioglitazone
sub-group (123.75 mg/dl) was similar to the adenine sub-group
(127.38 mg/dl; P>0.999) and to intact sub-group (59.52 mg/dl)
without significant difference (Fig.
2C). The eGFR was higher (1.06 ml/min) in relation to the
adenine (0.45 ml/min; P=0.539), indicating a possible restoration
of renal function. Moreover, a decrease in serum uric acid levels
was found in the adenine sub-group (1.58 mg/dl) compared with the
intact sub-group (2.41 mg/dl; P=0.529), while the levels in the
adenine/pioglitazone sub-group (3.61 mg/dl; P>0.999) were
similar to the intact sub-group (Fig.
2E).
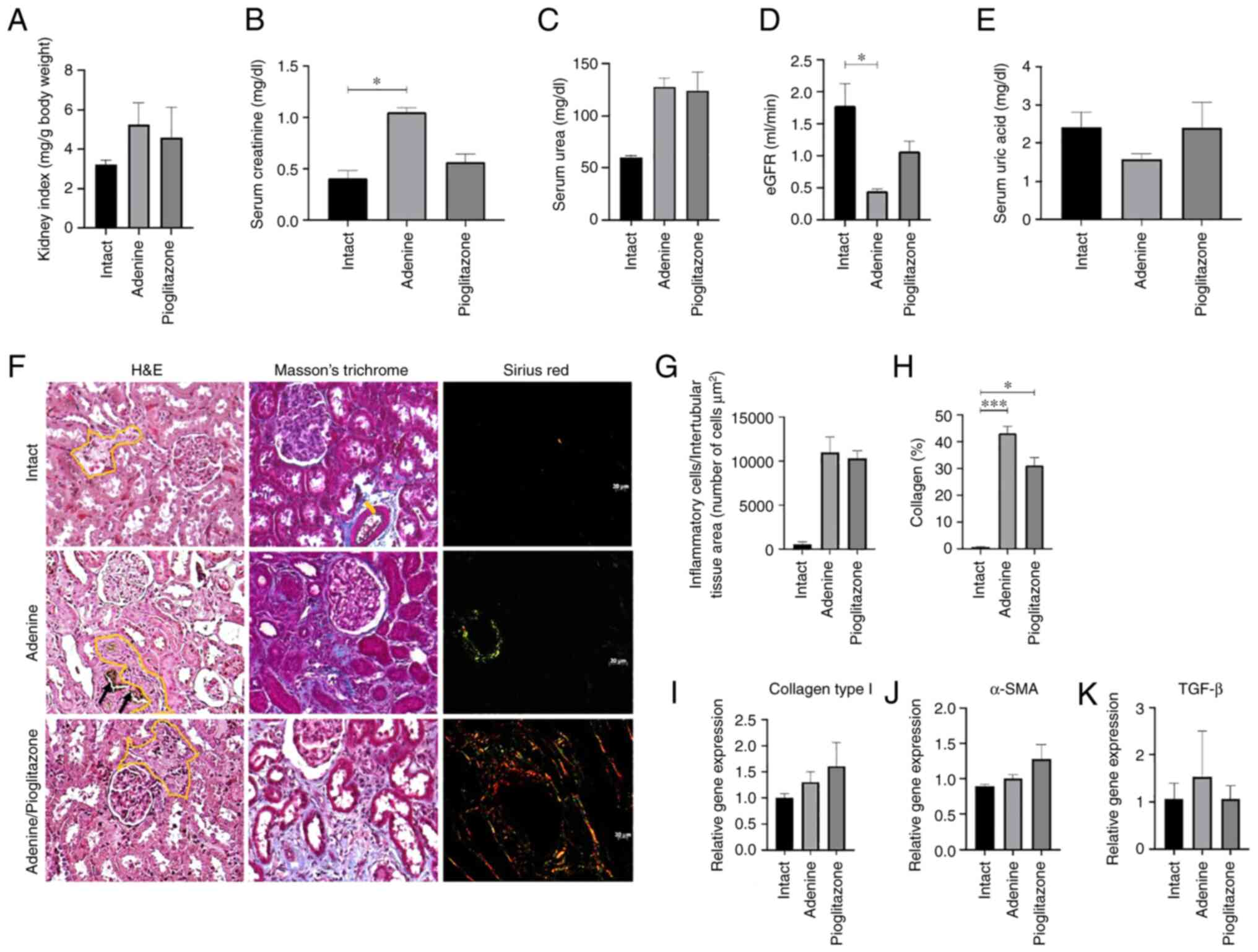 | Figure 2Pioglitazone restores renal function
by partially increasing eGFR and decreasing type 1 collagen
deposition in the therapeutic group. (A) Kidney index, with no
significant differences between the groups. (B-E) Biomarkers of
renal damage, measuring (B) creatinine, (C) blood urea, (D) eGFR
and (E) blood uric acid; eGFR was determined from the measurement
of creatinine and blood urea nitrogen. (F) Histopathological
analysis of renal tissues fixed in neutral formalin, stained with
hematoxylin/eosin, Masson's trichrome and Sirius red. Black arrows
indicate the deposition of 2,8-DHA crystals. Yellow line shows the
interstitial zone with inflammatory infiltrate. Magnification,
x200. (G) Quantification of inflammatory infiltrate cells in renal
interstitial zone. (H) Morphometric analysis of the amount of
collagen deposited in renal interstitial tissue by Masson's
trichrome stain. Expression of genes (I) collagen type 1, (J)
α-SMA, and (K) TGF-β. *P<0.05;
***P<0.001. eGFR, glomerular filtration rate. |
The histopathological analysis showed that
pioglitazone does not attenuate the inflammatory response, did not
decrease levels of cellular infiltration as abundant inflammatory
cells are observed in some glomeruli and the intertubular space of
the renal corticomedullary zone similar to what is observed in the
adenine sub-group (Fig. 2F and
G). The morphometric analysis of
Masson's staining shows a higher percentage of collagen fibers in
adenine-treated animals was markedly lower in pioglitazone-treated
animals (Fig. 2H). The analysis of
transcription levels of genes related to inflammation and fibrosis,
collagen type 1, α-SMA and TGF-β (Fig.
2I-K) did not show significant differences between the
sub-groups. However, TGF-β showed a lower level of expression in
adenine/pioglitazone sub-group compared with untreated animals.
Pioglitazone improves renal function
and attenuates the progression of damage in the prophylactic
group
The macroscopic analysis of the effect of
pioglitazone on the kidney is depicted in Fig. 3. The upper part of Fig. 3A shows kidney damage induced by
adenine in rats, with abundant deposits of 2,8-DHA crystals in the
cortico-medullary zone of the kidney. Conversely, the lower part of
Fig. 3A corresponds to a kidney
induced with adenine and treated with pioglitazone, demonstrating
the absence of abundant 2,8-DHA crystals. In the lower inset, the
presence of a crystal in the process of degradation in the renal
parenchyma is highlighted. On the other hand, the quantification of
2,8-DHA crystals in rats treated with adenine and
adenine/pioglitazone showed a higher number of crystals in
untreated rats compared to those treated (Fig. 3B). The rats treated with
pioglitazone had a lower kidney index, which was similar to the
intact sub-group (Fig. 3C).
Likewise, pioglitazone restores renal function, lowering serum
creatinine (0.704 mg/dl; P=0.895) and urea (66.34 mg/dl; P=0.023),
and concomitantly increases the eGFR (1.38 ml/min; P=0.0315) that
is mostly significant in relation to the adenine (0.452 ml/min) and
endogenous reversion (1.14 ml/min; P=0.2575). This reduction was
also found in the measurement of uric acid (2.06 mg/dl; P=0.044),
where it is significant in relation to the adenine (3.47 mg/dl)
(Fig. 3D-G). On the other hand,
the histopathological analysis demonstrated that pioglitazone
attenuated the inflammatory response and the cellular infiltration
by decreasing the number of inflammatory cells and deposition of
the extracellular matrix and collagen fibers, since the renal
morphology in adenine/pioglitazone sub-group is like that of the
intact sub-group (Fig. 3H).
Notably, inflammatory cells were counted per interstitial area of
kidneys treated with adenine and pioglitazone (Fig. 3I). Masson's morphometric staining
analysis showed a higher percentage of collagen fibers in the
adenine-treated animals while the percentage in the
pioglitazone-treated animals was significantly lower (Fig. 3J). This finding is also reflected
in the decrease of the expression of collagen type 1, α-SMA and
TGF-β (Fig. 3K-M). These effects
of pioglitazone were attributed to the drug administered and not
the recovery of the organism itself (endogenous reversion group),
since the results obtained in the endogenous reversion only reflect
the capacity of restoration of renal function, but not of the
regeneration of renal morphology (Fig.
3).
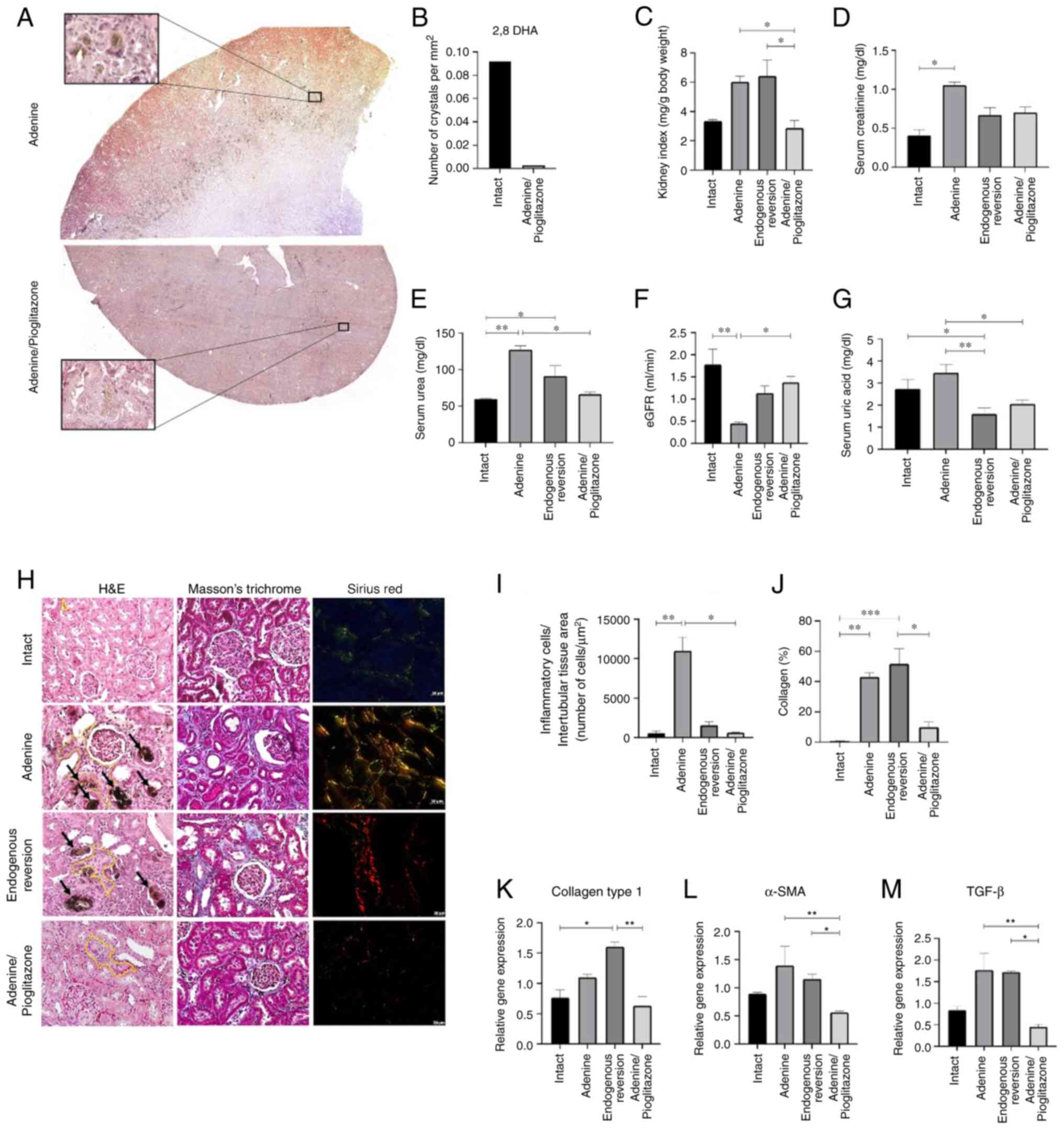 | Figure 3Pioglitazone reduces 2,8-DHA
crystals, restores renal function, and decreases inflammation and
fibrosis in the prophylactic group. (A) Panoramic images of kidneys
treated with adenine and adenine/pioglitazone increased
accumulation of 2,8-DHA crystals is observed in the
corticomedullary zone in the adenine group. Magnification, X5,
X400. (B) Quantification of 2,8-DHA crystals. (C) Kidney index.
Biomarkers of renal damage, measuring (D) creatinine, (E) blood
urea nitrogen, (F) eGFR and (G) uric acid; eGFR was determined from
the measurement of creatinine and blood urea nitrogen. (H)
Histopathological analysis of renal tissues fixed in neutral
formalin, stained with hematoxylin/eosin, Masson's trichrome and
Sirius red. Black arrows indicate the deposition of 2,8-DHA
crystals. Yellow line indicates the interstitial zone with
inflammatory infiltrate. Magnification, x200. (I) Quantification of
inflammatory infiltrate cells in renal interstitial zone. (J)
Morphometric analysis of the amount of collagen deposited in renal
interstitial tissue by Masson's trichrome stain. Expression of
genes (K) collagen type 1, (L) α-SMA and (M) TGF-β.
*P<0.05; **P<0.01;
***P<0.001. eGFR, glomerular filtration rate. |
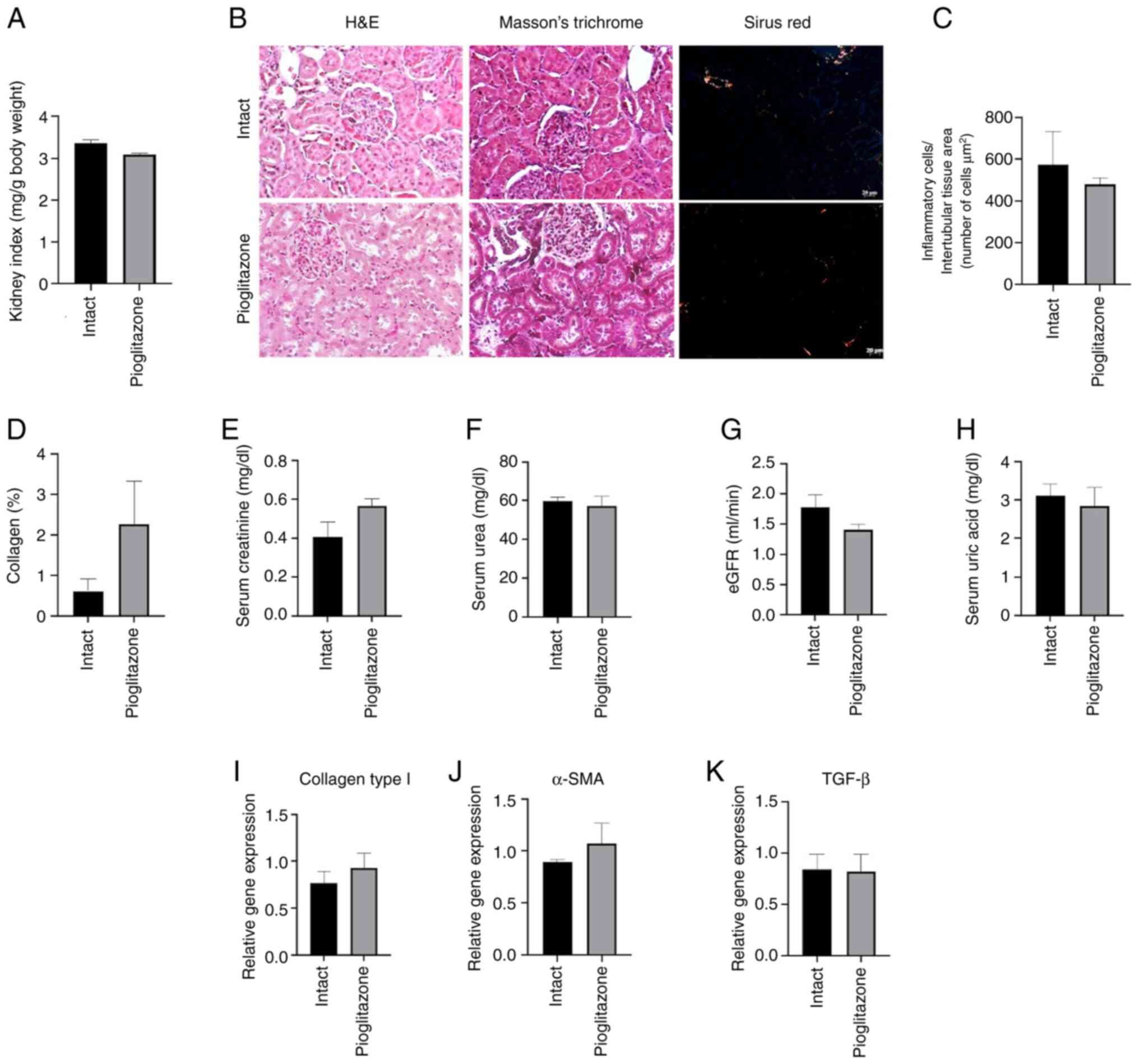
Pioglitazone does not show toxicity in
healthy kidneys at 7 weeks in a Wistar rat model
The renal function tests, the histopathological and
molecular analysis demonstrated that the administration of 60 mg/kg
of pioglitazone to healthy Wistar rats for 7 weeks did not generate
toxicity, since no significant differences were observed in
relation to the intact (Fig.
4).
Discussion
In tubulointerstitial injury, the cells commonly
involved are tubular epithelial cells, fibroblasts, fibrocytes,
myofibroblasts, monocytes, macrophages and mast cells. In addition,
molecular markers such as TGF-β, BMP, PDGF and HGF are involved
(42). Particularly, the increase
of TGF-β signaling correlates with the onset and progression of
fibrosis, as it promotes the activation of fibroblasts, synthesis
and expression of extracellular matrix proteins, such as collagen
(43). The mechanisms of tissue
repair and restoration in the presence of kidney damage trigger the
accumulation of dysfunctional connective tissue. The inflammatory
process and the increase of extracellular matrix impair the renal
parenchyma being a triggering mechanism of renal failure (44,45).
Some easy-to-use substances, such as neutral
electrolyzed saline, have been used to alleviate the renal
inflammatory/fibrotic process (46). Due to the high worldwide prevalence
of CKD and the poor effectiveness of current drugs in the
progression of the disease, it is for this reason that, in the
present study, the effects of pioglitazone as a potential treatment
of CKD were evaluated by repressing the inflammatory and fibrotic
processes triggered in two experimental groups of adenine-induced
renal damage (therapeutic and prophylactic groups). This was to
demonstrate the beneficial effects of the drug on the damage caused
by adenine. Studying two types of simultaneous comparative
experimental groups allowed the present study to identify the
pathophysiological mechanisms that lead to inflammation and
subsequent renal fibrosis (45)
and enabled the proposal of new drugs that counteract the damage in
early and late stage of CKD.
The results obtained from the therapeutic group of
the present study showed that pioglitazone did not manage to
reverse the damage in the renal tissue through the attenuation of
the inflammatory process and cellular infiltration, since,
according to the histopathological analysis in the Masson's
trichrome and Sirius red staining, the presence of extracellular
matrix and collagen fibers, predominantly type 1 and, were
observed. Likewise, the molecular analysis showed an increase in
the expression of collagen type 1, α-SMA and TGF-β of the rats
treated with pioglitazone. However, the renal function tests show a
marked improvement in the eGFR and creatinine. The eGFR is widely
accepted and used as a parameter that reflects renal function in
general (47) and can be
calculated from the concentrations of creatinine and urea.
Likewise, creatinine is a freely filtered molecule, so it is used
as a marker of renal function (48). Similar to creatinine, blood urea is
controlled by the eGFR since the concentration of this in the
ultrafiltrate is similar to that of plasma (49). The eGFR in the adenine sub-group
was low compared with the intact sub-group, indicating that renal
function decreased because of the induced damage. These findings
were also described by the authors Zhu et al (47), where they highlight the 57% renal
decrease after the administration of adenine in rats. Consistent
with this, the creatinine values were lower in the
adenine/pioglitazone sub-group with respect to the adenine
sub-group. Likewise, the sub-group treated with pioglitazone
presented with uremia at 5 weeks. As mentioned by Singh et
al (45), tubulointerstitial
fibrosis strongly correlates with renal function and represents a
complex change in the architecture of the kidney, which includes
the activation of proteases, production of MMP, collagen by
epithelial cells and activated myofibroblasts. However, the results
obtained in the present study show that, despite the interstitial
fibrosis generated by adenine, pioglitazone restores renal function
at 7 weeks by increasing the eGFR.
Consistent with this, the vasculoprotective
properties of pioglitazone have also been demonstrated in a
previous study, as it increases the expression of endothelial
nitric oxide synthase and neuronal nitric oxide synthase that
regulates glomerular blood flow and eGFR through the regulation of
vascular tone in the afferent arterioles (50). Unlike the therapeutic group, in the
present study, the results of the prophylactic group demonstrated
that pioglitazone at 7 weeks restored renal function and attenuated
inflammation, as the results of the biochemical analysis showed a
high value of eGFR compared with the adenine and endogenous
reversion animals. In adenine/pioglitazone sub-group the
histopathological analysis, a lower deposit of 2,8-DHA crystals was
observed during H&E staining, and a lower amount of type 1 and
3 collagen fibers were revealed using Sirius red staining compared
with the adenine and endogenous reversion. Likewise, the panoramic
images demonstrated the reduction of crystals in the
cortico-medullary zone of the group treated with pioglitazone
compared to the group treated with adenine.
According to Asplin et al (51) and Maalouf et al (52), by sensitizing insulin, pioglitazone
increases the pH of the urine and thus avoids the formation of uric
acid stones. The present study corroborated this with the results
obtained in the measurement of serum uric acid, where low values
were observed in the rats treated with pioglitazone compared with
the adenine and endogenous reversion groups. Similarly, the
molecular analysis corroborated that pioglitazone lowered the
extracellular matrix deposits and amount of collagen fibers
compared with adenine and endogenous reversion sub-groups, as it
showed the decrease of the expression of collagen type 1, α-SMA and
TGF-β.
This can be attributed to an inhibition of TGF-β
through two pathways: i) The signaling of TGF-β acts through the
SMAD pathway. The positive regulation of SMAD7 can suppress the
signaling of TGF-β-SMAD by activating the p65 subunit of NF-κB
(53). Studies have shown that
PPARγ agonists decrease the expression of TGF-β1 and the
phosphorylation of SMAD2/3 while increasing the expression of
SMAD7(54), as well as protein
Lefty-1(55). ii) The direct
inhibition of NF-κB through the PPARγ agonist. PPARγ act as an E3
ubiquitin ligase that interacts with the p65 subunit of NF-κB to
induce its ubiquitination and subsequent degradation, which
attenuates the inflammation caused by the NF-κB pathway (56). Pioglitazone, by inhibiting the
inflammatory pathway of NF-κB, attenuates the fibrotic process
(epithelial-mesenchymal transition and collagen deposit) (57). Also, the antioxidant properties of
pioglitazone have been revealed to increase the expression of SOD
levels and decrease the levels of MDA, which contributes to the
restoration of the antioxidant capacity (58) and cell regeneration.
Therefore, the results of the present suggested that
the administration of pioglitazone for 7 weeks in early stages
could slow down or prevent the progression of CKD, possibly due to
a pathway of cell regeneration that is increased by the metabolism
of lipids and carbohydrates (59).
This, in addition to improving the absorption of lipids and
carbohydrates through different pathways and mediating the
oxidative stress as aforementioned, under negative feedback of the
inflammatory pathway of NF-κB, could attenuate and/or reverse the
progression of interstitial fibrosis induced by adenine, as well as
restore the function of the renal parenchyma given by an increase
in eGFR.
In conclusion, in early stages of CKD, pioglitazone,
by stimulating the PPARγ pathway, counteracts the profibrotic and
inflammatory mechanisms triggered during the disease, improving
function and promoting the regeneration of renal morphology by
reducing the expression of TGF-β, α-SMA and type 1 collagen.
However, in the late phase of treatment, pioglitazone improved
renal function by increasing eGFR and slightly decreasing serum
creatinine.
Acknowledgements
Not applicable.
Funding
Funding: This research was funded by Autonomous University of
Aguascalientes (grant nos. PIBB22-10N, PIBB23-6) and National
Council for the Humanities, Science and Technology (grant no.
32029).
Availability of data of materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MPV, JVJ and SMH designed the article structure and
revised the manuscript. JMG, ABG and NGC made substantial
contributions to conception and design and wrote the manuscript.
JMG performed the literature review. MMO and ESA contributed to the
acquisition, analysis and interpretation of the data and prepared
the figures. MAB analyzed data. SMH and MAB confirm the
authenticity of all the raw data. All authors have read and
confirmed the final version of the manuscript.
Ethic approval and consent to
participate
All the experiments of this study were governed by
the NOM-062-ZOO-1999(34) (Mexican
standard) and were approved (approval no. CEADI-UAA-02-2023) by the
Institutional Bioethics Committee for the Management of Laboratory
Animals, which is based on the guidelines of the National
Institutes of Health for the care and use of Laboratory animals
(NIH publications no. 8023).
Patient consent for publication
Not applicable.
Competing of interests
The authors declare that they have no competing
interests.
References
|
1
|
Vaidya SR and Aeddula NR: Chronic Kidney
Disease. In: StatPearls. StatPearls Publishing, Treasure Island
(FL), 2023.
|
|
2
|
Naghavi M, Ong KL, Aali A, Ababneh HS,
Abate YH, Abbafati C, Abbasgholizadeh R, Abbasian M,
Abbasi-Kangevari M, Abbastabar H, et al: Global burden of 288
causes of death and life expectancy decomposition in 204 countries
and territories and 811 subnational locations, 1990-2021: A
systematic analysis for the Global Burden of Disease Study 2021.
Lancet. 403:2100–2132. 2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Murray CJL: The global burden of disease
study at 30 years. Nat Med. 28:2019–2026. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Colegio de Nefrólogos de México. Revista
nefrología Mexicana-colegio de nefrólogos de méxico. Nefrol Mex.
41(34)2020.
|
|
5
|
Liu BC, Lan HY and Lv LL: Renal fibrosis:
Mechanisms and therapies. Springer, Singapore, 2019.
|
|
6
|
Arreola-Guerra JM, Gutiérrez-Peña CM,
Zúñiga L, Ovalle-Robles I, García-Díaz AL, Macías-Guzmán MJ,
Delgado A, Macías D, Prado C, Vega A, et al: Enfermedad renal
Crónica en aguascalientes. ISEA México, 2019.
|
|
7
|
Awad AM, Saleh MA, Abu-Elsaad NM and
Ibrahim TM: Erlotinib can halt adenine induced nephrotoxicity in
mice through modulating ERK1/2, STAT3, p53 and apoptotic pathways.
Sci Rep. 10(11524)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Herman LL, Padala SA, Ahmed I and Bashir
K: Angiotensin-Converting Enzyme Inhibitors (ACEI). In: StatPearls.
StatPearls Publishing, Treasure Island (FL), 2024.
|
|
9
|
John M: Eisenberg Center for Clinical
Decisions and Communications Science: Medicamentos para la
enfermedad renal crónica en fase inicial. In: Las Guías Sumarias de
los Consumidores. Agency for Healthcare Research and Quality (US),
Rockville (MD), 2012.
|
|
10
|
Hill RD and Vaidya PN: Angiotensin II
Receptor Blockers (ARB). In: StatPearls. StatPearls Publishing,
Treasure Island (FL), 2024.
|
|
11
|
Crowley SD, Zhang J, Herrera M, Griffiths
R, Ruiz P and Coffman TM: Role of AT1 receptor-mediated salt
retention in angiotensin II-dependent hypertension. Am J Physiol
Renal Physiol. 301:F1124–F1130. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Brunton L, Knollmann B and Hilal-Dandan R:
Goodman and Gilman's the Pharmacological Basis of Therapeutics,
13th Edition. New York, 2017.
|
|
13
|
Niemi M, Kivistö KT, Backman JT and
Neuvonen PJ: Effect of rifampicin on the pharmacokinetics and
pharmacodynamics of glimepiride. Br J Clin Pharmacol. 50:591–595.
2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu J, Zhang J, Hou MH and Du WX: Clinical
efficacy of linagliptin combined with irbesartan in patients with
diabetic nephropathy. Pak J Med Sci. 38:52–56. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wanner C, Inzucchi SE, Lachin JM, Fitchett
D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC
and Zinman B: EMPA-REG OUTCOME Investigators. Empagliflozin and
progression of kidney disease in type 2 diabetes. N Engl J Med.
375:323–334. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Martin AE and Montgomery PA: Acarbose: An
alpha-glucosidase inhibitor. Am J Health Syst Pharm. 53:2277–2290.
1996.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lehmann JM, Moore LB, Smith-Oliver TA,
Wilkison WO, Willson TM and Kliewer SA: An antidiabetic
thiazolidinedione is a high affinity ligand for peroxisome
proliferator-activated receptor gamma (PPAR gamma). J Biol Chem.
270:12953–12956. 1995.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yamanouchi T: Concomitant therapy with
pioglitazone and insulin for the treatment of type 2 diabetes. Vasc
Health Risk Manag. 6:189–197. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
PubChem CID 60560 for Chemical Safety:
Pioglitazone Hydrochloride., 2024.
|
|
20
|
Ceddia RB, Somwar R, Maida A, Fang X,
Bikopoulos G and Sweeney G: Globular adiponectin increases GLUT4
translocation and glucose uptake but reduces glycogen synthesis in
rat skeletal muscle cells. Diabetologia. 48:132–139.
2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ho CC, Yang YS, Huang CN, Lo SC, Wang YH
and Kornelius E: The efficacy of pioglitazone for renal protection
in diabetic kidney disease. PLoS One. 17(e0264129)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kubota N, Terauchi Y, Kubota T, Kumagai H,
Itoh S, Satoh H, Yano W, Ogata H, Tokuyama K, Takamoto I, et al:
Pioglitazone ameliorates insulin resistance and diabetes by both
adiponectin-dependent and -independent pathways. J Biol Chem.
281:8748–8755. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yau H, Rivera K, Lomonaco R and Cusi K:
The future of thiazolidinedione therapy in the management of type 2
diabetes mellitus. Curr Diab Rep. 13:329–341. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Libby AE, Jones B, Lopez-Santiago I,
Rowland E and Levi M: Nuclear receptors in the kidney during health
and disease. Mol Aspects Med. 78(100935)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Platt C and Coward RJ: Peroxisome
proliferator activating receptor-γ and the podocyte. Nephrol Dial
Transplant. 32:423–433. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Németh Á, Mózes MM, Calvier L, Hansmann G
and Kökény G: The PPARγ agonist pioglitazone prevents TGF-β induced
renal fibrosis by repressing EGR-1 and STAT3. BMC Nephrol.
20(245)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kaplan J, Nowell M, Chima R and Zingarelli
B: Pioglitazone reduces inflammation through inhibition of NF-κB in
polymicrobial sepsis. Innate Immun. 20:519–528. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ko GJ, Kang YS, Han SY, Lee MH, Song HK,
Han KH, Kim HK, Han JY and Cha DR: Pioglitazone attenuates diabetic
nephropathy through an anti-inflammatory mechanism in type 2
diabetic rats. Nephrol Dial Transplant. 23:2750–2760.
2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sun L, Xu T, Chen Y, Qu W, Sun D, Song X,
Yuan Q and Yao L: Pioglitazone attenuates kidney fibrosis via
miR-21-5p modulation. Life Sci. 232(116609)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wyngaarden JB and Dunn JT:
8-Hydroxyadenine as the intermediate in the oxidation of adenine to
2,8-dihydroxyadenine by xanthine oxidase. Arch Biochem Biophys.
70:150–156. 1957.PubMed/NCBI View Article : Google Scholar
|
|
31
|
George J: Role of urate, xanthine oxidase
and the effects of allopurinol in vascular oxidative stress. Vasc
Health Risk Manag. 5:265–272. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Herlitz LC, D'Agati VD and Markowitz GS:
Crystalline nephropathies. Arch Pathol Lab Med. 136:713–720.
2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang Q, Su S, Luo N and Cao G:
Adenine-induced animal model of chronic kidney disease: Current
applications and future perspectives. Ren Fail.
46(2336128)2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Muñoz LIO: Norma Oficial Mexicana
NOM-062-ZOO-1999, especificaciones técnicas para la producción,
cuidado y uso de los animales de laboratorio., 2001.
|
|
35
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US), Washington (DC), 2011.
|
|
36
|
Center For Drug Evaluation and Research:
APPLICATION NUMBER: 21-073/S023., 2004.
|
|
37
|
Peng XH, Liang PY, Ou SJ and Zu XB:
Protective effect of pioglitazone on kidney injury in diabetic
rats. Asian Pac J Trop Med. 7:819–822. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Afraz S, Kamran A, Moazzami K, Nezami BG
and Dehpour AR: Protective effect of pharmacologic preconditioning
with pioglitazone on random-pattern skin flap in rat is mediated by
nitric oxide system. J Surg Res. 176:696–700. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Leary S, Pharmaceuticals F, Underwood W,
Anthony R, Cartner S, Johnson CL and Patterson-Kane E: AVMA
guidelines for the euthanasia of animals: 2020. Edition., 2020.
|
|
40
|
Besseling PJ, Pieters TT, Nguyen ITN, de
Bree PM, Willekes N, Dijk AH, Bovée DM, Hoorn EJ, Rookmaaker MB,
Gerritsen KG, et al: A plasma creatinine- and urea-based equation
to estimate glomerular filtration rate in rats. Am J Physiol Renal
Physiol. 320:F518–F524. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Farris AB and Colvin RB: Renal
interstitial fibrosis: Mechanisms and evaluation. Curr Opin Nephrol
Hypertens. 21:289–300. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Budi EH, Schaub JR, Decaris M, Turner S
and Derynck R: TGF-β as a driver of fibrosis: Physiological roles
and therapeutic opportunities. J Pathol. 254:358–373.
2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Nogueira A, Pires MJ and Oliveira PA:
Pathophysiological mechanisms of renal fibrosis: A review of animal
models and therapeutic strategies. In Vivo. 31:1–22.
2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Singh MP, Sharma C and Kang SC: Morin
hydrate attenuates adenine-induced renal fibrosis via targeting
cathepsin D signaling. Int Immunopharmacol.
90(107234)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Aurelien-Cabezas NS, Paz-Michel BA,
Jacinto-Cortes I, Delgado-Enciso OG, Montes-Galindo DA,
Cabrera-Licona A, Zaizar-Fregoso SA, Paz-Garcia J, Ceja-Espiritu G,
Melnikov V, et al: Protective effect of neutral electrolyzed saline
on Gentamicin-Induced nephrotoxicity: Evaluation of histopathologic
parameters in a murine model. Medicina (Kaunas).
59(397)2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhu CZ, Doyle KJ, Nikkel AL, Olsen L,
Namovic MT, Salte K, Widomski D, Su Z, Donnelly-Roberts DL,
Gopalakrishnan MM and McGaraughty S: Short-term oral gavage
administration of adenine induces a model of fibrotic kidney
disease in rats. J Pharmacol Toxicol Methods. 94:34–43.
2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Poulsen HE, Weimann A, Henriksen T, Kjær
LK, Larsen EL, Carlsson ER, Christensen CK, Brandslund I and Fenger
M: Oxidatively generated modifications to nucleic acids in vivo:
Measurement in urine and plasma. Free Radic Biol Med. 145:336–341.
2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Weiner ID, Mitch WE and Sands JM: Urea and
ammonia metabolism and the control of renal nitrogen excretion.
Clin J Am Soc Nephrol. 10:1444–1458. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kvandova M, Barancik M, Balis P, Puzserova
A, Majzunova M and Dovinova I: The peroxisome
proliferator-activated receptor gamma agonist pioglitazone improves
nitric oxide availability, renin-angiotensin system and aberrant
redox regulation in the kidney of pre-hypertensive rats. J Physiol
Pharmacol: 69, 2018 doi: 10.26402/jpp.2018.2.09. Epub 2018 Jul
4.
|
|
51
|
Asplin JR and Goldfarb DS: Effect of
thiazolidinedione therapy on the risk of uric acid stones. Kidney
Int. 95:1022–1024. 2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Maalouf NM, Poindexter JR, Adams-Huet B,
Moe OW and Sakhaee K: Increased production and reduced urinary
buffering of acid in uric acid stone formers is ameliorated by
pioglitazone. Kidney Int. 95:1262–1268. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Freudlsperger C, Bian Y, Contag Wise S,
Burnett J, Coupar J, Yang X, Chen Z and Van Waes C: TGF-β and NF-κB
signal pathway cross-talk is mediated through TAK1 and SMAD7 in a
subset of head and neck cancers. Oncogene. 32:1549–1559.
2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ni XX, Li XY, Wang Q and Hua J: Regulation
of peroxisome proliferator-activated receptor-gamma activity
affects the hepatic stellate cell activation and the progression of
NASH via TGF-β1/Smad signaling pathway. J Physiol Biochem.
77:35–45. 2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhang L, Xu C, Hu W, Wu P, Qin C and Zhang
J: Anti-inflammatory effects of Lefty-1 in renal tubulointerstitial
inflammation via regulation of the NF-κB pathway. Int J Mol Med.
41:1293–1304. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Hou Y, Moreau F and Chadee K: PPARγ is an
E3 ligase that induces the degradation of NFκB/p65. Nat Commun.
3(1300)2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang HB, Zhang Y, Chen C, Li YQ, Ma C and
Wang ZJ: Pioglitazone inhibits advanced glycation end
product-induced matrix metalloproteinases and apoptosis by
suppressing the activation of MAPK and NF-κB. Apoptosis.
21:1082–1093. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Sun L, Yuan Q, Xu T, Yao L, Feng J, Ma J,
Wang L, Lu C and Wang D: Pioglitazone, a peroxisome
proliferator-activated receptor γ agonist, ameliorates chronic
kidney disease by enhancing antioxidative capacity and attenuating
angiogenesis in the kidney of a 5/6 nephrectomized rat model. Cell
Physiol Biochem. 38:1831–1840. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Miyamae Y: Insights into dynamic mechanism
of ligand binding to peroxisome proliferator-activated receptor γ
toward potential pharmacological applications. Biol Pharm Bull.
44:1185–1195. 2021.PubMed/NCBI View Article : Google Scholar
|