Introduction
Among the numerous novel immunotherapies, immune
checkpoint inhibitors (ICIs) have emerged as critical treatments
for cancer. The two most studied ICIs, cytotoxic T
lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death
protein 1 (PD-1), serve roles in maintaining the balance between
peripheral tolerance and immune response under normal conditions as
co-inhibitory molecules to prevent over-activation of immune system
(1). Since the initial approval of
nivolumab and pembrolizumab by the U.S. Food and Drug
Administration, a number of ICIs have been approved in China such
as: PD-1 monoclonal antibodies (mAbs) including nivolumab,
pembrolizumab, tislelizumab, sintilimab, camrelizumab, toripalimab,
penpulimab, zimberelimab and serplulimab; programmed cell death 1
ligand 1 mAbs including durvalumab, atezolizumab and envafolimab;
and CTLA-4 mAbs including ipilimumab. The occurrence of
immune-related adverse events (irAEs) is linked to overactivation
of the immune system, which leads to changes in T-cell activity and
cytokine responses (2). It is
theorized that there are several mechanisms behind the development
of irAEs, including the presence of identical antigens in both
tumour and normal tissues, the activation of pre-existing humoral
autoimmunity in patients, heightened expression of the CTLA-4
antigen in normal tissues, other genetic factors and the microbiota
(3). Higher infiltration of
CD4+ T cells is reportedly more usually a result of
anti-CTLA-4 therapy, while CD8+ T cells are a result of
anti-PD-1 therapy (4).
In general, irAEs occur more frequently in the
dermatological, gastrointestinal (GI) and endocrine systems, with
GI tract irAEs accounting for 30-50% of all irAEs (2,5).
Most GI adverse events typically occur within 3 months of treatment
initiation. The incidence of GI toxicity is particularly high
during combination therapy with nivolumab and ipilimumab (6). Typical irAEs in the digestive system
include immune-mediated colitis (8-22%), hepatotoxicity (4-11%),
pancreatitis (10-15%) and non-specific symptoms such as nausea,
vomiting and diarrhoea (27-54%) (2). However, toxicities affecting the
upper GI tract, particularly peptic ulcers, have rarely been
reported.
The treatment choice for GI irAEs depends on the
specific disease presentation (7).
Supportive treatments such as anti-motility agents (including
atropine/diphenoxylate and loperamide) and dietary adjustments
(including low-fibre and bland diets) are recommended for grade 1
GI irAEs. In cases of grade 2 GI irAEs, it is crucial to rule out
infectious factors, and systemic corticosteroids (including
prednisone/intravenous methylprednisolone at 1-2 mg/kg/day) should
be initiated until symptoms improve to grade 1 or lower (8,9).
After the improvement of adverse events, it is recommended to
discontinue anti-CTLA-4 agents permanently. Whether anti-PD-1
agents can be resumed depends on their previous efficacy (10,11).
For grade 3-4 toxicities, administration of ICIs should be
immediately and permanently halted (10-13).
Alongside intravenous methylprednisolone, maintaining electrolyte
balance and implementing aggressive fluid resuscitation are
required for the management of grade 3-4 toxicities. These
strategies have been reported to lead to the regression of GI irAEs
in 40-70% of patients (14). For
patients experiencing grade 2-4 toxicities, it is essential to
assess response to corticosteroid treatment after 3 days of
administration to promptly identify patients with a poor response.
In cases where corticosteroids are ineffective, additional
treatment options include biological agents, such as infliximab and
vedolizumab, should be considered (15).
In the present case series, the diagnosis and
treatment of 3 patients with ICI-induced peptic ulcers at West
China Hospital (Chengdu, China) is presented. All patients were
diagnosed by GI endoscopy and treated with corticosteroids. Patient
follow-up was performed through outpatient visits or telephone
calls.
Case report
Materials and methods
The cancer tissue used was fixed with 4%
paraformaldehyde at 25˚C for 24 h and embedded with paraffin.
Embedded tissue was cut into 5 µm sections. Then the slides were
stained by hematoxylin for 5 min and eosin for 25 sec at 25˚C. The
stained slides were observed under light microscope.
Patient 1. In October 2018, a 60-year-old man
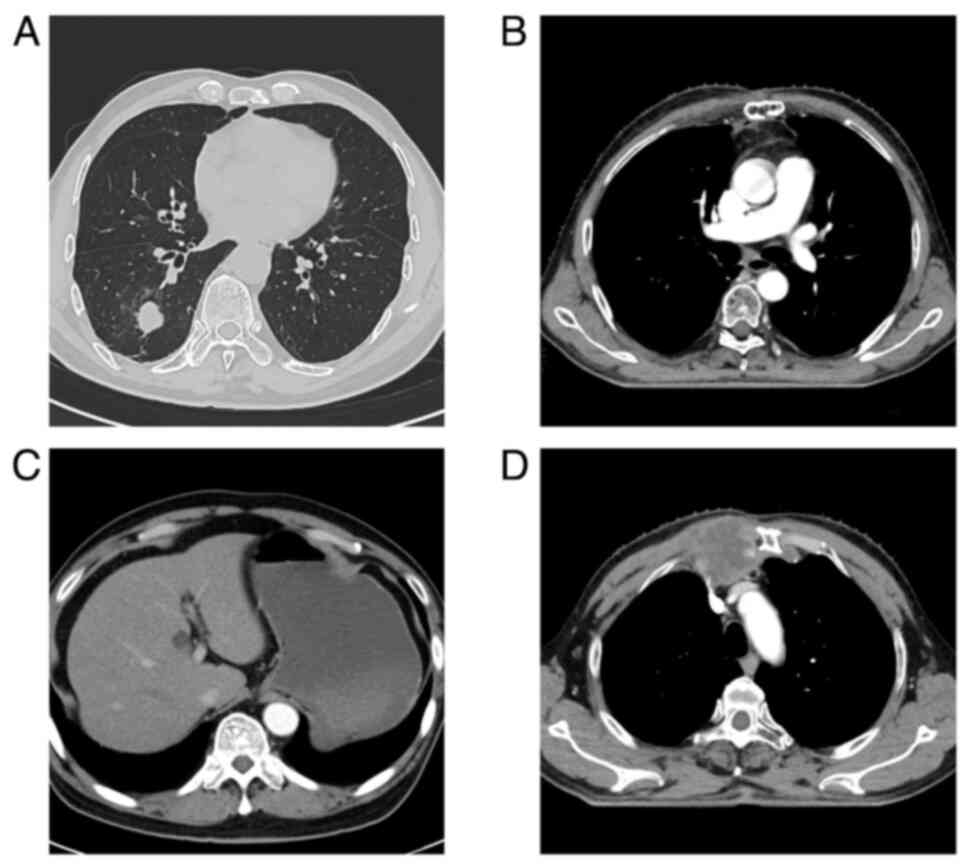
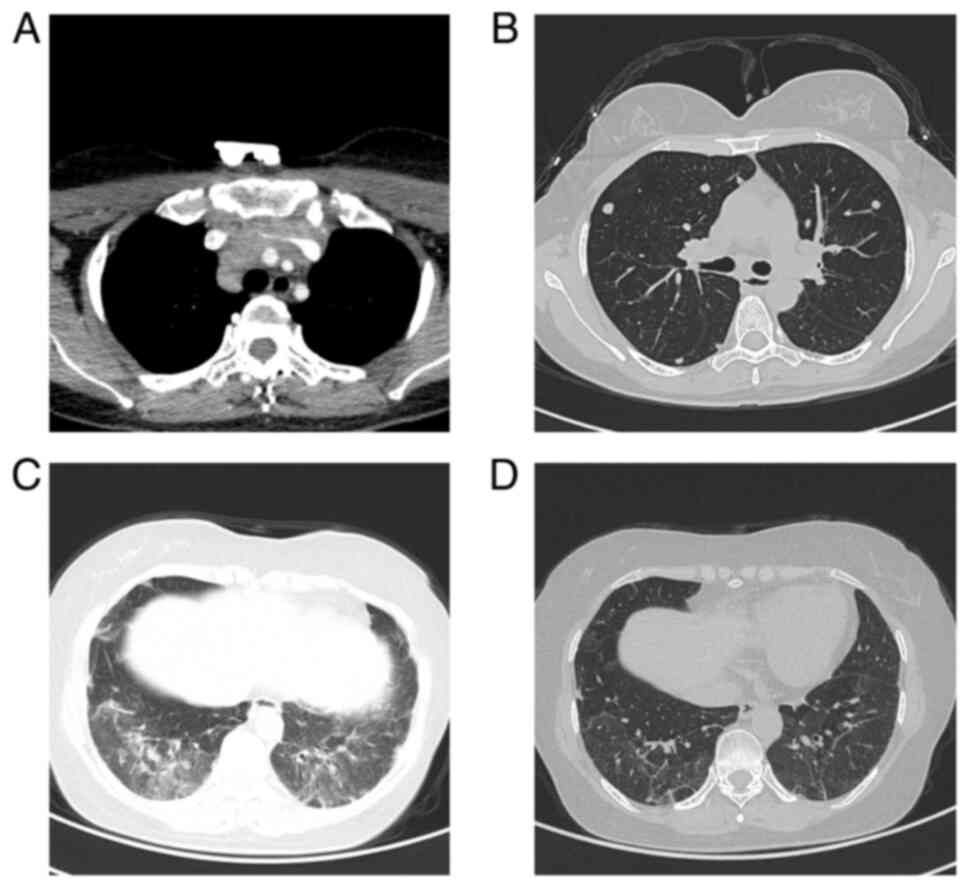
presented with a mass in the lower lobe of the right lung on CT
scan (Fig. 1A) at West China
Hospital (Chengdu, China). The patient underwent thoracoscopic
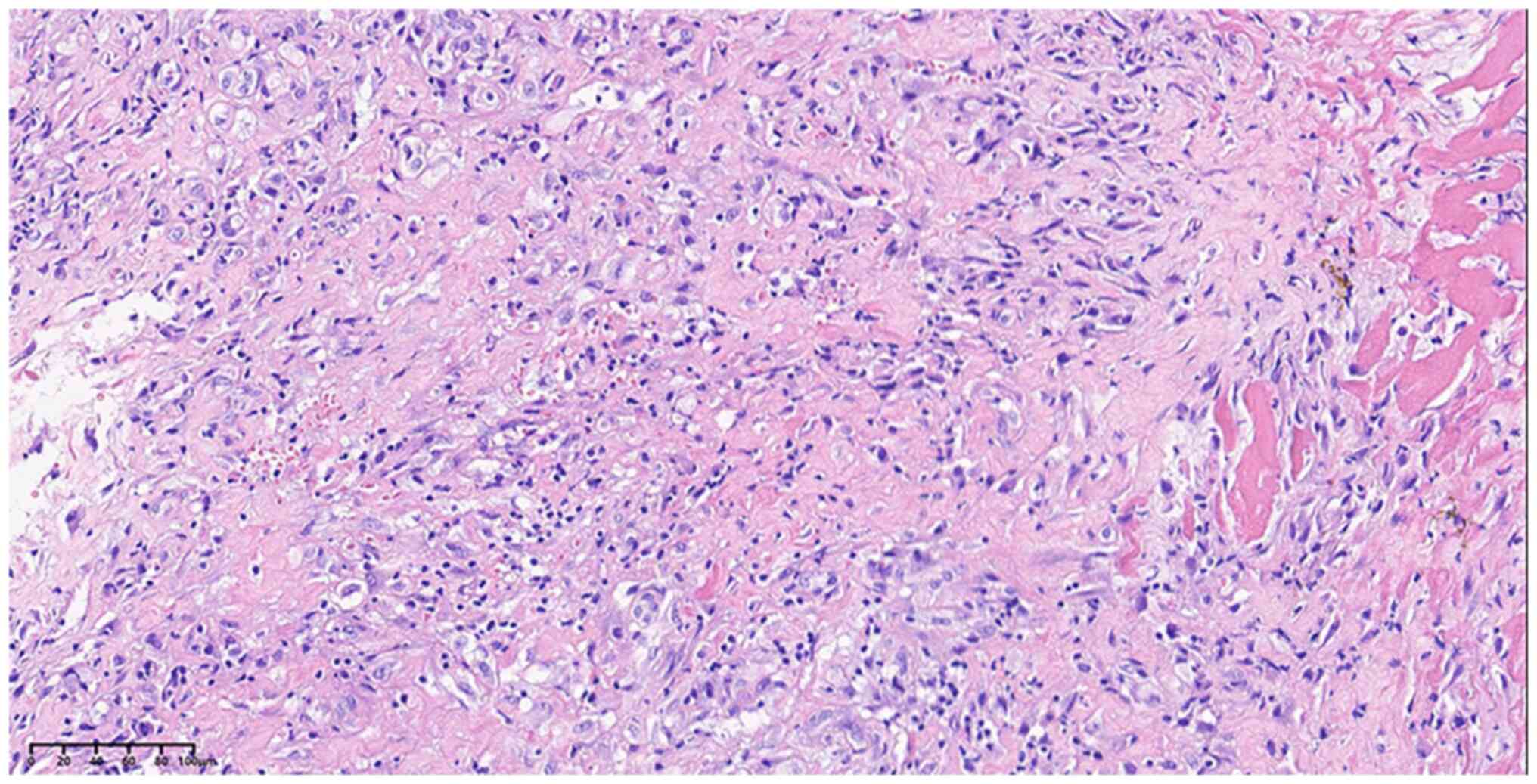
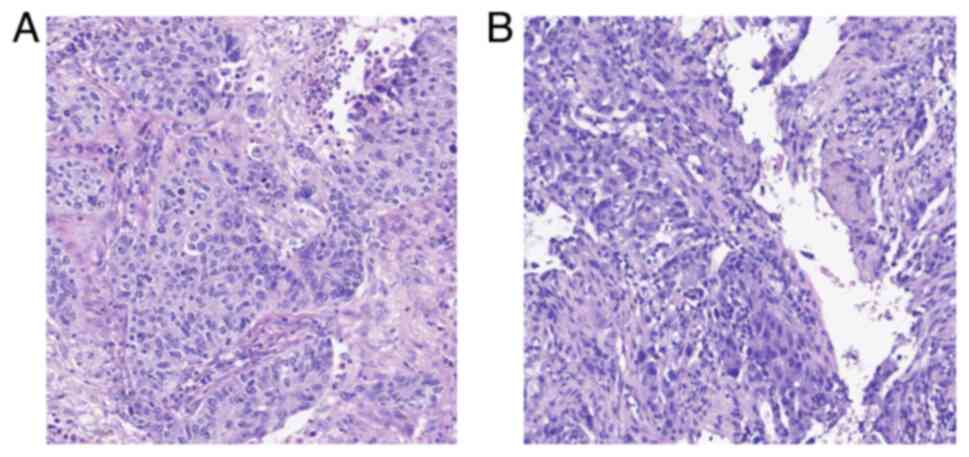
right lower pneumonectomy. The pathological result suggested
non-keratinised squamous cell carcinoma [pT1cN0M0; stage IA3, AJCC
8th edition (16)] (Fig. 2). At 1 year post surgery, a
mediastinal metastasis was discovered (Fig. 1B), prompting the administration of
three cycles of chemotherapy (paclitaxel albumin 440 mg every 21
days and carboplatin 400 mg/21 days), with immunotherapy
(pembrolizumab 200 mg every 21 days). Subsequently, the patient
received seven cycles of pembrolizumab (200 mg every 21 days) as
maintenance therapy. After the third cycle of maintenance therapy,
anlotinib hydrochloride (12 mg, days 1-14, every 21 days) was
introduced due to tumour progression. Subsequently, the patient
underwent incomplete excision of the mediastinal metastasis
followed by concurrent chemoradiotherapy (paclitaxel albumin 400 mg
every 21 days and carboplatin 380 mg every 21 days, with
radiotherapy administered for cardiophrenic angle lesions at a dose
of [45 Gy/25 fractions (f)]. Moreover, Tislelizumab (200 mg every
21 days) was initiated after the second cycle of chemotherapy upon
the discovery of liver metastasis (Fig. 1C). The patient underwent a total of
five cycles of chemotherapy and six cycles of tislelizumab.
Subsequently, sternal metastases were detected (Fig. 1D), leading to sternum radiotherapy
(30 Gy/10f), followed by six cycles of paclitaxel albumin (400 mg
every 21 days) and tislelizumab (200 mg every 21 days) as
maintenance therapy.
After completing the last treatment cycle, the
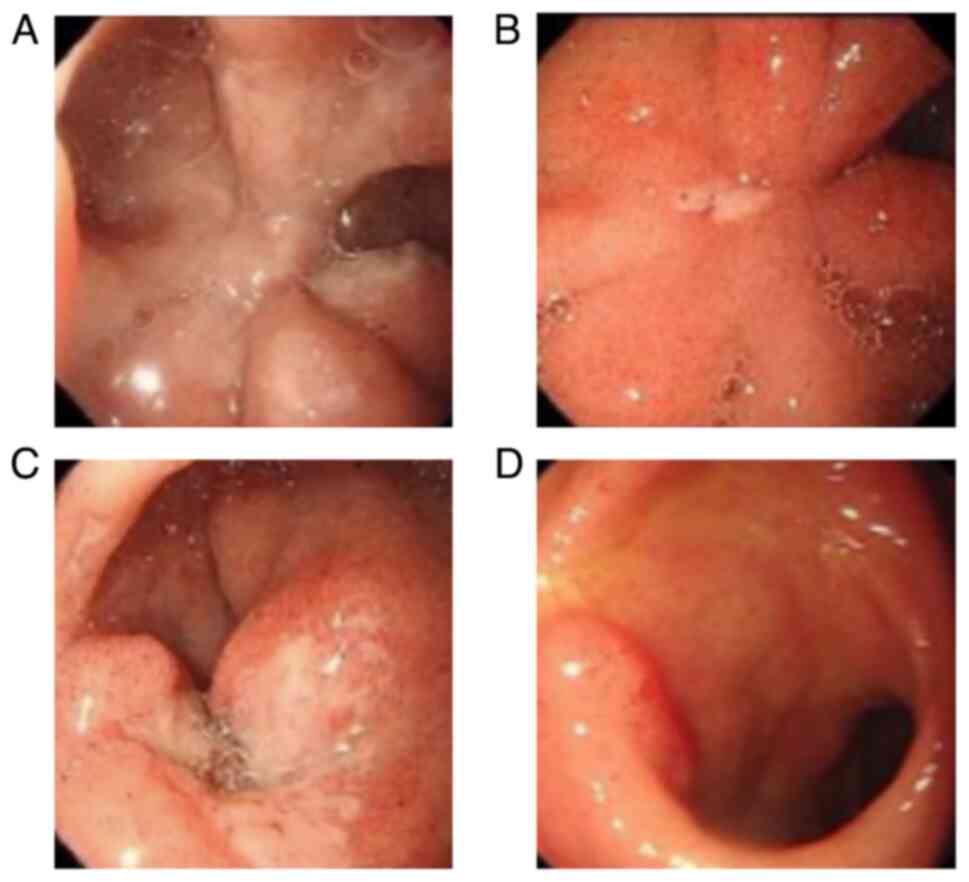
patient reported experiencing abdominal pain. GI endoscopy revealed
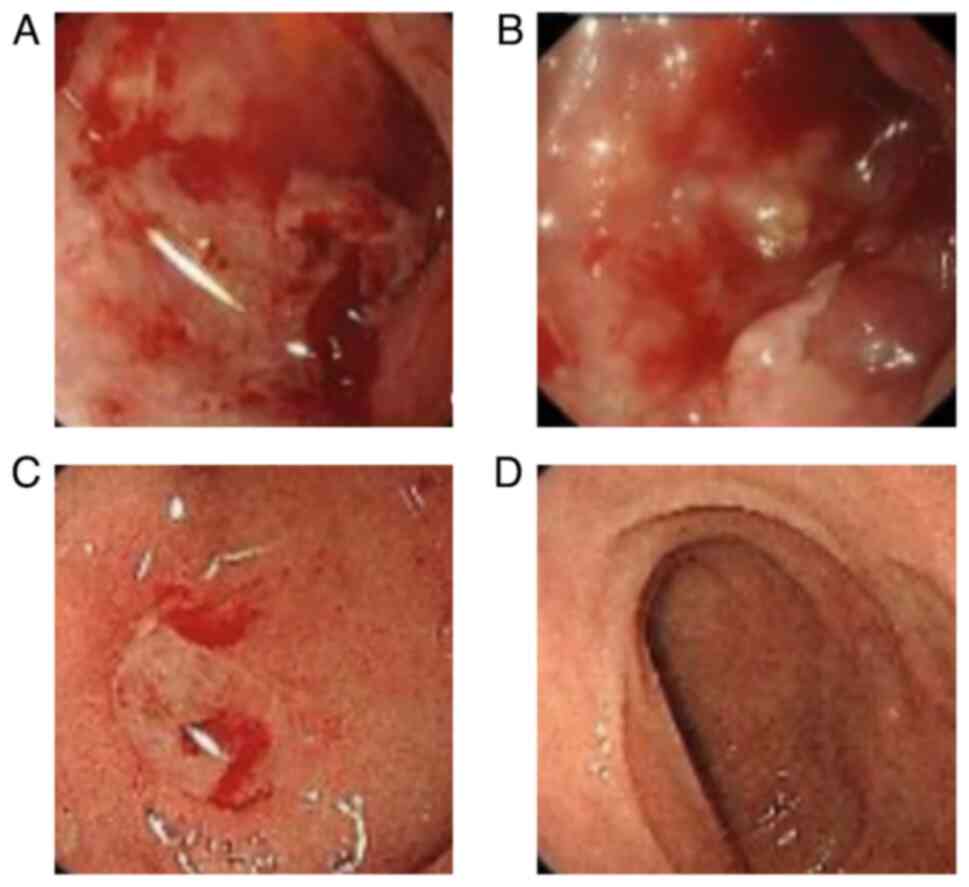
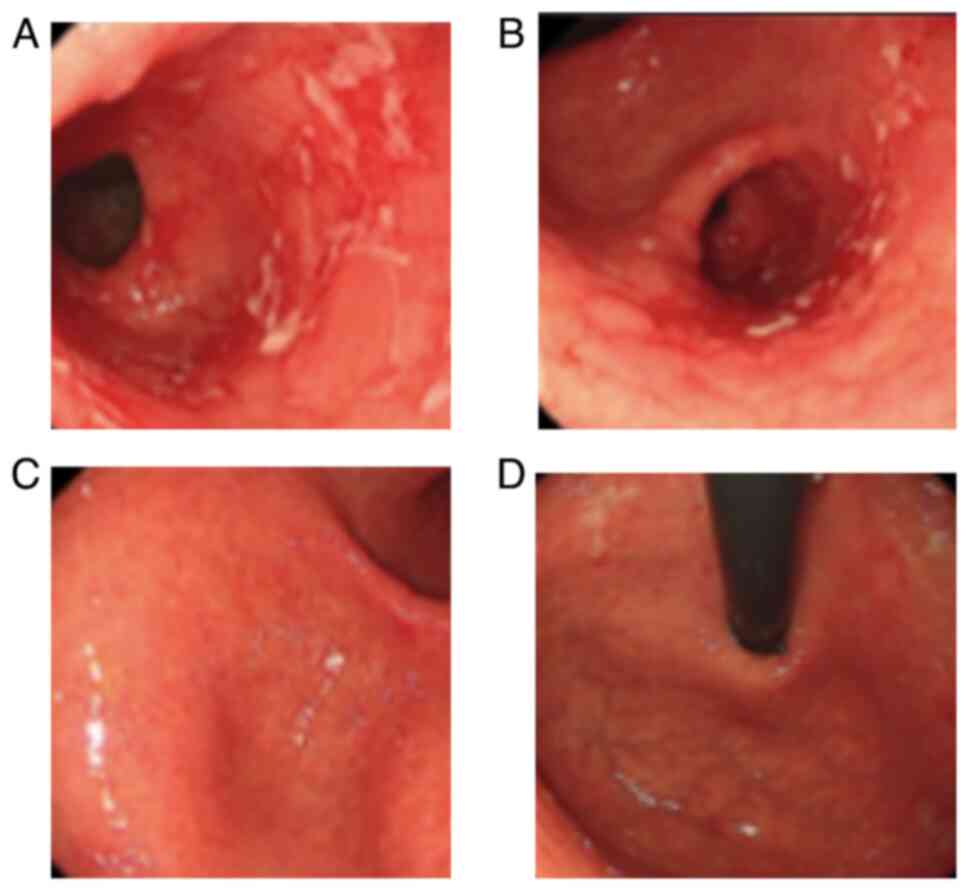
the presence of a duodenal bulbar ulcer (Fig. 3A). Helicobacter pylori (HP)
infection was negative and confirmed via the C14 breath test.
Neither non-steroidal anti-inflammatory drugs (NSAIDS) nor steroids
were prescribed to the patient, as administration of NSAIDS is also
a common cause of peptic ulcer and the treatment is different.
Given that other causes besides ICIs were deemed to not explain the
duodenal bulbar ulcer, the patient was diagnosed with an
ICI-induced duodenal bulbar ulcer. Treatment was initiated with a
daily administration of 40 mg methylprednisolone, 40 mg omeprazole
and teprenone 50 mg three times daily. After 1 month of treatment,
GI endoscopy revealed an improvement in the duodenal bulbar ulcer
(Fig. 3B). Then, 1 month later,
progression of the right lung metastasis was identified and
tislelizumab (200 mg every 21 days) was reintroduced for two
cycles. However, the patient experienced shoulder and chest pain
and GI endoscopy identified a new duodenal bulbar ulcer (Fig. 3C). Consequently, the patient was
treated with a daily administration of 40 mg methylprednisolone and
40 mg omeprazole. Following a 2-month-treatment period, the
duodenal ulcer healed (Fig. 3D).
Subsequently, the patient was administered oral afatinib (30 mg
every day) to control the tumor. The patient was then followed up
regularly at West China Hospital for 27 months until October 2023,
without disease progression.
Patient 2. A 52-year-old woman presented at
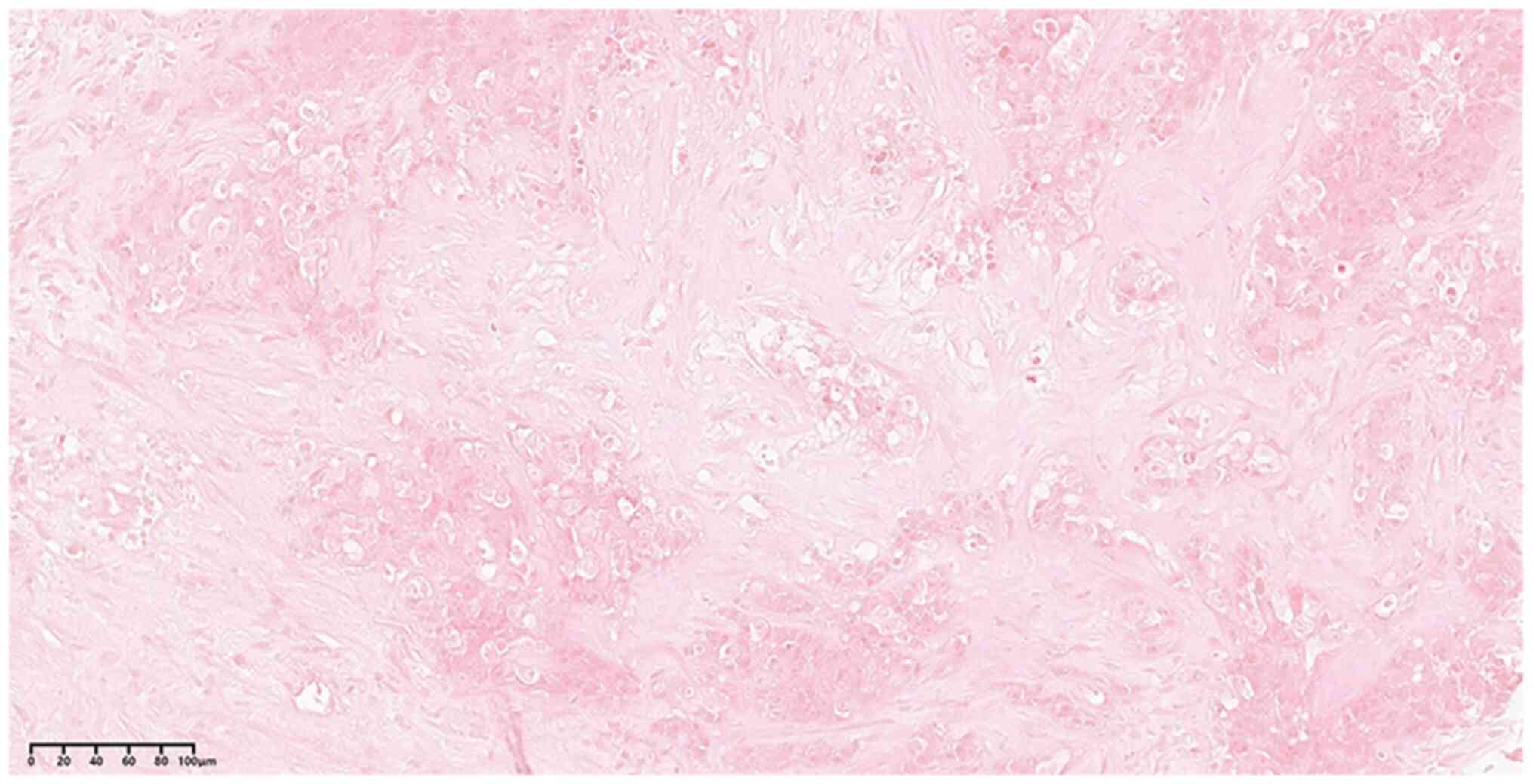
West China Hospital with a neck mass in November, 2016. Based on
cervical lymph node biopsy (Fig.
4) and CT scans (Fig. 5A and
B), the patient was diagnosed with
thymic squamous cell carcinoma with cervical lymph node and lung
metastasis [cT3N2M1; stage IV, AJCC 8th edition (16)]. The patient underwent five cycles
of chemotherapy (gemcitabine 1,600 mg every 21 days and cisplatin
40 mg days 1-3 every 21 days), resulting in partial remission (PR),
as determined by CT scan. Following chemotherapy, the patient
received apatinib (750 mg every day) as a maintenance therapy for
10 days. However, the patient experienced symptoms of headache,
fatigue and albuminuria, leading to the discontinuation of
apatinib. After 2 months, CT scan showed that the tumor was larger
than before. Then, the patient underwent six cycles of paclitaxel
(320 mg every 21 days) and carboplatin (600 mg every 21 days).
Subsequently, the patient was treated with sunitinib (37.5 mg every
day). However, due to intolerance to its side effects, the
treatment was changed to 27 cycles of pembrolizumab (200 mg every
21 days). After the sixth cycle, the patient was diagnosed with
ICI-related pneumonia (Fig. 5C and
D). This condition was
successfully treated with a 40-day course of prednisone (50 mg
every day). Moreover, the patient received radiotherapy for the
supraclavicular and mediastinal lymph nodes (5,040 cGy/28f). After
completing 27 cycles of pembrolizumab (200 mg every 21 days), the
patient developed nausea, vomiting, melena and upper abdominal
discomfort. The subsequent HP test was positive and GI endoscopy
revealed a duodenal bulbar ulcer (Fig.
6A). Consequently, the patient was diagnosed with an HP
infection and anti-HP therapy was initiated (amoxicillin 1,000 mg
twice every day, levofloxacin 500 mg once every day, bismuth
potassium cirtrate 300 mg 4 times every day, omeprazole 40 mg once
every day). However, despite 4-week-treatment, the symptoms and the
duodenal bulbar ulcers persisted (Fig.
6B). Given that neither NSAIDS nor steroids were prescribed and
the stool test for parasitic and bacterial infection showed
negative results, the patient was diagnosed with an ICI-induced
duodenal bulbar ulcer. After 3 days of 40 mg methylprednisolone
treatment daily, the symptoms subsided and the duodenal bulbar
ulcer was successfully healed, as confirmed by the GI endoscopy
results (Fig. 6C and D). The patient was followed up regularly
thereafter for 52 months until March, 2023 with no disease
progression.
Patient 3. A 68-year-old man presented at
West China Hospital with persistent cough and sputum production in
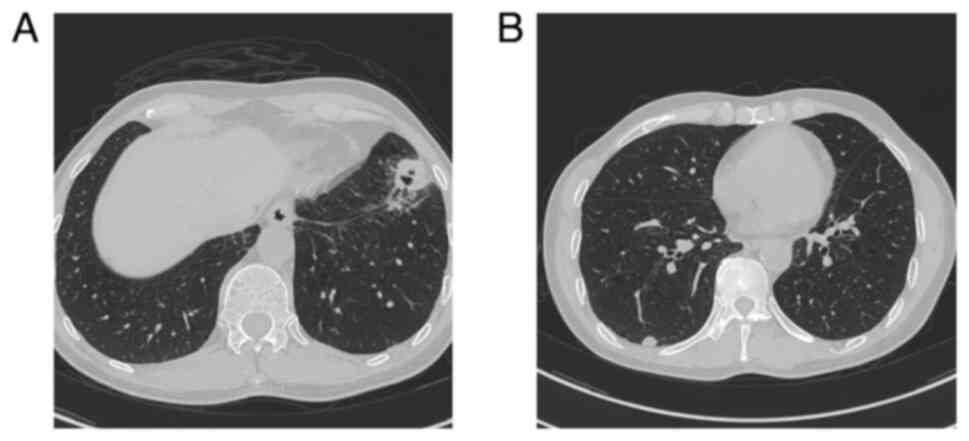
April, 2020. A CT scan identified a bilateral lung mass (Fig. 7). The patient was diagnosed with
poorly differentiated squamous carcinoma of the upper lobe of the
left lung via bronchofiberscopy biopsy (Fig. 8A) and poorly differentiated
adenocarcinoma of the lower lobe of the left lung through
percutaneous lung needle biopsy (Fig.
8B). The patient underwent six cycles of pemetrexed disodium
(860 mg every 21 days) and carboplatin (475 mg every 21 days) in
combination with pembrolizumab (200 mg every 21 days), followed by
five cycles of pemetrexed disodium (860 mg every 21 days) and
pembrolizumab (200 mg every 21 days) as maintenance therapy.
Throughout the treatment process, the CT scan consistently
indicated PR. Due to the slight enlargement of the pleural nodule,
the patient received local radiotherapy (24 Gy/3f). After 31 cycles
of maintenance therapy, the patient reported upper abdominal pain,
hiccups and weight loss. Despite a 2-month administration of
omeprazole (40 mg every day), the symptoms persisted, and GI
endoscopy identified a gastric ulcer (Fig. 9A and B). Neither NSAIDS nor steroids had been
prescribed to this patient. Consequently, the patient was diagnosed
with an ICI-induced gastric ulcer and was treated with
dexamethasone (11.25 mg every day). After 1 month, the patient
reported that the discomfort had subsided, and GI endoscopy
confirmed the disappearance of the gastric ulcer (Fig. 9C and D). Subsequently, the patient was followed
up for 18 months until March, 2024 and demonstrated consistent
PR.
Discussion
With the increasing utilization of ICIs, irAEs have
become more prevalent and have garnered heightened attention.
However, upper immunotherapy-related upper GI ulcers are rare and
there is currently no established standard treatment. Previously
reported cases are summarised in Table
I, in which 6 reported cases of ICI-induced peptic ulcers are
listed. Among them, 3 patients received glucocorticoids and
experienced symptomatic relief. The symptoms of 2 patients improved
after receiving infliximab therapy. However, 1 patient succumbed to
gastric ulcer perforation, despite receiving dexamethasone
treatment. Initially, this patient was diagnosed with
NSAIDS-related gastric ulcers due to recent daily NSAIDS use,
highlighting the importance of timely diagnosis of ICI-induced
peptic ulcers.
 | Table ISummary of previous cases of peptic
ulcers induced by ICIs. |
Table I
Summary of previous cases of peptic
ulcers induced by ICIs.
| First author/s,
year | Age/sex | Tumour type | ICI | Onset, months | Symptoms | Endoscopic
findings | Treatment | Outcome | (Refs.) |
|---|
| Collins et al,
2020 | 64/F | Lung
adenocarcinoma | Pembrolizumab | 24 | Peripheral edema,
ascites, bloating and diarrhoea | Loss of villi,
erythema, congestion and ulcers in the duodenum | Budesonide | Improved | (14) |
| Young et al,
2021 | 71/M | Colon
adenocarcinoma | Atezolizumab | / | Abdominal pain,
diarrhoea, haematochezia and haemorrhagic shock | Duodenal and jejunal
ulcers | Small bowel resection
+ infliximab | Improved | (28) |
| Trystram et
al, 2022 | 62/M | Melanoma | Ipilimumab +
nivolumab | 1.5 | Fever, frontal
syndrome, intestinal obstruction, and haemorrhagic shock | Jejunoileum ulcers
and Meckel diverticulum | Infliximab | Improved | (29) |
| Liu et al,
2022 | 49/F | Squamous cell
carcinoma of the tongue | Pembrolizumab | 6 | Epigastric abdominal
pain and decreased appetite | Diffuse mucosal
erythema at the cardia with exudate and ulcers at the antrum | Prednisolone +
pantoprazole | Improved | (30) |
| Kato et al,
2018 | 68/M | Lung squamous cell
carcinoma | Nivolumab | 17 | Melena and
anaemia | Gastric ulcer
bleeding at the antrum | Prednisone +
cyclophosphamide | Improved | (31) |
| Desai et al,
2019 | 74/M | Non-small cell lung
cancer | Nivolumab | 2 | Recurrent abdominal
pain, nausea and vomiting | Gastric ulcer and
cryptitis | Dexamethasone | Dead from
perforation of gastric ulcer | (32) |
Peptic ulcers can be induced by numerous factors,
including ICIs, radiotherapy, anti-angiogenesis-targeting drugs,
NSAIDS, steroids, HP infection, bacteria and parasites. Early
diagnosis of ICI-induced peptic ulcers is crucial as treatment
approaches for peptic ulcers vary depending on the underlying
cause. Usually, peptic ulcers may necessitate acid suppression and
stomach protection treatment. Ulcers induced by other drugs require
immediate discontinuation of the responsible medication followed by
acid suppression and stomach protection therapy. Ulcers caused by
pathogens require identification of the specific etiology and
targeted treatment. For instance, HP-related ulcers typically
require standard anti-HP therapy (17). Due to the unique mechanisms of
ICIs, ICI-induced ulcers require not only anti-acid treatment but
also prompt initiation of immunosuppressive therapy. Some
immunosuppressants, such as corticosteroids, are contraindicated in
ulcers caused by other factors, as they may cause secondary ulcers.
Compared with irAEs affecting other systems, initial symptoms of
irAEs affecting the digestive system, such as nausea, vomiting and
anaemia, may not be typical. This complexity arises from the
medical history of the patient with the symptoms often being
mistaken for chemotherapy side effects. Hence, the careful
consideration and differential diagnosis of ICI-induced ulcers pose
challenges and necessitates the extensive experience of the
clinicians, attentive listening to complaints from patients and
thorough knowledge of their medical history (18). In the present case series, it is
noteworthy that the 3 patients had not been previously exposed to
NSAIDs or steroids and stool test for enteric bacterial and
parasitic infections were negative. Moreover, the radiotherapy
fields of these 3 patients were distant from the GI tract. Despite
the positive HP test result for patient 2, the lesion and symptoms
did not ameliorate following anti-HP therapy. Notably, patient 2
had previously received anti-angiogenetic drugs, but the onset of
the duodenal bulbar ulcers occurred over a year after the
administration of these drugs and are therefore likely unrelated.
Standard peptic ulcer treatments, including acid inhibitory drugs,
anti-motility agents and dietary adjustments, failed to yield
improvement for all 3 patients. Consequently, all 3 patients were
diagnosed with ICI-induced upper GI ulcers.
The positive aspect of GI irAEs is their
relationship with longer overall survival times (OS) compared with
those without GI toxicity (19,20).
Moreover, previous study reported that high grade of diarrhoea was
associated with improved OS times (P=0.04). Similarly, patients who
experienced serious or recurrent irAEs exhibited longer OS times
compared with those with mild symptoms (28.5 vs. 13 months;
P=0.015) (21). Furthermore,
patients who encounter two simultaneous or sequential irAEs may
have more favourable clinical outcomes. For instance, patients
experiencing both colitis and a rash caused by immunotherapy had a
significantly improved OS time (28.6 vs. 19.9 months; P=0.018) and
progression-free survival time (16.1 vs. 3.2 months; P=0.001)
compared with those with colitis alone (22). The specific mechanisms underlying
irAEs and their prognoses remain elusive. However, previous
research indicates that interleukin 6 (IL-6) might serve a role in
elucidating the connection between irAEs and patient prognosis. A
previous study compared the RNA expression in colonic tissues from
patients with ICI-induced colitis and paired normal colonic tissue,
including patients ‘responding’ and ‘not-responding’ to ipilimumab
treatment, and it was reported that IL-6 expression was upregulated
in inflamed colon tissue (23).
Moreover, increased IL-6 expression was reported in patient with
‘non-responding’ tumours compared with patient with ‘responding’
tumours. IL-6 is a cytokine involved in the differentiation of
naïve CD4+ T cells into the T helper 17 (Th17) cell
lineage, which serves a role in the pathogenesis of several
autoimmune diseases, such as systemic lupus erythematosus and giant
cell arteritis (24). A
prospective study of 140 patients indicated that a lower baseline
IL-6 serum level was an independent risk factor for irAEs (odds
ratio=2.84; 95% confidence interval, 1.34-6.03; P=0.007) (25). A previous study indicated that
blocking IL-6 expression was associated with decreased tumour size,
a higher density of CD4+/CD8+ effector T
cells and reduced infiltration of Th17 cells, macrophages and
myeloid cells in murine models (26). Furthermore, a retrospective study
that analysed 92 patients who received therapeutic anti-IL-6R
antibodies (tocilizumab or sarilumab) indicated that IL-6 blockade
may be a promising method to treat a number of irAEs without
reducing antitumour immunity (27). In addition, two phase II clinical
studies assessing the safety and efficacy of tocilizumab in
combination with ICIs are ongoing (NCT04940299 and
NCT03999749).
The present case series only discussed 3 patients
with ICI-induced peptic ulcers and did not further evaluate the
mechanisms underlying ulcer development, indicating certain
limitations of the study. However, ICI-induced peptic ulcers are
relatively rare among GI irAEs and are not easily identified at an
early stage. In the present study, all 3 patients were diagnosed at
an early stage and demonstrated improvement after treatment,
without any effect on treatment efficacy of tumor. This aspect of
the present study provides some level of innovation.
By reporting these cases, the aim of the present
case series was to contribute to the knowledge base and provide
valuable insights for other physicians involved in the diagnosis
and treatment of peptic ulcers. Similar literature reports and
listed symptoms, treatment methods and prognosis were also
summarised to collate the relevant information in the literature.
In the present study, 3 patients diagnosed with ICI-induced peptic
ulcers through GI endoscopy effectively treated with
corticosteroids are presented. Peptic ulcer is a rare adverse event
of ICI and can lead to serious complications, underscoring the
importance of early diagnosis, accurate assessment and timely
intervention.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Author's contributions
Conceptualisation, supervision and project
administration were performed by SZ. Methodology, resources and
data curation were performed by MY. Investigation, data validation
and visualization were performed by QW. The manuscript draft was
prepared by QW and edited by SZ. QW and MY confirm the authenticity
of all the raw data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Informed consent for treatment information and
results of examination were obtained from all participants involved
in the study. Written informed consent was obtained from the
patients for the publication of this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Som A, Mandaliya R, Alsaadi D, Farshidpour
M, Charabaty A, Malhotra N and Mattar MC: Immune checkpoint
inhibitor-induced colitis: A comprehensive review. World J Clin
Cases. 7:405–418. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Durrechou Q, Domblides C, Sionneau B,
Lefort F, Quivy A, Ravaud A, Gross-Goupil M and Daste A: Management
of immune checkpoint inhibitor toxicities. Cancer Manag Res.
12:9139–9158. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Coutzac C, Adam J, Soularue E, Collins M,
Racine A, Mussini C, Boselli L, Kamsukom N, Mateus C and Charrier
M: Colon immune-related adverse events: Anti-CTLA-4 and Anti-PD-1
blockade induce distinct immunopathological entities. J Crohns
Colitis. 11:1238–1246. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Darnell EP, Mooradian MJ, Baruch EN,
Yilmaz M and Reynolds KL: Immune-related adverse events (irAEs):
Diagnosis, management, and clinical pearls. Curr Oncol Rep.
22(39)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shieh C, Chalikonda D, Block P, Shinn B
and Kistler CA: Gastrointestinal toxicities of immune checkpoint
inhibitors: A multicenter retrospective analysis. Ann
Gastroenterol. 34:46–52. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Thompson JA, Schneider BJ, Brahmer J,
Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M,
Dunnington D, et al: Management of immunotherapy-related
toxicities, version 1.2019. J Natl Compr Canc Netw. 17:255–289.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pernot S, Ramtohul T and Taieb J:
Checkpoint inhibitors and gastrointestinal immune-related adverse
events. Curr Opin Oncol. 28:264–268. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kähler KC, Hassel JC, Heinzerling L,
Loquai C, Mössner R, Ugurel S, Zimmer L and Gutzmer R: ‘Cutaneous
Side Effects’ Committee of the Work Group Dermatological Oncology
(ADO). Management of side effects of immune checkpoint blockade by
anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J
Dtsch Dermatol Ges. 14:662–681. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Puzanov I, Diab A, Abdallah K, Bingham CO
III, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR,
et al: Managing toxicities associated with immune checkpoint
inhibitors: Consensus recommendations from the society for
immunotherapy of cancer (SITC) toxicity management working group. J
Immunother Cancer. 5(95)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Haanen J, Carbonnel F, Robert C, Kerr KM,
Peters S, Larkin J and Jordan K: ESMO Guidelines Committee.
Management of toxicities from immunotherapy: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 28
(Suppl 4):iv119–iv142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Grover S, Rahma OE, Hashemi N and Lim RM:
Gastrointestinal and hepatic toxicities of checkpoint inhibitors:
Algorithms for management. Am Soc Clin Oncol Educ Book. 38:13–19.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Collins M, Soularue E, Marthey L and
Carbonnel F: Management of patients with immune checkpoint
inhibitor-induced enterocolitis: A systematic review. Clin
Gastroenterol Hepatol. 18:1393–1403.e1. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dougan M, Wang Y, Rubio-Tapia A and Lim
JK: AGA clinical practice update on diagnosis and management of
immune checkpoint inhibitor colitis and hepatitis: Expert review.
Gastroenterology. 160:1384–1393. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
American Joint Committee on Cancer (AJCC):
AJCC Cancer Staging Manual. 8th edition. Springer, Cham, p1032,
2017.
|
|
17
|
Kamada T, Satoh K, Itoh T, Ito M, Iwamoto
J, Okimoto T, Kanno T, Sugimoto M, Chiba T, Nomura S, et al:
Evidence-based clinical practice guidelines for peptic ulcer
disease 2020. J Gastroenterol. 56:303–322. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cheng Y, Ling F, Li J, Chen Y, Xu M, Li S
and Zhu L: An updated review of gastrointestinal toxicity induced
by PD-1 inhibitors: From mechanisms to management. Front Immunol.
14(1190850)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Abu-Sbeih H, Ali FS, Qiao W, Lu Y, Patel
S, Diab A and Wang Y: Immune checkpoint inhibitor-induced colitis
as a predictor of survival in metastatic melanoma. Cancer Immunol
Immunother. 68:553–561. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS,
Qiao W, Lum P, Raju G, Shuttlesworth G, Stroehlein J and Diab A:
Immune-checkpoint inhibitor-induced diarrhea and colitis in
patients with advanced malignancies: Retrospective review at MD
Anderson. J Immunother Cancer. 6(37)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tang L, Wang J, Lin N, Zhou Y, He W, Liu J
and Ma X: Immune checkpoint inhibitor-associated colitis: From
mechanism to management. Front Immunol. 12(800879)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Molina GE, Allen IM, Hughes MS, Zubiri L,
Lee H, Mooradian MJ, Reynolds KL, Dougan M and Chen ST: Prognostic
implications of co-occurring dermatologic and gastrointestinal
toxicity from immune checkpoint inhibition therapy for advanced
malignancies: A retrospective cohort study. J Am Acad Dermatol.
82:743–746. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Johnson DH, Hailemichael Y, Foo WC, Hess
KR, Haymaker CL, Wani KM, Lazar AJ, Saberian CM, Bentebibel SE,
Burton EM, et al: Interleukin-6 is potential target to de-couple
checkpoint inhibitor-induced colitis from antitumor immunity. J
Clin Oncol. 37(2616)2019.
|
|
24
|
Miossec P, Korn T and Kuchroo VK:
Interleukin-17 and type 17 helper T cells. N Engl J Med.
361:888–898. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Valpione S, Pasquali S, Campana LG, Piccin
L, Mocellin S, Pigozzo J and Chiarion-Sileni V: Sex and
interleukin-6 are prognostic factors for autoimmune toxicity
following treatment with anti-CTLA4 blockade. J Transl Med.
16(94)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hailemichael Y, Johnson DH, Abdel-Wahab N,
Foo WC, Bentebibel SE, Daher M, Haymaker C, Wani K, Saberian C,
Ogata D, et al: Interleukin-6 blockade abrogates immunotherapy
toxicity and promotes tumor immunity. Cancer Cell. 40:509–523.e6.
2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fa'ak F, Buni M, Falohun A, Lu H, Song J,
Johnson DH, Zobniw CM, Trinh VA, Awiwi MO, Tahon NH, et al:
Selective immune suppression using interleukin-6 receptor
inhibitors for management of immune-related adverse events. J
Immunother Cancer. 11(e006814)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Young K, Lin E, Chen E, Brinkerhoff B,
Scott G and Yu J: Small bowel hemorrhage from check point inhibitor
enteritis: A case report. BMC Gastroenterol. 21(345)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Trystram N, Laly P, Bertheau P, Baroudjian
B, Aparicio T and Gornet JM: Haemorrhagic shock secondary to a
diffuse ulcerative enteritis after Ipilimumab and Nivolumab
treatment for metastatic melanoma: A case report. Ann Palliat Med.
11:837–842. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu WT, Li YF and Hsieh TY: Unusual severe
gastritis and gastric ulcers caused by pembrolizumab. J Postgrad
Med. 68:38–40. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kato R, Hayashi H, Sano K, Handa K, Kumode
T, Ueda H, Okuno T, Kawakami H, Matsumura I, Kudo M and Nakagawa K:
Nivolumab-induced hemophilia A presenting as gastric ulcer bleeding
in a patient with NSCLC. J Thorac Oncol. 13:e239–e241.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Desai M, Chitnavis V and Grider D: Gastric
ulcer in a patient with metastatic lung cancer. Gastroenterology.
156:1572–1573. 2019.PubMed/NCBI View Article : Google Scholar
|