Introduction
Chronic myeloid leukemia (CML) is a type of
malignant tumor formed by the clonal proliferation of bone marrow
hematopoietic stem cells (1). A
majority of patients with CML exhibit slow disease progression and
remain asymptomatic in the early stages of disease (2). The pathogenesis of CML is attributed
to ectopic rearrangement of chromosomes 9 and 22, which produce an
abnormal BCR activator of RhoGEF and GTPase-ABL proto-oncogene
(BCR-ABL) fusion gene (3). This
fusion gene can produce an abnormal protein with tyrosine kinase
activity, which prompts increased cell proliferation and may lead
to CML pathogenesis (4).
Therefore, tyrosine kinase inhibitors (TKIs) targeting BCR-ABL have
exhibited high efficacy in CML treatment. Imatinib (IM) is the
first-line treatment for CML (4,5). The
use of IM and second-generation TKIs, such as nilotinib and
dasatinib, has considerably prolonged disease-free survival in
patients with CML (4,5). However, despite its excellent safety
profile, IM can cause certain adverse reactions, including edema,
nausea, vomiting, joint and muscle pain, rash, fatigue, abdominal
distension, diarrhea, muscle spasms, liver and kidney dysfunction,
bleeding, ascites and exfoliative dermatitis (6). Rare, but potentially adverse
reactions with a risk of morbidity, have also been reported. These
rare adverse effects include splenic rupture, severe rash, hair
repigmentation, bone marrow necrosis, acute fulminant hepatitis,
breast carcinoma and male gynecomastia (7-9).
The present study reports a case of IM-induced gynecomastia and
aims to discuss the therapeutic considerations in this unique
population.
Case report
A 48-year-old male patient was admitted to the
Department of Gastroenterology of The First Affiliated Hospital of
Jishou University (Jishou, China) in March 2016 with a complaint of
abdominal distension persisting for over 2 months. Physical
examinations showed splenomegaly with three transverse fingers
under the splenic ribs. Routine blood tests showed a white blood
cell count of 342.48x109/l (normal range,
~4.00-10.00x109/l), a hemoglobin count of 82 g/l (normal
range, 110-150 g/l), a platelet count of 242x109/l
(normal range, ~100-400x109/l) and a mean corpuscular
volume size of 104.90 fl (normal range, 80.00-100.00 fl). Abdominal
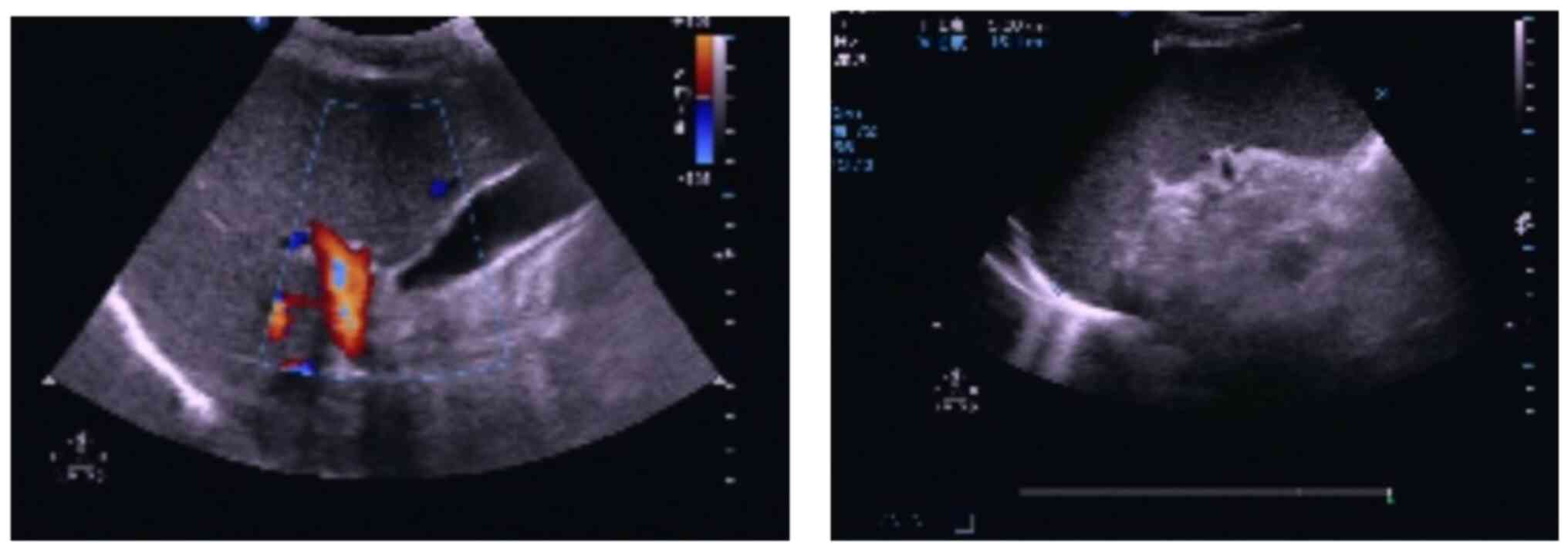
color ultrasound results demonstrated an enlarged spleen measuring
~164 mm in length and ~52 mm in thickness (Fig. 1). The patient was advised to
undergo treatment at the Department of Hematology of The First
Affiliated Hospital of Jishou University.
The patient had a history of hepatitis B and
hepatitis E infection, and took entecavir capsule to control the
proliferation of HBV, but their liver function (Hitachi 7060
automatic biochemical analyzer; Shanghai Kehua Bio-Engineering Co.,
Ltd.) and liver color ultrasound were normal, and the nucleic
results of hepatitis B virus were <2.00 IU/ml (normal range,
<2.00 IU/ml). The patient had no history of alcohol consumption.
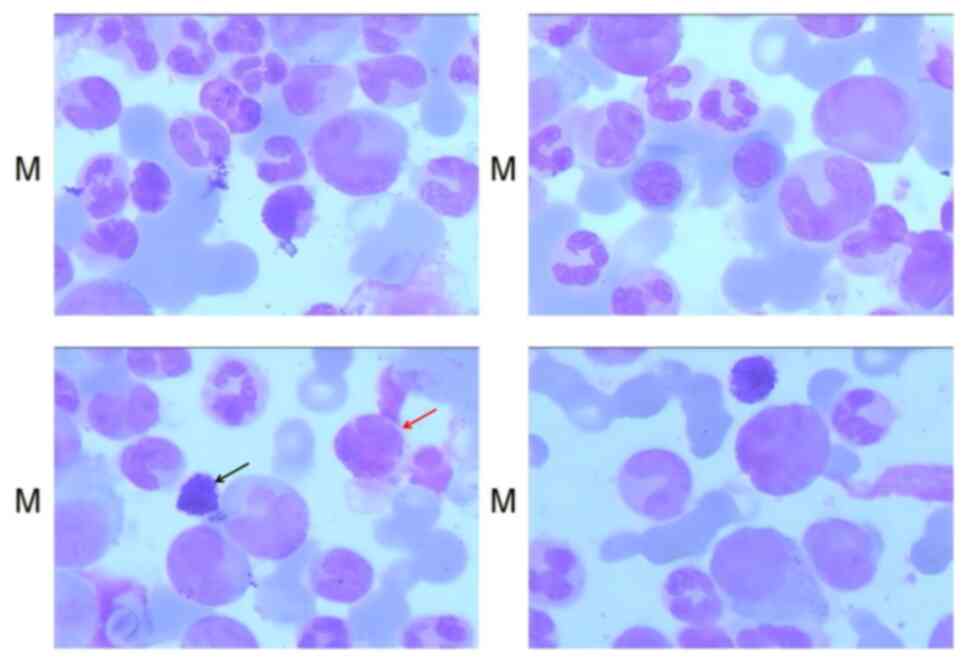
Bone marrow testing (10) was
performed after admission to hospital. Hyperactive bone marrow
proliferation was shown, with the proliferation of various stages
of the neutral myelocytes (5% acidophils and 3% alkalophils; 200
cells were manually counted under a microscope, and the specific
values are the ratio of acidophils and alkalophils in 200 cells
multiplied by 100%) (Fig. 2).
These findings were consistent with the symptoms of CML. The
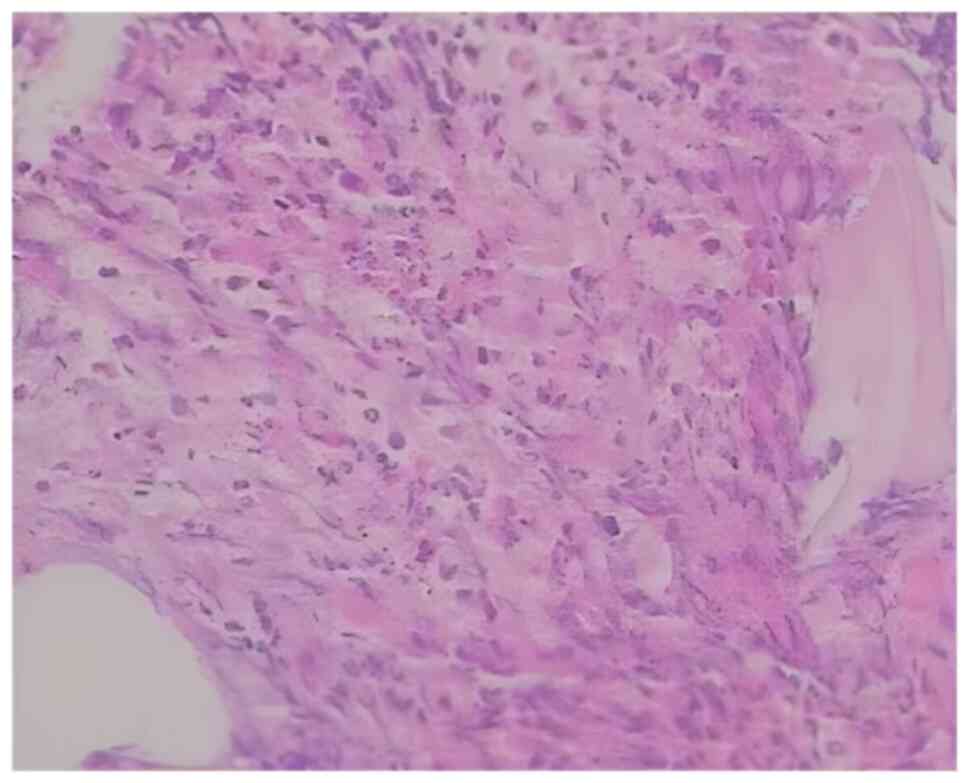
results of a bone marrow biopsy (fixed with Bouin fluid; thickness,
3 µm; lined separately for hepatocyte growth factor, H&E and
Gomori staining) (11-13)
demonstrated active bone marrow hyperplasia with an increased
granulocyte/erythrocyte ratio and granular hyperplasia, containing
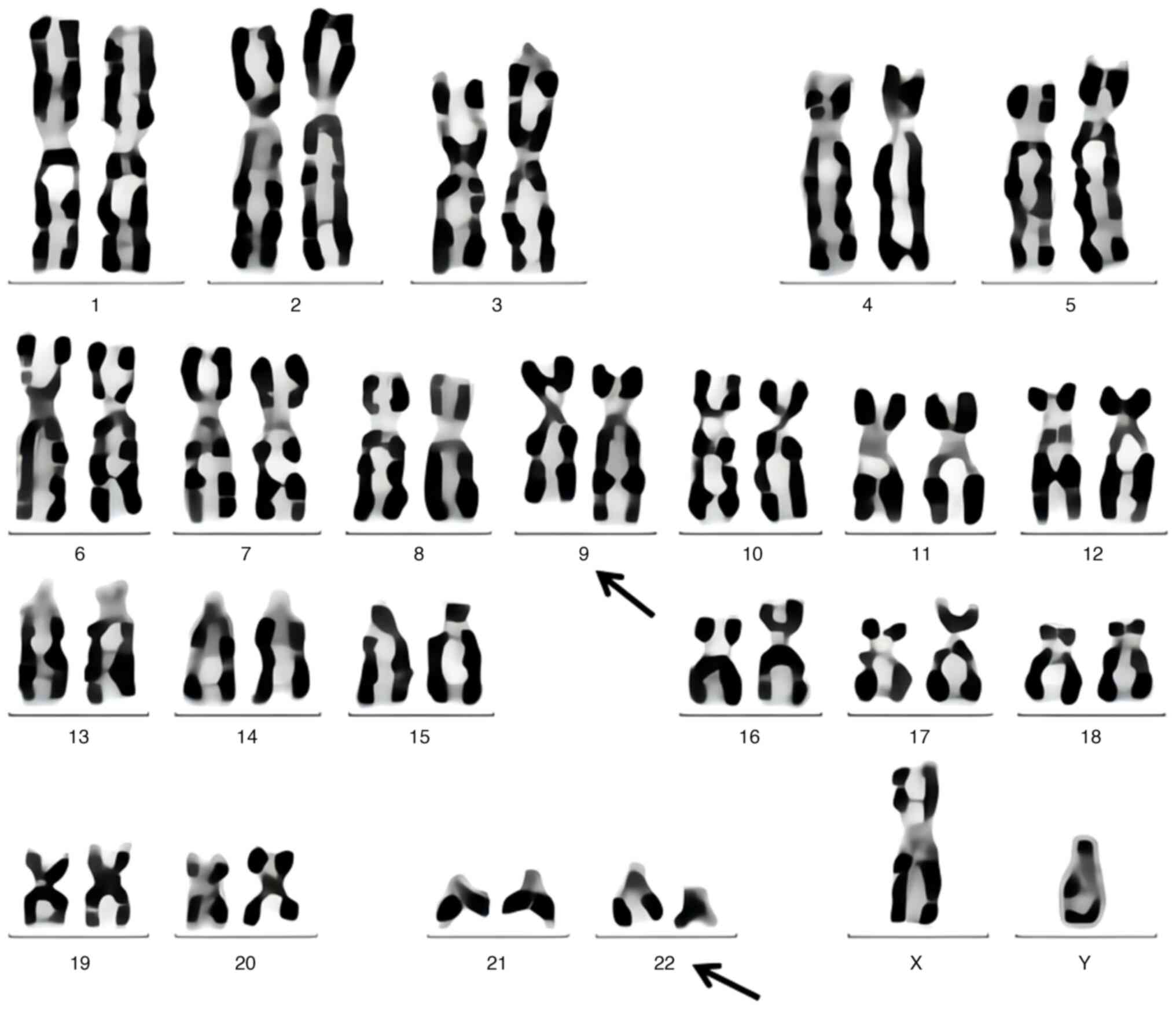
mature cells and acidophilic granulocytes (Fig. 3). The bone marrow chromosome test
results (GTG banding; ISCN 2009) (14,15)
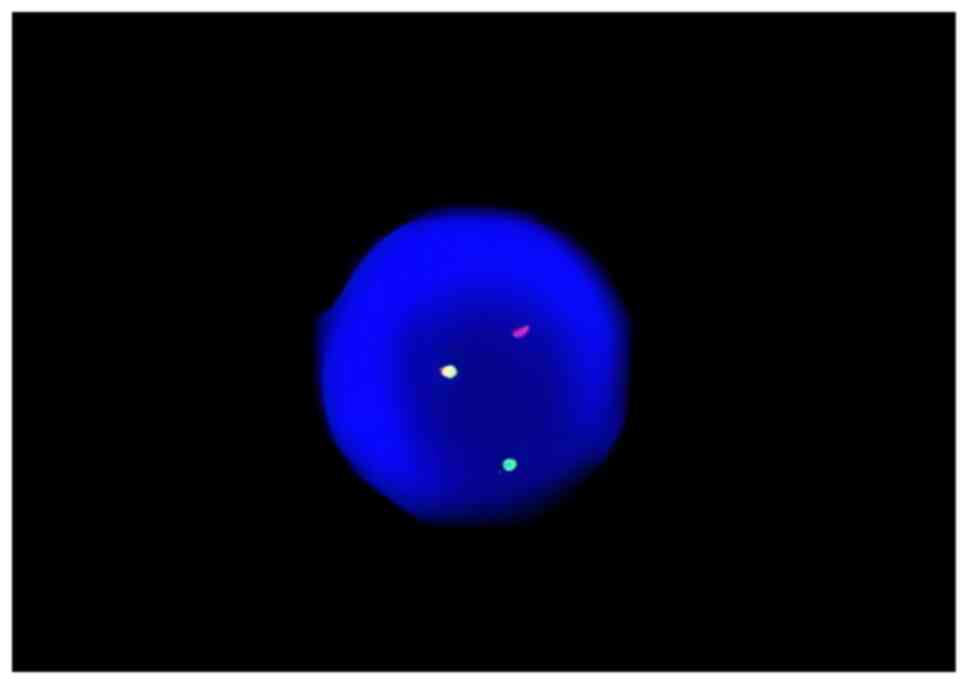
demonstrated the presence of the Philadelphia chromosome (Fig. 4). The results of bone marrow
fluorescence in situ hybridization experiment to detect
BCR/ABL fusion were positive (Fig.
5). Quantification of the BCR/ABL fusion gene P210 was
performed using a two-color dual-fusion DNA probe (Beijing Jinpujia
Medical Technology Co., Ltd.) and imaged using an OLYMPUS-BX51
fluorescence microscope (Olympus Corporation) (16). The quantification of the BCR/ABL
fusion gene (BCR-ABL copy number/ABL copy number x100%) was
demonstrated to be 251.9126% (17). Considering the patient's symptoms
and the results of blood and bone marrow-related tests, a diagnosis
of CML-chronic phase with a Sokal score of 1.2(18) was suggested. After admission, the
patient was treated with hydroxyurea (1.0 g orally three times a
day) for 2 weeks in March 2016 and then with 400 mg of IM/day since
the CML diagnosis in March 2016 (400 mg/day from March 2016; 300
mg/day from April 2017; 400 mg/day from January 2018). Routine
treatment with 400 mg of IM/day was continued after hospital
discharge. In July 2016, October 2016, January 2017 and April 2017,
the peripheral blood BCR/ABL fusion gene P210 quantification was
3.20, 0.16, 0.05 and 0.125%.
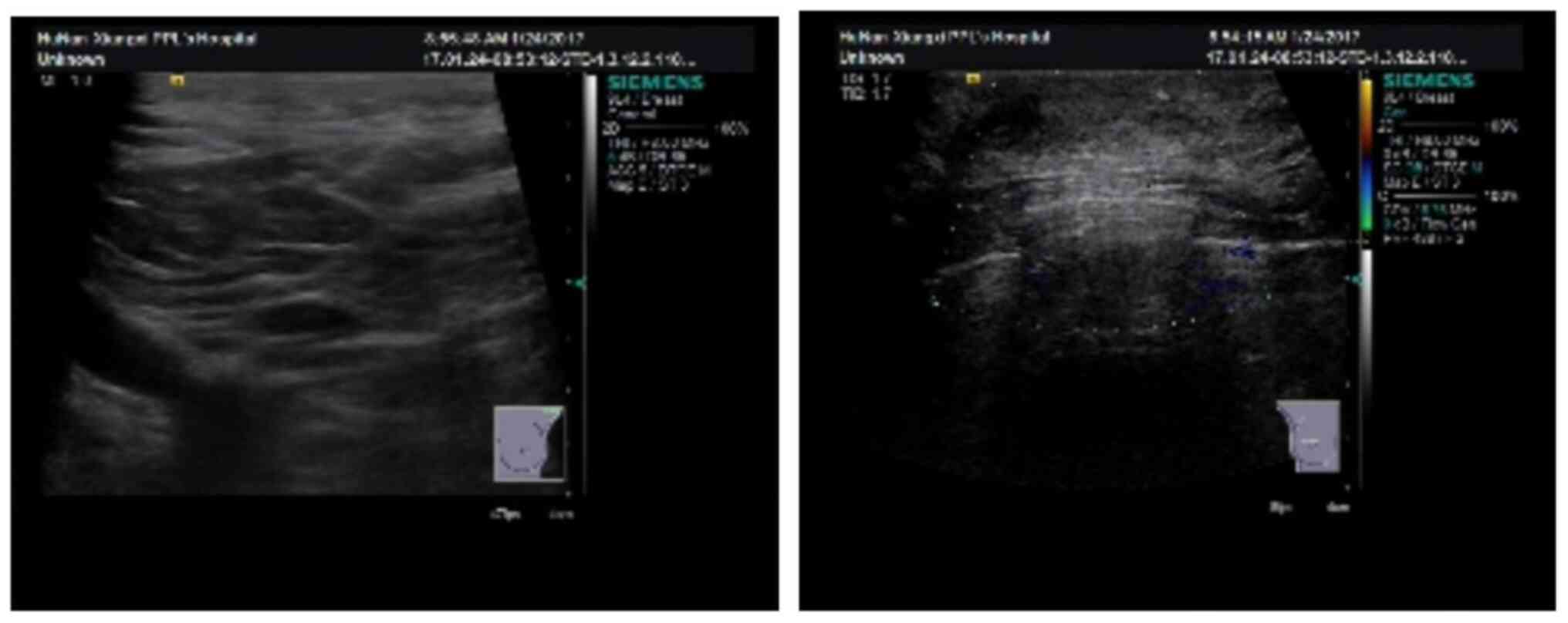
In January 2017, the patient visited the breast and
thyroid clinic at the aforementioned hospital with a complaint of
double breast pain. Color ultrasound of breast tissues demonstrated
that the size of the left breast was ~14x13x6 mm and the size of
the right breast was ~27x29x8 mm. Right breast enlargement was
observed (Fig. 6). The patient was
then advised to undergo breast surgery, but refused. A color
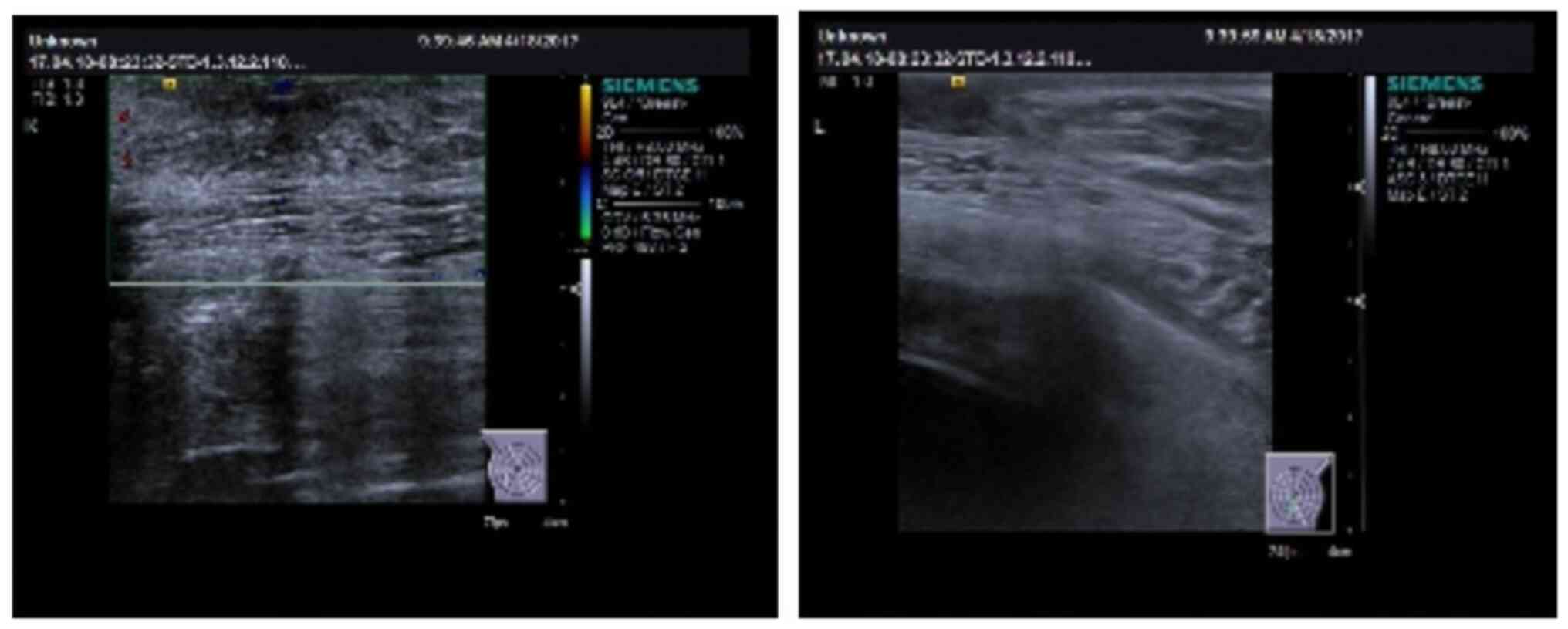
ultrasound performed in April 2017 to determine whether the mammary
gland exhibited an enlargement trend showed that the right breast
gland had a size of 34x27x11 mm (Fig.
7). A sex hormone test of a peripheral blood sample
demonstrated a marginal increase in progesterone levels (Table I). After follow-up, the potential
adverse effects caused by IM were considered. As the androgen level
was not decreased, testosterone undecanaic acid was not
administered. A reduction of the IM dose to 300 mg/day was
recommended to the patient to reduce the adverse side effects.
After this change in the treatment plan, the severity of the
patient's symptoms and the size of both breasts decreased, and the
patient reported no breast pain. Quantification of peripheral
BCR/ABL fusion gene expression was reported to be 0.067, 0.122 and
0.454% in July 2017, December 2017 and January 2018, respectively.
Subsequently, the IM dose was increased to 400 mg/day. Owing to the
side effects of IM and poor treatment efficacy, the patient
underwent dasatinib (orally; 100 mg/day) treatment since March
2018. A subsequent BCR/ABL quantification test showed negative
results and the patient reported no abnormal breast development.
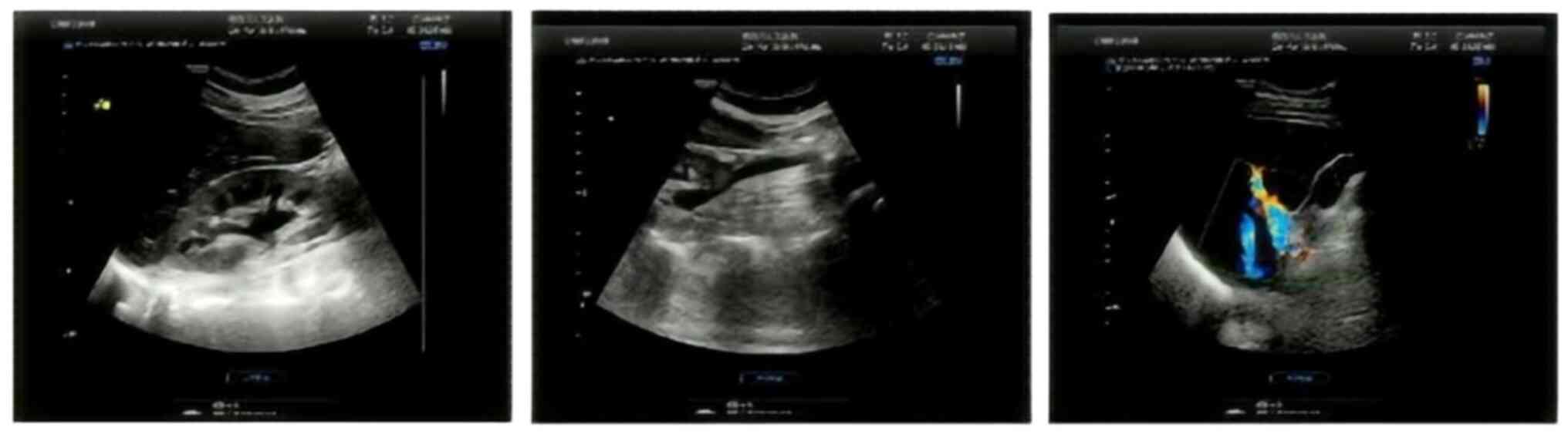
Whilst undergoing dasatinib treatment, a color ultrasound showed
normal liver, gallbladder and splenic function (Fig. 8). Peripheral blood liver and renal
function and HBV-DNA results were normal (Table II). In May 2024, the patient
underwent follow-up in the outpatient clinic. No specific symptoms
were observed during the last follow-up appointment. The patients
will be followed up in the outpatient clinic every 1-2 months.
 | Table ISex hormone test results. |
Table I
Sex hormone test results.
| Sex hormone | Patient result | Normal laboratory
reference value |
|---|
| Serum prolactin,
ng/ml | 7.66 | 4.79-23.30 |
| Folkopoietin,
mIU/ml | 12.26a | 25.80-134.80 |
| Luteinizing hormone,
mIU/ml | 4.89a | 7.70-58.50 |
| Estradiol, pg/ml | 28.00b | <5.00 |
| Progesterone,
ng/ml | 0.98b | 0.10-0.80 |
| Testosterone,
ng/ml | 9.28b | 0.08-0.48 |
 | Table IILaboratory test results of the
patient. |
Table II
Laboratory test results of the
patient.
| Variable | Patient result | Normal laboratory
reference value |
|---|
| Alanine
aminotransferase, U/l | 12.00 | 0.00-40.00 |
| Aspartate
aminotransferase, U/l | 17.00 | 0.00-40.00 |
| Total bilirubin,
µmol/l | 8.80 | 3.40-20.50 |
| Direct bilirubin,
µmol/l | 2.00 | 0.00-6.84 |
| Indirect bilirubin,
µmol/l | 6.80 | 0.00-18.00 |
| Cholinesterase,
U/l | 8,516.00 |
4,000.00-11,000.00 |
| Total bile acid,
mmol/l | 2.00 | 0.00-12.00 |
| Carbamide,
µmol/l | 4.50 | 2.50-7.14 |
| Creatinine,
µmol/l | 68.60 | 40.00-120.00 |
| Hepatitis B virus
DNA IU/ml | <2.00 | <2.00 |
Discussion
Gynecomastia is highly prevalent in the elderly and
may affect up to 24-65% of men among those aged 50-80 years
(19). Unlike in children and
young adults, gynecomastia in older adults is more likely to be
associated with certain pathological factors. Pathological
gynecomastia is associated with several factors, including liver
cirrhosis, drug therapy, hypogonadism, malnutrition,
hyperthyroidism, testicular tumors and chronic kidney disease
(20). IM is an antitumor-targeted
chemotherapeutic agent that inhibits tyrosine kinase receptors,
including c-Kit, BCR-ABL and platelet-derived growth factor
receptors (PDGFRs). These receptors regulate a number of cellular
processes, including cell proliferation, differentiation and
survival (21). Despite its
excellent safety profile, IM can cause distinct side effects, such
as edema, nausea, vomiting, fatigue, splenic rupture, severe rash,
breast cancer and gynecomastia (8,22).
The present study reported a rare case of IM-induced gynecomastia
in a patient with CML.
A limited number of studies have reported findings
on gynecomastia occurring in response to IM treatment (9,23-29).
The patient in the present study developed gynecomastia ~10 months
after starting treatment with IM. During IM administration, the
patient took the ‘entecavir capsule’ to control HBV replication and
without any solid tumors. Therefore, it was hypothesized that
gynecomastia was an adverse reaction caused by IM. However, owing
to the small number of cases currently published, the relationship
between IM and gynecomastia remains unclear. Therefore, the
relevant literature was reviewed using PubMed (https://pubmed.ncbi.nlm.nih.gov/?term=imatinib+and+gynecomastia&size=50;
key words, imatinib and gynecomastia) and discussed.
The literature suggested that free testosterone
levels decrease and estrogen levels increase in response to IM
administration, thus indicating that an imbalance in sex hormone
levels may lead to gynecomastia (26-29).
IM, as a TKI, can selectively block the c-Kit receptor and PDGFR
via the same mechanism (30), both
of which are expressed in both male and female gonads. By binding
with its ligands, IM can promote the proliferation and development
of primordial germ cells, thus maintaining their normal
physiological function and survival. In addition, IM also promotes
the secretion of testosterone by testicular stromal cells and
ovarian endometrial cells (31,32).
A number of clinical trials conducted on animals and patients have
reported that the serum levels of testosterone, follicular hormone
and luteinizing hormone increase significantly in response to IM
use (33). Hormone tests conducted
on patients treated with IM in China showed that the
triiodothyronine levels of some patients decreased and their
thyroid-stimulating hormone levels increased, whilst the
testosterone levels of some male patients decreased (34). These changes in hormone levels were
related to a number of corresponding clinical symptoms, such as
body temperature drop, cold hands and feet, loss of appetite,
fatigue, dizziness, memory loss, and edema for low triiodothyronine
levels, fear, myxoedema, weight gain and increased heart rate for
high thyroid-stimulating hormone levels, and decreased sexual
desire, lack of energy, mood abnormalities and osteoporosis for low
testosterone levels. These effects were also related to the
duration of drug administration; testosterone decreased with
administration time and was negatively associated with
administration time (34). Among
second-generation TKIs, dasatinib and nilotinib are also
multitarget inhibitors of target receptors such as c-Kit, tyrosine
kinases and PDGFR (35). Caocci
et al (36) reported a case
of gynecomastia in a patient with CML receiving dasatinib.
Therefore, the aforementioned studies indicated that IM may affect
the function of certain receptors, such as c-Kit and PDGFR, which
causes an imbalance in physiological sex hormone levels and can
lead to the abnormal development of breasts in male patients.
It has also been reported that gynecomastia caused
by IM may be dose-dependent. Gambacorti-Passerini et al
(27) reported a significant
association between the therapeutic dose of IM and gynecomastia,
with IM doses of 600-800 mg/day reducing testosterone levels more
than 400 mg/day IM. Zhao et al (29) achieved satisfactory treatment
efficacy by subsequently reducing the IM dose from 400 to 300 mg in
patients with CML. In the present case, the IM dose was reduced
from 400 to 300 mg/day, which improved the symptoms reported by the
patient. Subsequently, the patient was recommended an alternative
treatment, dasatinib, owing to the poor efficacy and side effects
of IM. Upon dasatinib treatment, the patient did not show further
signs of gynecomastia. This suggested that gynecomastia in the
patient in the present report was likely a side effect of IM
treatment.
Gynecomastia can be treated using radiotherapy,
surgery and medication. Drugs such as androgens, antiestrogens,
aromatase inhibitors, tamoxifen and danazol are reported to be
effective for the treatment gynecomastia (25,32,37,38).
With the continuous use of new and increasingly
effective TKIs, published reports on IM-induced male breast
development have decreased. However, clinicians should be aware of
such adverse reactions. Based on the results of the present case
report, it could be recommended that clinicians assess the hormonal
status of each patient before and during treatment and take timely
measures to compensate for the adverse consequences of sex hormone
imbalance.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Innovation
Platform and talent program of Hunan Province (grant no.
2021SK4050) and the Natural Science Foundation of Hunan Province
(grant nos. 2023JJ30608 and 2023JJ30609).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XLL, ML and ZWS were responsible for collecting
clinical, imaging and pathological data of the patient and drafting
the manuscript. HJF, SKT and LZW analyzed the data. KS designed the
study. JT participated in making the pathological diagnosis and was
involved in data collection and analysis. XLL and KS confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written consent for publication of the case report
and any accompanying images, without any potentially identifying
information, was provided by the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun J, Hu R, Han M, Tan Y, Xie M, Gao S
and Hu JF: Mechanisms underlying therapeutic resistance of tyrosine
kinase inhibitors in chronic myeloid leukemia. Int J Biol Sci.
20:175–181. 2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arzoun H, Srinivasan M, Thangaraj SR,
Thomas SS and Mohammed L: The progression of chronic myeloid
leukemia to myeloid sarcoma: A systematic review. Cureus.
14(e21077)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhou T, Medeiros LJ and Hu S: Chronic
myeloid leukemia: Beyond BCR-ABL1. Curr Hematol Malig Rep.
13:435–445. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jabbour E and Kantarjian H: Chronic
myeloid leukemia: 2016 Update on diagnosis, therapy, and
monitoring. Am J Hematol. 91:252–265. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stagno F, Stella S, Spitaleri A, Pennisi
MS, Di Raimondo F and Vigneri P: Imatinib mesylate in chronic
myeloid leukemia: Frontline treatment and long-term outcomes.
Expert Rev Anticancer Ther. 16:273–278. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kantarjian H, Sawyers C, Hochhaus A,
Guilhot F, Schiffer C, Gambacorti-Passerini C, Niederwieser D,
Resta D, Capdeville R, Zoellner U, et al: Hematologic and
cytogenetic responses to imatinib mesylate in chronic myelogenous
leukemia. N Engl J Med. 346:645–652. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kaygusuz-Atagunduz I, Toptas T, Yumuk F,
Firatli-Tuglular T and Bayik M: Newly diagnosed breast cancer in a
patient receiving imatinib mesylate. J Cancer Res Ther.
10:1107–1108. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pan L, Duan J, Qiao W, Bi L, Wu D, Fan Z
and Yang M: Secondary breast carcinoma after completely remitted
chronic myeloid leukemia following targeted tyrosine kinase
inhibitor therapy. Breast Cancer. 24:790–793. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tanriverdi O, Unubol M, Taskin F, Meydan
N, Sargin G, Guney E and Barutca S: Imatinib-associated bilateral
gynecomastia and unilateral testicular hydrocele in male patient
with metastatic gastrointestinal stromal tumor: A literature
review. J Oncol Pharm Pract. 18:303–310. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li N, Zhao L, Li J, Ding Y, Shen Y, Huang
X, Wang X and Wang J: Turner syndrome caused by rare complex
structural abnormalities involving chromosome X. Exp Ther Med.
14:2265–2270. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gala JL, Chenut F, Hong KB, Rodhain J,
Camby P, Philippe M and Scheiff JM: A panel of antibodies for the
immunostaining of Bouin's fixed bone marrow trephine biopsies. J
Clin Pathol. 50:521–524. 1997.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Narang NC, Rusia U, Sikka M and Kotru M:
Morphological changes in bone marrow post imatinib therapy in
chronic phase CML: A follow up study on sequential bone marrow
aspirates and Biopsies. J Clin Diagn Res. 11:EC25–EC29.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu YW and Duan MH: A unique bone marrow
lymphoma patient presenting with an isolated mass: A case report.
Oncol Lett. 15:2529–2533. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Al-Achkar W, Wafa A and Nweder MS: A
complex translocation t(5;9;22) in philadelphia cells involving the
short arm of chromosome 5 in a case of chronic myelogenous
leukemia. J Exp Clin Cancer Res. 26:411–415. 2007.PubMed/NCBI
|
|
15
|
Shaffer LG, Slovak ML and Campbell LJ
(eds): ISCN 2009: An international system for human cytogenetic
nomenclature. S. Karger, Basel, 2009.
|
|
16
|
Ewers E, Yoda K, Hamid AB, Weise A,
Manvelyan M and Liehr T: Centromere activity in dicentric small
supernumerary marker chromosomes. Chromosome Res. 18:555–562.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shen X, Zhang M, Shen Y, Shi W, Liu W and
Wei WU: Nilotinib rapidly reverses breakpoint cluster
region-Abelson oncogene fusion gene and M244V mutations in a
patient with chronic myelogenous leukemia: A case report. Exp Ther
Med. 10:1479–1482. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Syed NN, Usman M, Khaliq G, Adil SN and
Khurshid M: Clinico-pathologic features of chronic myeloid leukemia
and risk stratification according to Sokal score. J Coll Physicians
Surg Pak. 16:336–339. 2006.PubMed/NCBI
|
|
19
|
Leung AKC and Leung AAC: Gynecomastia in
infants, children, and adolescents. Recent Pat Endocr Metab Immune
Drug Discov. 10:127–137. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Karamchandani MM, De La Cruz Ku G, Sokol
BL, Chatterjee A and Homsy C: Management of gynecomastia and male
benign diseases. Surg Clin North Am. 102:989–1005. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kadivar A, Ibrahim Noordin M, Aditya A,
Kamalidehghan B, Davoudi ET, Sedghi R and Akbari Javar H:
Antiproliferative effects of imatinib mesylate on ZR-75-1 and
MDA-MB-231 cell lines via PDGFR-β, PDGF-BB, c-Kit and SCF
expression. Int J Mol Med. 42:414–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ma Z, Zhao J, Li S, Gao F, Zhang C, Wu L
and Lin Y: Imatinib-induced ulcerative colitis. J Oncol Pharm
Pract: 10781552241255290, 2024 (Epub ahead of print).
|
|
23
|
Gupta P, Cherian KE, Kapoor N, Fouzia NA
and Paul TV: IM-induced gynecomastia. Indian J Endocrinol Metab.
23(648)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kim H, Chang HM, Ryu MH, Kim TW, Sohn HJ,
Kim SE, Kang HJ, Park S, Lee JS and Kang YK: Concurrent male
gynecomastia and testicular hydrocele after imatinib mesylate
treatment of a gastrointestinal stromal tumor. J Korean Med Sci.
20:512–515. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu H, Liao G and Yan Z: Gynecomastia
during imatinib mesylate treatment for gastrointestinal stromal
tumor: A rare adverse event. BMC Gastroenterol.
11(116)2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jain A, Varma S, Garg R and Malhotra P:
Unilateral gynaecomastia in a young man with chronic myeloid
leukemia. Indian J Hematol Blood Transfus. 33:448–450.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gambacorti-Passerini C, Tornaghi L,
Cavagnini F, Rossi P, Pecori-Giraldi F, Mariani L, Cambiaghi N,
Pogliani E, Corneo G and Gnessi L: Gynaecomastia in men with
chronic myeloid leukaemia after imatinib. Lancet. 361:1954–1956.
2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tavil B, Kınık S, Gözen A and Olcay L:
Gynecomastia in a boy with chronic myeloid leukemia during imatinib
therapy. Turk J Haematol. 30:336–337. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhao D, Wang G, Li C and Meng L: Bilateral
masculine mastoplasia associated with imatinib mesylate: A case
report and literature review. J Huazhong Univ Sci Technolog Med
Sci. 31:145–146. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Griffin J: The biology of signal
transduction inhibition: Basic science to novel therapies. Semin
Oncol. 28 (5 Suppl 17):S3–S8. 2001.PubMed/NCBI
|
|
31
|
Sette C, Dolci S, Geremia R and Rossi P:
The role of stem cell factor and of alternative c-kit gene products
in the establishment, maintenance and function of germ cells. Int J
Dev Biol. 44:599–608. 2000.PubMed/NCBI
|
|
32
|
Basciani S, Brama M, Mariani S, De Luca G,
Arizzi M, Vesci L, Pisano C, Dolci S, Spera G and Gnessi L:
Imatinib mesylate inhibits Leydig cell tumor growth: Evidence for
in vitro and in vivo activity. Cancer Res. 65:1897–1903.
2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ghalaut VS, Prakash G, Bansal P, Dahiya K,
Dokwal S, Ghalaut PS, Bala M and Dhankhar R: Effect of imatinib on
male reproductive hormones in BCR-ABL positive CML patients: A
preliminary report. J Oncol Pharm Pract. 20:243–248.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hou Z, Zhu HL and Liu T: Effects of
imatinib mesylate on the levels of endocrine hormones. Zhonghua Xue
Ye Xue Za Zhi. 34:762–766. 2013.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
35
|
Lindauer M and Hochhaus A: Dasatinib.
Recent Results Cancer Res. 212:29–68. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Caocci G, Atzeni S, Orrù N, Azzena L,
Martorana L, Littera R, Ledda A and La Nasa G: Gynecomastia in a
male after dasatinib treatment for chronic myeloid leukemia.
Leukemia. 22:2127–2128. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Prasetyono TOH, Andromeda I and
Budhipramono AG: Approach to gynecomastia and pseudogynecomastia
surgical techniques and its outcome: A systematic review. J Plast
Reconstr Aesthet Surg. 75:1704–1728. 2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
McLeod DG and Iversen P: Gynecomastia in
patients with prostate cancer: A review of treatment options.
Urology. 56:713–720. 2000.PubMed/NCBI View Article : Google Scholar
|