In 2020, global cancer statistics reported prostate
cancer as the second most widespread cancer in American men,
representing 7.3% of all cases. Lung cancer was the most prevalent
cancer, accounting for 11.4% of all cases (1,2).
These statistics indicate an estimate of 1,141,259 (7.3%) new cases
and 375,304 mortalities worldwide attributed to prostate cancer
among men (1). Prostate cancer
affects a large number of men every year and the incidence and
mortality rates can be influenced by various factors, such as age,
ethnicity, genetic background and staging (3). Prostate cancer exhibits higher
incidence and mortality rates in developed countries compared with
developing countries (4). The
improvement of prostate cancer treatment and the understanding of
its pathogenesis are challenging tasks. Researchers have identified
exosomes and the tumor microenvironment (TME) as crucial areas of
study and active topics of current literature.
Exosomes are nano-sized organelles encased in a
single membrane, typically ranging between 30 to 200 nanometers in
diameter. These organelles contain various chemicals, including
proteins, lipids, nucleic acids and other substances such as amino
acids, and metabolites (5).
Exosomes are essential for various cellular activities and have the
capacity to transmit information between cells. These
single-membrane organelles can be secreted by various cell types
and mediate intracellular communication signaling (6). Exosomes are natural nanoparticles
that facilitate communication between cells, aiding in the
regulation of cancerous growth. These cellular messengers transfer
proteins and other biological substances through tissue fluids,
affecting the development of cancer (7). Exosomes have the potential to respond
to the growth and progression of tumor cells and can also have an
impact on the metastasis of tumor cells that are located in a
remote location (8). Exosomes have
a crucial function in controlling the TME by affecting various
processes, such as metastasis, angiogenesis and immunity. These
functions are crucial in altering the state of the TME (9). The scientific community and clinical
practitioners have shown considerable interest in the mechanism of
exosome function in tumors.
The TME encompasses tumor cells, surrounding cells
and their cytokine secretions, creating a conducive and abundant
environment for tumor survival and proliferation (10). It is important to highlight the
intricate and constantly altering nature of the TME, as well as the
influence that the type of tumor may have on its individual
components (11). However, the
essential components of the TME include immune and stromal cells,
blood vessels and the extracellular matrix (12). The composition of the TME and its
existence are critical for the tumor development, advancement and
spread (13). The understanding of
the impact of the TME on cancer development and progression is
critical for identifying novel treatment strategies. This
relationship can be considered similar to the connection between
soil and seeds, with exosomes serving as essential messengers
between the tumor and its environment (14). Previous research has revealed a
strong interaction between TME and exosomes (15). The interaction between exosomes and
TME remains unclear. As a result, the current review aimed to
examine the role of exosomes on the regulation of prostate cancer
cells within the TME.
The structure of exosomes and their inherent
biological activity render them significant to intercellular
communication. Their discovery and significance first gained
prominence in a 1983 article by Pan and Johnstone, which indicated
that exosomes released alongside transferrin receptors are involved
in sheep reticulocyte formation (16). Subsequent research has uncovered
the ability of exosomes to transport various RNA molecules,
including but not limited to mRNA, microRNA (miR), transfer RNA,
ribosomal RNA, small nuclear RNA, small nucleolar RNA,
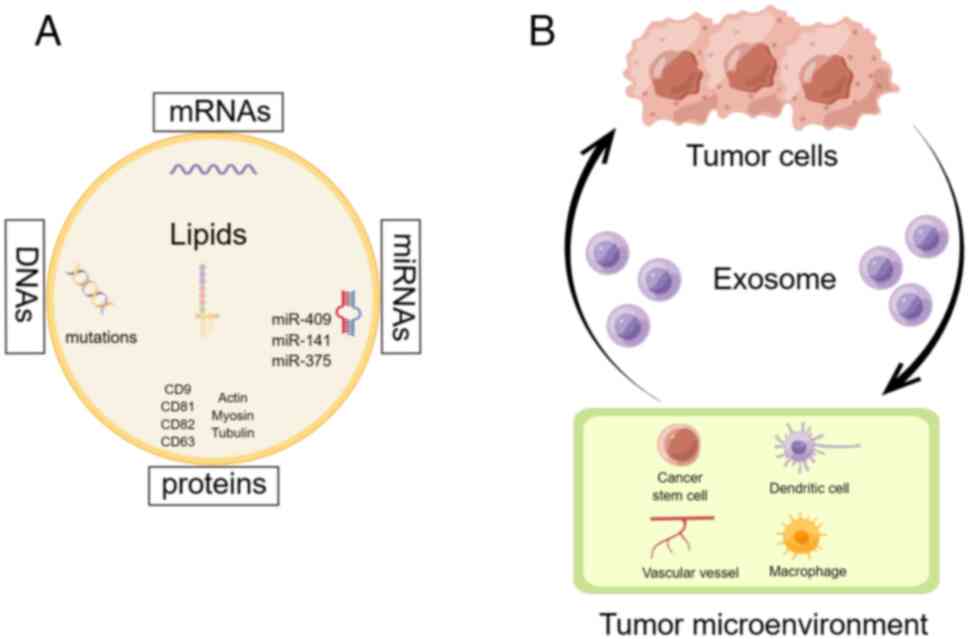
piwi-interacting RNA and small Cajal body-specific RNA (17). As shown in Fig. 1A, exosomes consist of various
components, such as miRs (miR-409, miR-141 and miR-375), mRNAs,
DNAs, lipids and functional proteins including cluster of
differentiation (CD) 9, CD81, CD82, CD83, actin, myosin and tubulin
(18).
These vesicles serve as important players in various
physiological and pathological processes, posing challenges for
researchers to fully comprehend their functions. Exosome formation
encompasses initiation, endocytosis, multivesicular formation and
secretion (19). Early endosomes
are formed during the initiation stage through the invagination of
cell membrane sites that contain ubiquitination surface receptors.
This process allows for membrane fusion, which creates a platform
for the detection of Fab1-YOTB-Vac1-EEA1 domain-containing
proteins, facilitated by the protein Rad5(20). As multivesicular bodies mature,
they can either undergo degradation by lysosomes or be secreted
from the cell via Golgi processing or exosomal release. The
proteins Rab GTPase (Rab) 6 and Rab7 have significant influence on
multivesicular bodies; Rab6 directs them towards lysosomal
degradation, while Rab7 guides them towards Golgi processing
(21). The involvement of the Rad
protein family is crucial in various stages of exosome
biogenesis.
Within the TME, tumor-related fibroblasts represent
a prominent cell population (22).
The functioning mechanism of fibroblasts associated with tumors
remains unknown. Fig. 1B
demonstrates the significant role of exosomes in enabling
communication between tumor cells and the neighboring
microenvironment. A study conducted by Baroni et al
(23) revealed that exosomes can
transport miR-9, which in turn transforms human breast fibroblasts
into cells resembling cancer-associated fibroblasts. Zhu et
al (24) discovered that
breast cancer cells can trigger cancer-associated fibroblast-like
characteristics in human breast fibroblasts through exosomal
miR-425-5p. The TGFβ1/reactive oxygen species (ROS) signaling
pathway is the mediator of this process (24). According to Yan et al
(25), exosomal miR-18b derived
from cancer-related fibroblasts can stimulate invasion and
metastasis of breast cancer by modulating transcription elongation
factor A-like 7.
Exosomes have been identified as significant players
in various cancers and diseases, including breast cancer. Kang
et al (26) reported that
exosomes derived from human umbilical cord mesenchymal stem cells
(hucMSCs) can boost neural function restoration in rats with spinal
cord injuries. According to their findings, exosomes obtained from
hucMSCs have potential therapeutic benefits in enhancing motor
function through their antiapoptotic and anti-inflammatory
properties. It is hypothesized that hucMSC exosomes may exert their
protective effects through modulation of the Bcl2/Bax and
Wnt/β-catenin signaling pathways, which are implicated in spinal
cord injury (26).
Moreover, endometrial cancer is impacted by
exosomes, as evidenced by a previous research study (30). Pan et al (30) demonstrated that exosomes containing
miR-503-3p from hucMSCs can impede the advancement of endometrial
cancer cells by suppressing mesoderm-specific transcripts. Exosomes
have been shown by numerous studies to enhance the functions of
tumor stromal cells in the microenvironment, leading to the
promotion of tumor progression (31,32).
Earlier studies have suggested that exosomes are
involved in the growth of tumors. A previous study conducted by
Giovannelli et al (33)
indicates that exosomes derived from prostate cancer cells can
affect the TME, leading to tumor progression. Nevertheless, low
oxygen levels and acidic conditions in the TME can influence the
production and absorption of exosomes by cancer cells (34,35).
Low pH will increase the yield of exosome separation (36,37).
A previous study has shown that an increase in the
pH levels in the TME can enhance the therapeutic effects of
pharmacological ascorbic acid on castration-resistant prostate
cancer cells (38). Xi et
al (39) have demonstrated
that hypoxia-induced activation of ataxia-telangiectasia mutated
regulates the secretion of exosomes that are involved in autophagy
by cancer-associated fibroblasts, thereby promoting cancer cell
invasion. Tumor-derived related exosomes can induce
immunosuppressive macrophages to promote the progression of
intrahepatic cholangiocarcinoma; however the specific underlying
mechanisms have not been fully elucidated (40). The intracellular signaling
mechanism of macrophages, the processes involving exosome uptake
and the regulatory payload of the TME have not been specifically
elucidated.
Dendritic cells are an important component present
in the TME. A previous study has shown that tumor-derived exosomes
can promote tumor metastasis and development by acting on dendritic
cells through the heat shock protein (HSP) 72/HSP105-toll-like
receptor (TLR) 2/TLR4 pathway (41). Pancreatic cancer is characterized
by a reduction in the number and function of dendritic cells, which
affects antigen presentation and contributes to immune tolerance
(42). Concomitantly,
hypoxia-induced exosome secretion promotes the survival of prostate
cancer cells in both African-American and native American
populations in the USA (43).
Three distinct pathways have been elucidated by which exosomes can
enter prostate cancer cells (44).
One mechanism of prostate cancer cell communication involves direct
fusion with the recipient cell membrane (45). The second pathway involves the
entrance of several molecules in the prostate cancer cell by
binding to its membrane surface (46). The third pathway involves the
process of endocytosis of prostate cancer cells that allows
exosomes to enter and secrete exosome contents (2). It should be mentioned that p53 has a
significant function in enhancing the dispersion of exosomes
(47,48). In the following sections, the role
of exosomes is examined in regulating intercellular communication
between prostate cancer cells and TME, focusing on three crucial
perspectives.
Normal prostate epithelial cells secrete exosomes
that prevent bone metastasis by failing to transport them to bone
stromal cells (54). A study
conducted by Karlsson et al (55) revealed that exosomes extracted from
the TRAMP-C1 mouse prostate cancer cell line can considerably
impede the advancement of mononuclear osteoclast precursors by
obstructing their maturation, leading to a deceleration in their
progression. Zhang et al indicated (2) that exosomes play a crucial role in
the progression of androgen-independent prostate cancer by
activating heme oxygenase 1. Exosomes have multiple functions,
which facilitate the spread of prostate cancer and promote the
proliferation of prostate cancer cells through the activity of
miRs. Therefore, the presence of miRs in exosomes may provide a
novel diagnostic method for prostate cancer (56,57).
The circ_0044516 exosome is highly promising as a biomarker, as it
has the capacity to increase the proliferation and metastasis of
prostate cancer cells (58). The
contribution of exosomes to prostate cancer metastasis is yet not
fully understood, indicating a need for further research in this
area.
The immune system plays a crucial role in the growth
of cancer. Effective communication between tumors and the immune
system is imperative for the development and spread of the tumor,
as well as its metastasis. Exosomes present in the environment
surrounding the tumor, can cause both chemotherapy resistance, as
well as immune system suppression (59). A detailed comprehension of the way
by which exosomes mediate immune responses in cancer is essential
to further examine the development of exosome-based
immunotherapies. Notably, the binding of programmed death-ligand-1
(PD-L1) to programmed cell death protein 1 (PD-1) and the
subsequent signaling to CD8+ cells aims to alleviate
immunosuppression (60). Numerous
research studies have revealed that prostate cancer cells increase
PD-1 expression to evade the immune system (61,62).
Simultaneously, the findings of Liu et al (63) have corroborated this assertion.
This study suggests that exosomes originating from gastric cancer
cells can elicit immune suppression by altering the gene expression
levels in CD8+ cells and the patterns of cytokine
secretion (63).
Exosome immunotherapy has gained significant
attention in recent years, particularly with regard to the impact
of nasopharyngeal carcinoma cell-derived exosome PD-L1 on
CD8+ T cell activity and immune evasion (64). The exosome PD-L1 has been
identified as a mechanism of immune resistance in non-small cell
lung cancer, which promotes the progression of tumors (65). Exosomal miRs serve as mediators in
the process of immune evasion in neuroblastoma (66). The immune evasion of breast cancer
is facilitated by the increase of exosome miR-27a-3p, which is
induced by endoplasmic reticulum stress. These exosomes regulate
PD-L1 expression in macrophages, thus reducing immune response
(67).
In patients with prostate cancer, myeloid suppressor
and dendritic cells have been observed in the TME and are known to
exhibit immunosuppressive effects. A previous study has indicated
that these cells may contribute significantly to the proliferation
and metastasis of cancer cells (68). Previous studies have indicated that
a large quantity of PD-L1 within exosomes can potentially signify
the advanced stages of prostate cancer, leading to a lower rate of
survival and negative outcome (69,70).
It should be emphasized that exosomes originating from prostate
cancer cells have the potential to alter the behavior of
macrophages and adjust the immune response (71).
Hypoxia-induced angiogenesis is a critical process
that drives the advancement and dissemination of prostate cancer
(72). Three stages of
angiogenesis have been identified: i) The formation of blood
vessels; ii) the angiogenesis stage; iii) and the maturation stage
(73). Angiogenesis requires the
synchronization of regulatory factors and activating signals
(74). Exosomes are known to carry
angiogenic factors, including VEGF and fibroblast growth factors,
which promote the growth of new blood vessels (75,76).
Early detection of prostate cancer is possible by utilizing
biomarkers that are derived from proteins present in plasma
exosomes associated with survival (77).
The Src tyrosine kinase is crucial for the
development and growth of prostate cancer (78). Src tyrosine kinases impact the
process of angiogenesis by activating signaling pathways through
integrin (79). Earlier
investigations have indicated that exosomes can contain Src
tyrosine kinases and facilitate the progression of prostate cancer
(80,81). Alcayaga-Miranda et al
(82) revealed that exosomes
extracted from menstrual stem cells have the ability to effectively
suppress angiogenesis caused by prostate cancer. A previous study
has indicated that exosomes can decrease ROS production and promote
VEGF release, while also lowering NF-κB activity (83). Exosomes have been shown to possess
an impact on the formation of blood vessels and as a result
influence the multiplication of cells in prostate cancer. However,
the precise mechanisms responsible for this effect require further
investigation. The in-depth understanding of the angiogenesis
mechanisms can be beneficial in improving prostate cancer treatment
and prognosis.
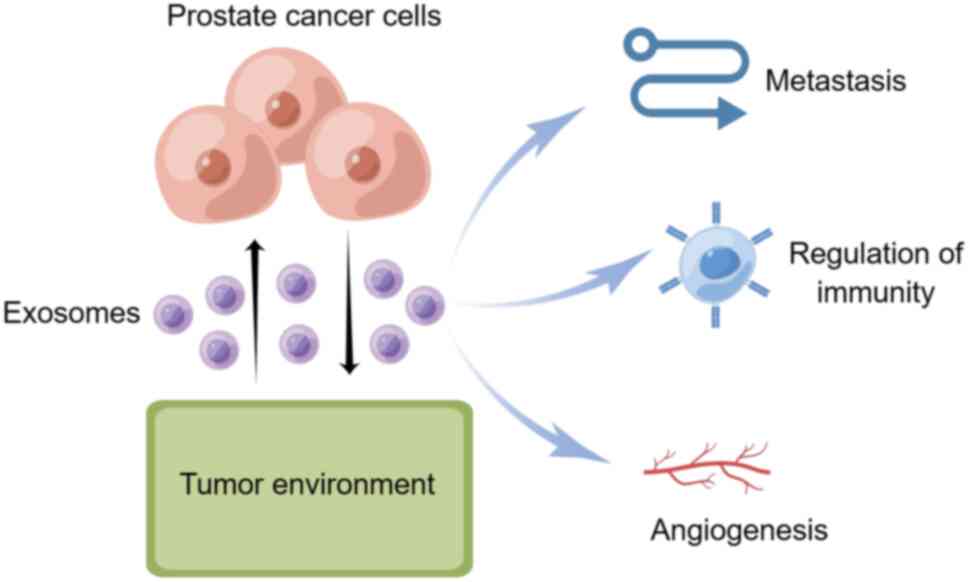
Exosomes have been found to impact cancer metastasis
and progression and regulate immunity and angiogenesis, which are
involved in the spread of prostate cancer cells (Fig. 2). Nonetheless, the specific
mechanisms underlying this correlation are currently uncertain.
Further analysis of these mechanisms has the potential to advance
the management and prediction of prostate cancer outcomes (Table I). Exosomes play a key inhibitory
role in the progression of androgen-independent prostate cancer by
activating heme oxygenase-1(2).
Effective communication between tumors and the immune system is
essential for tumor development and spread, as well as metastasis.
Exosomes in the tumor microenvironment promote the progression of
prostate cancer by secreting PD-L1, but macrophages in the immune
system are able to slow this process. Exosomes in the prostate
cancer microenvironment can promote the release of VEGF by reducing
the production of ROS, and at the same time reduce the activity of
NF-κB, inhibit angiogenesis, and effectively reduce the
proliferation of prostate cancer.
Not applicable.
Funding: No funding was received.
Not applicable.
YQW and XHL wrote the manuscript and made
substantial contributions to conception and design. XW and YZ drew
the pictures and analyzed and interpretated the data. YQW revised
the manuscript. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang Y, Chen B, Xu N, Xu P, Lin W, Liu C
and Huang P: Exosomes promote the transition of androgen-dependent
prostate cancer cells into androgen-independent manner through
up-regulating the heme oxygenase-1. Int J Nanomedicine. 16:315–327.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rebello RJ, Oing C, Knudsen KE, Loeb S,
Johnson DC, Reiter RE, Gillessen S, Van der Kwast T and Bristow RG:
Prostate cancer. Nat Rev Dis Primers. 7(9)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sandhu S, Moore CM, Chiong E, Beltran H,
Bristow RG and Williams SG: Prostate cancer. Lancet. 398:1075–1090.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pegtel DM and Gould SJ: Exosomes. Annu Rev
Biochem. 88:487–514. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Beit-Yannai E, Tabak S and Stamer WD:
Physical exosome:Exosome interactions. J Cell Mol Med.
22:2001–2006. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Terrasini N and Lionetti V: Exosomes in
critical illness. Crit Care Med. 45:1054–1060. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Soung YH, Ford S, Zhang V and Chung J:
Exosomes in cancer diagnostics. Cancers (Basel).
9(8)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
No authors listed. Exosomes. Nat
Biotechnol. 38(1150)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang L: Tumor microenvironment and
metabolism. Int J Mol Sci. 18(2729)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Anderson NM and Simon MC: The tumor
microenvironment. Curr Biol. 30:R921–R925. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jiang X, Wang J, Deng X, Xiong F, Zhang S,
Gong Z, Li X, Cao K, Deng H, He Y, et al: The role of
microenvironment in tumor angiogenesis. J Exp Clin Cancer Res.
39(204)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hu C, Chen M, Jiang R, Guo Y, Wu M and
Zhang X: Exosome-related tumor microenvironment. J Cancer.
9:3084–3092. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lau AN and Vander Heiden MG: Metabolism in
the tumor microenvironment. Annu Rev Cancer Biol. 4:17–40.
2020.
|
|
15
|
Ugel S, Canè S, De Sanctis F and Bronte V:
Monocytes in the tumor microenvironment. Annu Rev Pathol.
16:93–122. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pan BT and Johnstone RM: Fate of the
transferrin receptor during maturation of sheep reticulocytes in
vitro: Selective externalization of the receptor. Cell. 33:967–978.
1983.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wróblewska JP, Lach MS, Kulcenty K, Galus
Ł, Suchorska WM, Rösel D, Brábek J and Marszałek A: The analysis of
inflammation-related proteins in a cargo of exosomes derived from
the serum of uveal melanoma patients reveals potential biomarkers
of disease progression. Cancers (Basel). 13(3334)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Javed A, Kong N, Mathesh M, Duan W and
Yang W: Nanoarchitectonics-based electrochemical aptasensors for
highly efficient exosome detection. Sci Technol Adv Mater.
25(2345041)2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kakarla R, Hur J, Kim YJ, Kim J and Chwae
YJ: Apoptotic cell-derived exosomes: Messages from dying cells. Exp
Mol Med. 52:1–6. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kiral FR, Kohrs FE, Jin EJ and Hiesinger
PR: Rab GTPases and membrane trafficking in neurodegeneration. Curr
Biol. 28:R471–R486. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shikanai M, Yuzaki M and Kawauchi T: Rab
family small GTPases-mediated regulation of intracellular logistics
in neural development. Histol Histopathol. 33:765–771.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vitale I, Manic G, Coussens LM, Kroemer G
and Galluzzi L: Macrophages and metabolism in the tumor
microenvironment. Cell Metab. 30:36–50. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Baroni S, Romero-Cordoba S, Plantamura I,
Dugo M, D'Ippolito E, Cataldo A, Cosentino G, Angeloni V, Rossini
A, Daidone MG and Iorio MV: Exosome-mediated delivery of miR-9
induces cancer-associated fibroblast-like properties in human
breast fibroblasts. Cell Death Dis. 7(e2312)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhu Y, Dou H, Liu Y, Yu P, Li F, Wang Y
and Xiao M: Breast cancer exosome-derived miR-425-5p Induces
cancer-associated fibroblast-like properties in human mammary
fibroblasts by TGF β 1/ROS signaling pathway. Oxid Med Cell Longev.
2022(5266627)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yan Z, Sheng Z, Zheng Y, Feng R, Xiao Q,
Shi L, Li H, Yin C, Luo H, Hao C, et al: Cancer-associated
fibroblast-derived exosomal miR-18b promotes breast cancer invasion
and metastasis by regulating TCEAL7. Cell Death Dis.
12(1120)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kang J and Guo Y: Human umbilical cord
mesenchymal stem cells derived exosomes promote neurological
function recovery in a rat spinal cord injury model. Neurochem Res.
47:1532–1540. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang G, Yuan J, Cai X, Xu Z, Wang J,
Ocansey DKW, Yan Y, Qian H, Zhang X, Xu W and Mao F:
HucMSC-exosomes carrying miR-326 inhibit neddylation to relieve
inflammatory bowel disease in mice. Clin Transl Med.
10(e113)2020.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Zhang Z, Chen L, Chen X, Qin Y, Tian C,
Dai X, Meng R, Zhong Y, Liang W, Shen C, et al: Exosomes derived
from human umbilical cord mesenchymal stem cells (HUCMSC-EXO)
regulate autophagy through AMPK-ULK1 signaling pathway to
ameliorate diabetic cardiomyopathy. Biochem Biophys Res Commun.
632:195–203. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang X, Cui Z, Zeng B, Qiong Z and Long Z:
Human mesenchymal stem cell derived exosomes inhibit the survival
of human melanoma cells through modulating miR-138-5p/SOX4 pathway.
Cancer Biomark. 34:533–543. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pan Y, Wang X, Li Y, Yan P and Zhang H:
Human umbilical cord blood mesenchymal stem cells-derived exosomal
microRNA-503-3p inhibits progression of human endometrial cancer
cells through downregulating MEST. Cancer Gene Ther. 29:1130–1139.
2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Y, Huo M, Li W, Zhang H, Liu Q,
Jiang J, Fu Y and Huang C: Exosomes in tumor-stroma crosstalk:
Shaping the immune microenvironment in colorectal cancer. FASEB J.
38(e23548)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen XJ, Guo CH, Wang ZC, Yang Y, Pan YH,
Liang JY, Sun MG, Fan LS, Liang L and Wang W: Hypoxia-induced ZEB1
promotes cervical cancer immune evasion by strengthening the
CD47-SIRPα axis. Cell Commun Signal. 22(15)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Giovannelli P, Di Donato M, Galasso G,
Monaco A, Licitra F, Perillo B, Migliaccio A and Castoria G:
Communication between cells: Exosomes as a delivery system in
prostate cancer. Cell Commun Signal. 19(110)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Parolini I, Federici C, Raggi C, Lugini L,
Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A,
et al: Microenvironmental pH is a key factor for exosome traffic in
tumor cells. J Biol Chem. 284:34211–3422. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang X, Sun C, Huang X, Li J, Fu Z, Li W
and Yin Y: The advancing roles of exosomes in breast cancer. Front
Cell Dev Biol. 9(731062)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ban JJ, Lee M, Im W and Kim M: Low pH
increases the yield of exosome isolation. Biochem Biophys Res
Commun. 461:76–79. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Saber SH, Ali HEA, Gaballa R, Gaballah M,
Ali HI, Zerfaoui M and Abd Elmageed ZY: Exosomes are the driving
force in preparing the soil for the metastatic seeds: Lessons from
the prostate cancer. Cells. 9(564)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li Z, He P, Luo G, Shi X, Yuan G, Zhang B,
Seidl C, Gewies A, Wang Y, Zou Y, et al: Increased tumoral
microenvironmental pH improves cytotoxic effect of pharmacologic
ascorbic acid in castration-resistant prostate cancer cells. Front
Pharmacol. 11(570939)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xi L, Peng M, Liu S, Liu Y, Wan X, Hou Y,
Qin Y, Yang L, Chen S, Zeng H, et al: Hypoxia-stimulated ATM
activation regulates autophagy-associated exosome release from
cancer-associated fibroblasts to promote cancer cell invasion. J
Extracell Vesicles. 10(e12146)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Luo C, Xin H, Zhou Z, Hu Z, Sun R, Yao N,
Sun Q, Borjigin U, Wu X, Fan J, et al: Tumor-derived exosomes
induce immunosuppressive macrophages to foster intrahepatic
cholangiocarcinoma progression. Hepatology. 76:982–999.
2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shen Y, Guo D, Weng L, Wang S, Ma Z, Yang
Y, Wang P, Wang J and Cai Z: Tumor-derived exosomes educate
dendritic cells to promote tumor metastasis via
HSP72/HSP105-TLR2/TLR4 pathway. OncoImmunology.
6(e1362527)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Deicher A, Andersson R, Tingstedt B,
Lindell G, Bauden M and Ansari D: Targeting dendritic cells in
pancreatic ductal adenocarcinoma. Cancer Cell Int.
18(85)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Panigrahi GK, Praharaj PP, Peak TC, Long
J, Singh R, Rhim JS, Abd Elmageed ZY and Deep G: Hypoxia-induced
exosome secretion promotes survival of African-American and
Caucasian prostate cancer cells. Sci Rep. 8(3853)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Llorente A, Skotland T, Sylvänne T,
Kauhanen D, Róg T, Orłowski A, Vattulainen I, Ekroos K and Sandvig
K: Molecular lipidomics of exosomes released by PC-3 prostate
cancer cells. Biochim Biophys Acta. 1831:1302–1309. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bertokova A, Svecova N, Kozics K, Gabelova
A, Vikartovska A, Jane E, Hires M, Bertok T and Tkac J: Exosomes
from prostate cancer cell lines: Isolation optimisation and
characterisation. Biomed Pharmacother. 151(113093)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Spetzler D, Pawlowski TL, Tinder T,
Kimbrough J, Deng T, Kim J, Moran B, Conrad A, Esmay P and Kuslich
C: The molecular evolution of prostate cancer cell line exosomes
with passage number. J Clin Oncol. 28 (15 Suppl)(e21071)2010.
|
|
47
|
Müller JS, Burns DT, Griffin H, Wells GR,
Zendah RA, Munro B, Schneider C and Horvath R: RNA exosome
mutations in pontocerebellar hypoplasia alter ribosome biogenesis
and p53 levels. Life Sci Alliance. 3(e202000678)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ogawa K, Lin Q, Li L, Bai X, Chen X, Chen
H, Kong R, Wang Y, Zhu H, He F, et al: Aspartate β-hydroxylase
promotes pancreatic ductal adenocarcinoma metastasis through
activation of SRC signaling pathway. J Hematol Oncol.
12(144)2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Adekoya TO and Richardson RM: Cytokines
and chemokines as mediators of prostate cancer metastasis. Int J
Mol Sci. 21(4449)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Dann J, Castronovo FP, McKusick KA,
Griffin PP, Strauss HW and Prout GR Jr: Total bone uptake in
management of metastatic carcinoma of the prostate. J Urol.
137:444–448. 1987.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Chau CH and Figg WD: Molecular and
phenotypic heterogeneity of metastatic prostate cancer. Cancer Biol
Ther. 4:166–167. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bilen MA, Pan T, Lee YC, Lin SC, Yu G, Pan
J, Hawke D, Pan BF, Vykoukal J, Gray K, et al: Proteomics profiling
of exosomes from primary mouse osteoblasts under proliferation
versus mineralization conditions and characterization of their
uptake into prostate cancer cells. J Proteome Res. 16:2709–2728.
2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Renzulli JF II, Del Tatto M, Dooner G,
Aliotta J, Goldstein L, Dooner M, Colvin G, Chatterjee D and
Quesenberry P: Microvesicle induction of prostate specific gene
expression in normal human bone marrow cells. J Urol.
184:2165–2171. 2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Duan Y, Tan Z, Yang M, Li J, Liu C, Wang
C, Zhang F, Jin Y, Wang Y and Zhu L: PC-3-derived exosomes inhibit
osteoclast differentiation by downregulating miR-214 and blocking
NF-κB signaling pathway. Biomed Res Int.
2019(8650846)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Karlsson T, Lundholm M, Widmark A and
Persson E: Tumor cell-derived exosomes from the prostate cancer
cell line TRAMP-C1 impair osteoclast formation and differentiation.
PLoS One. 11(e0166284)2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Lee J, Kwon MH, Kim JA and Rhee WJ:
Detection of exosome miRNAs using molecular beacons for diagnosing
prostate cancer. Artif Cells Nanomed Biotechnol. 46 (Suppl
3):S52–S63. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Che Y, Shi X, Shi Y, Jiang X, Ai Q, Shi Y,
Gong F and Jiang W: Exosomes derived from miR-143-Overexpressing
MSCs inhibit cell migration and invasion in human prostate cancer
by downregulating TFF3. Mol Ther Nucleic Acids. 18:232–244.
2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Li T, Sun X and Chen L: Exosome
circ_0044516 promotes prostate cancer cell proliferation and
metastasis as a potential biomarker. J Cell Biochem. 121:2118–2126.
2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Petanidis S, Domvri K, Porpodis K,
Anestakis D, Freitag L, Hohenforst-Schmidt W, Tsavlis D and
Zarogoulidis K: Inhibition of kras-derived exosomes downregulates
immunosuppressive BACH2/GATA-3 expression via RIP-3 dependent
necroptosis and miR-146/miR-210 modulation. Biomed Pharmacother.
122(109461)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ayala-Mar S, Donoso-Quezada J and
González-Valdez J: Clinical implications of exosomal PD-L1 in
cancer immunotherapy. J Immunol Res. 2021(8839978)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang J, Zeng H, Zhang H and Han Y: The
role of exosomal PD-L1 in tumor immunotherapy. Transl Oncol.
14(101047)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Bai W, Tang X, Xiao T, Qiao Y, Tian X, Zhu
B, Chen J, Chen C, Li Y, Lin X, et al: Enhancing antitumor efficacy
of oncolytic virus M1 via albendazole-sustained CD8+ T
cell activation. Mol Ther Oncol. 32(200813)2024.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Liu J, Wu S, Zheng X, Zheng P, Fu Y, Wu C,
Lu B, Ju J and Jiang J: Immune suppressed tumor microenvironment by
exosomes derived from gastric cancer cells via modulating immune
functions. Sci Rep. 10(14749)2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Yang J, Chen J, Liang H and Yu Y:
Nasopharyngeal cancer cell-derived exosomal PD-L1 inhibits CD8+
T-cell activity and promotes immune escape. Cancer Sci.
113:3044–3054. 2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kim DH, Kim H, Choi YJ, Kim SY, Lee JE,
Sung KJ, Sung YH, Pack CG, Jung MK, Han B, et al: Exosomal PD-L1
promotes tumor growth through immune escape in non-small cell lung
cancer. Exp Mol Med. 51:1–13. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Schmittgen TD: Exosomal miRNA cargo as
mediator of immune escape mechanisms in neuroblastoma. Cancer Res.
79:1293–1294. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Yao X, Tu Y, Xu Y, Guo Y, Yao F and Zhang
X: Endoplasmic reticulum stress-induced exosomal miR-27a-3p
promotes immune escape in breast cancer via regulating PD-L1
expression in macrophages. J Cell Mol Med. 24:9560–9573.
2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Palicelli A, Bonacini M, Croci S, Bisagni
A, Zanetti E, De Biase D, Sanguedolce F, Ragazzi M, Zanelli M,
Chaux A, et al: What do we have to know about PD-L1 expression in
prostate cancer? A systematic literature review. Part 7: PD-L1
expression in liquid biopsy. J Pers Med. 11(1312)2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Xu W, Lu M, Xie S, Zhou D, Zhu M and Liang
C: Endoplasmic reticulum stress promotes prostate cancer cells to
release exosome and up-regulate PD-L1 expression via PI3K/Akt
signaling pathway in macrophages. J Cancer. 14:1062–1074.
2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Li D, Zhou X, Xu W, Chen Y, Mu C, Zhao X,
Yang T, Wang G, Wei L and Ma B: Prostate cancer cells
synergistically defend against CD8+ T cells by secreting
exosomal PD-L1. Cancer Med. 12:16405–16415. 2023.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Hosseini R, Asef-Kabiri L, Yousefi H,
Sarvnaz H, Salehi M, Akbari ME and Eskandari N: The roles of
tumor-derived exosomes in altered differentiation, maturation and
function of dendritic cells. Mol Cancer. 20(83)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Felmeden DC, Blann AD and Lip GYH:
Angiogenesis: Basic pathophysiology and implications for disease.
Eur Heart J. 24:586–603. 2003.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Kargozar S, Baino F, Hamzehlou S, Hamblin
MR and Mozafari M: Nanotechnology for angiogenesis: Opportunities
and challenges. Chem Soc Rev. 49:5008–5057. 2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Jin Y, Xing J, Xu K, Liu D and Zhuo Y:
Exosomes in the tumor microenvironment: Promoting cancer
progression. Front Immunol. 13(1025218)2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Yu L, Gui S, Liu Y, Qiu X, Zhang G, Zhang
X, Pan J, Fan J, Qi S and Qiu B: Exosomes derived from
microRNA-199a-overexpressing mesenchymal stem cells inhibit glioma
progression by down-regulating AGAP2. Aging (Albany NY).
11:5300–5318. 2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zhang C, Ji Q, Yang Y, Li Q and Wang Z:
Exosome: Function and role in cancer metastasis and drug
resistance. Technol Cancer Res Treat.
17(1533033818763450)2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Khan S, Jutzy JMS, Valenzuela MMA, Turay
D, Aspe JR, Ashok A, Mirshahidi S, Mercola D, Lilly MB and Wall NR:
Plasma-derived exosomal survivin, a plausible biomarker for early
detection of prostate cancer. PLoS One. 7(e46737)2012.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Bagnato G, Leopizzi M, Urciuoli E and
Peruzzi B: Nuclear functions of the tyrosine kinase Src. Int J Mol
Sci. 21(2675)2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Rivera-Torres J and San José E: Src
tyrosine kinase inhibitors: New perspectives on their immune,
antiviral, and senotherapeutic potential. Front Pharmacol.
10(1011)2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
DeRita RM, Zerlanko B, Singh A, Lu H,
Iozzo RV, Benovic JL and Languino RL: c-Src, insulin-like growth
factor I receptor, G-protein-coupled receptor kinases and focal
adhesion kinase are enriched into prostate cancer cell exosomes. J
Cell Biochem. 118:66–73. 2016.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Larssen P, Wik L, Czarnewski P, Eldh M,
Löf L, Ronquist KG, Dubois L, Freyhult E, Gallant CJ, Oelrich J, et
al: Tracing cellular origin of human exosomes using multiplex
proximity extension assays. Mol Cell Proteomics. 16:502–511.
2017.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Alcayaga-Miranda F, González PL,
Lopez-Verrilli A, Varas-Godoy M, Aguila-Díaz C, Contreras L and
Khoury M: Prostate tumor-induced angiogenesis is blocked by
exosomes derived from menstrual stem cells through the inhibition
of reactive oxygen species. Oncotarget. 7:44462–44477.
2016.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Maisto R, Oltra M, Vidal-Gil L,
Martínez-Gil N, Sancho-Pellúz J, Filippo CD, Rossi S, D Amico M,
Barcia JM and Romero FJ: ARPE-19-derived VEGF-containing exosomes
promote neovascularization in HUVEC: The role of the melanocortin
receptor 5. Cell Cycle. 18:413–424. 2019.PubMed/NCBI View Article : Google Scholar
|